Abstract
Mycoplasma genitalium is an emerging sexually transmitted infection and in women is associated with notable reproductive tract syndromes such as cervicitis, pelvic inflammatory disease, and infertility. Investigations into the causal relationships of M. genitalium infections and clinical disease have been hindered largely by the lack of a well-established small-animal model of genital tract infection. To establish a murine model, female Swiss Webster mice were conditioned with either progesterone or estradiol and then inoculated intravaginally with M. genitalium type strain G37 or a contemporary Danish strain, M2300. Persistent lower tract infection was observed at up to 77 days postinoculation (d.p.i.). Upper reproductive tract colonization was observed as early as 3 d.p.i., with long-term infection observed in estradiol-treated (65%) and progesterone-treated (18%) animals. In the upper tract, more than 90% of M. genitalium PCR-positive samples were from the uterus and oviducts. Ultimately, gross hydrosalpinx was observed 21 days to 10 weeks p.i. in approximately 60% of infected animals, suggesting the presence of tubal occlusion. In addition, dissemination of M. genitalium to the knee tissues was observed as early as 7 d.p.i., with persistent infection detected at up to 28 d.p.i. Mice infected with M. genitalium also developed specific antibodies to the major antigenic outer membrane protein MgPa, elongation factor Tu, pyruvate dehydrogenase E1α, and DnaK (Hsp70), indicating persistent infection despite robust humoral responses to infection. These findings provide strong experimental evidence that M. genitalium can establish long-term infection of reproductive tract and joint tissues, with preliminary evidence of pathological reproductive tract outcomes.
Mycoplasma genitalium is an emerging sexually transmitted pathogen that was first identified as a cause of inflammatory urogenital disease in men (reviewed in references 15 and 18). Importantly, M. genitalium infections in women have also been associated with inflammatory syndromes, including cervicitis (8, 24, 27, 32, 50) and pelvic inflammatory disease (12), and (serologically) with impaired fertility (4, 37). Mechanistically, M. genitalium has previously been shown to activate highly expressed Toll-like receptors in reproductive tract epithelia, leading to inflammation (28, 29). Collectively, these associations have led to an increased awareness of M. genitalium as a pathogen that could adversely affect reproductive health. It may also be of considerable importance that M. genitalium is strongly associated with HIV infections in men and women (reviewed in reference 31), suggesting that reproductive tract infections by M. genitalium may increase the likelihood of acquiring or transmitting other genital pathogens.
Although the genital tract seems to be a preferred site of colonization, M. genitalium also has been a suspected cause of reactive arthritis, since DNA was previously detected in the knee joints of arthritic patients (45, 47) and in synovial fluid from temporomandibular joints (23). It is possible that M. genitalium may be a cause of sexually acquired reactive arthritis, but to date, no direct evidence exists for an association with an arthritic condition. Furthermore, no published reports have addressed the ability of M. genitalium to disseminate from the vagina to colonize the joint tissues or upper genital tract tissues. Similarly, there is a lack of experimental evidence for the causal associations of M. genitalium infection with inflammatory disease syndromes in women.
As epidemiological data continue to implicate M. genitalium as a cause of reproductive tract disease, relevant animal models to investigate pathogenesis and evaluate therapeutic interventions are of utmost importance. Five years after the initial isolation of M. genitalium from men with urethritis (48), several large-animal species, including male cynomolgus monkeys (Macaca fascicularis), male chimpanzees (Pan troglodytes), female squirrel monkeys (Saimiri sciureus), female tamarins (Saguinus mystax), and female marmosets (Callithrix jacchus [44]), were found to be susceptible to experimental urogenital infection. These studies provided excellent preliminary evidence that M. genitalium could establish infection of female reproductive tract tissues. However, the cost of developing and maintaining primate and large-animal models prohibits experimentation employing larger-scale studies to address biological variability as part of an effective model of reproductive tract disease. In contrast, rodent models are cost-effective and afford the opportunity to investigate larger study populations of animals with specific genetic characteristics. Such models also allow evaluation of vaccines and interventions to prioritize lead compounds for subsequent study of larger species. Experiments by Furr and Taylor-Robinson and colleagues provided preliminary evidence that the type strain of M. genitalium (G37) could establish genital tract infection in inbred female BALB/c mice (10). Considering the emerging clinical associations with upper reproductive tract disease, it now seems imperative to address the capacity of M. genitalium to disseminate from the vagina and establish upper reproductive tract infection.
Systemic regulation of sex hormones prior to vaginal inoculation affords several important advantages for experimental manipulation, including rendering otherwise-resistant animals susceptible to infection and arresting the reproductive cycle to synchronize the estrus phase of animals within a study (43). With regard to mycoplasmas, it is unclear why some species, such as M. pneumoniae and M. pulmonis, require progesterone treatment whereas others, including the genital pathogen M. fermentans, colonize the genital tract only following estrogen treatment (10). The mouse model proposed by Taylor-Robinson's group showed that BALB/c mice were susceptible to vaginal M. genitalium infection only following progesterone treatment and not after estradiol benzoate treatment (40, 41). To our knowledge, this model has not been employed since for investigation of M. genitalium genital tract disease.
In the present paper, we describe results compiled from more than 3 years of experimentation that show the successful establishment of a reproducible murine model to investigate M. genitalium infection of the female reproductive tract. The presented findings provide strong evidence that, following vaginal exposure of M. genitalium, the lower and upper reproductive tract tissues become persistently colonized, resulting in long-term shedding from the vagina and serious disease in more than half of the members of groups of infected animals. Animals conditioned with either estradiol or progesterone were susceptible to infection, resulting in differential rates of dissemination to upper tract tissues and the development of hydrosalpinx. Importantly, colonization of the knee joints was also observed, providing evidence that M. genitalium can disseminate from the vagina to colonize synovial sites as well. A reproducible small-animal model to study M. genitalium pathobiology would be indispensable for continued investigation into the mechanisms of disease and coinfection with other sexually transmitted infection (STI) pathogens and for evaluation of novel therapeutics, preventatives, and vaccines.
MATERIALS AND METHODS
Propagation of M. genitalium.
M. genitalium type strain G37 (ATCC 33530) or the low-passage-number, clinically derived Danish M2300 strain (kindly provided by Jørgen Jensen; Staten Serum Institut, Copenhagen, Denmark) was propagated in modified Friis FB medium as described previously (19) with 100 U/ml of penicillin G and 100 μg/ml of streptomycin sulfate. Immediately following a pH-mediated color change of the culture medium, adherent microcolonies were washed extensively with sterile phosphate-buffered saline (PBS) before being scraped into fresh PBS for immediate inoculation. M. genitalium was passaged in culture minimally prior to inoculation of animals to reduce further adaptation to axenic culture conditions. Following receipt from the ATCC, M. genitalium G37 was passaged once to establish frozen stocks and then a second time to generate the log-phase M. genitalium mouse inocula. The same conservative passage measures were employed for M. genitalium strain M2300 as well. Viable M. genitalium titers were quantified using a color-changing-unit (CCU) assay (10) in Friis medium (∼2 × 107/20 μl of inoculum) and confirmed by quantitative PCR as described below.
Hormone treatment and vaginal inoculation paradigm.
For all studies, animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved quarters and provided with unlimited access to food and water and environmental enrichment. All procedures were performed humanely in concordance with a University of Texas Medical Branch (UTMB) Animal Care and Use Committee-approved protocol. For all studies, 5-week-old female Swiss Webster mice (Harlan Sprague Dawley, Inc., Indianapolis, IN) were administered a subcutaneous injection of either estradiol cypionate (Depo-estradiol; Pfizer, New York, NY) (0.25 mg/20 g of animal weight) or medroxyprogesterone acetate (Sicor, Irvine, CA) (3 mg/20 g) at 7 days and at 1 day prior to M. genitalium inoculation. Immediately prior to inoculation, mice were swabbed once with a PBS-soaked calcium alginate swab to remove vaginal debris and then once with a “dry” swab to remove excess liquid from the vagina to better expose the mucosa for inoculation. Animals were inoculated intravaginally with 2 × 107 CCU/20 μl of M. genitalium G37 or M2300 in sterile PBS. In each independent study, mock-infected mice were hormone treated but received only the PBS vehicle to control for nonspecific PCR detection and false positives in viability testing.
Determining the ratio of M. genitalium genomes to viable organisms.
Quantitative PCR (described below) was used to determine primary outcomes for several experiments presented in this paper. In order to approximate the ratio of genomes to viable organisms, M. genitalium strain G37 or M2300 bacteria were seeded into fresh Friis FB medium in a 48-well plate and incubated at 37°C for 3 days. At 24, 48, and 72 h postinoculation (p.i.), triplicate wells for each strain were individually harvested by removal of the culture supernatant and scraping adherent microcolonies into 0.5 ml of sterile PBS. Next, 0.1 ml was removed from the culture well and added to lysis buffer for DNA purification and PCR quantification as described above. For viable titration, 10 μl was removed from the culture well and titrated by serial 10-fold dilution in Friis FB medium. Titration plates were then incubated at 37°C for 14 days. The last well in the dilution series that showed a color change and adherent microcolony formation was used to calculate the approximate number of viable organisms in the original sample.
Early dynamics of vaginal M. genitalium infection.
In order to better understand the acute-phase dynamics of vaginal infection, female mice were inoculated intravaginally with M. genitalium and then vaginal titers were monitored using quantitative PCR and viability assays. Because PCR was used as a tool for quantification, we also inoculated mice with UV-killed M. genitalium G37 by the use of a Stratalinker 2400 UV crosslinker (Stratagene, La Jolla, CA) (254 nm; total energy of 720,000 microjoules/cm2) or with purified genomic DNA to determine the longevity of nonviable organisms or M. genitalium genomes in the vagina. Loss of bacterial viability after UV irradiation was verified by an absence of M. genitalium growth in Friis FB medium after 21 days of incubation at 37°C.
Following inoculation of viable M. genitalium, UV-killed M. genitalium, or purified M. genitalium DNA, vaginal swabs were collected at the indicated times and placed into 0.5 ml of sterile PBS. For viability testing, each swab was vortexed and then 0.2 ml was removed and placed into 1 ml Friis FB medium. Samples for viable outgrowth were incubated at 37°C for 21 days and observed daily for a color change of the medium and the formation of adherent microcolonies. M. genitalium detection by PCR utilized 0.2 ml of the original swab volume. DNA was extracted from the swab specimens per the manufacturer's instructions and eluted into 100 μl of the kit-provided elution buffer (described below). M. genitalium DNA was quantified using real-time PCR (also described below).
Determining M. genitalium viability from swab and tissue specimens.
We first investigated whether M. genitalium was disseminating from the vagina to the upper reproductive tract and knee tissues by culture of M. genitalium collected directly from tissue homogenates. Following estradiol or progesterone treatment and vaginal inoculation, M. genitalium viability was determined from vaginal swabs (at 3 d.p.i.) and upper reproductive tract tissues (at 3 to 4 weeks p.i.). Reproductive tract and knee tissues were aseptically dissected from each mouse by the use of multiple sets of instruments that were thoroughly cleaned and sterilized prior to dissection. Immediately following euthanasia, mice were doused and rinsed with 70% ethanol three times before careful dissection of each tissue using aseptic techniques. A small border on each side of the tissue juncture (e.g., between the uterus and oviduct) was maintained when the reproductive tract tissues were separated so that no tissue from the adjacent site was collected. With dissecting microscope assistance, a new razor blade was used for each animal to make fine separations from each tissue sample to prevent cross-contamination. Similarly, each reproductive tract tissue sample was minced thoroughly with a new razor blade. For knee tissues, the muscle and connective tissues were removed from the long bones and knee joint with a new razor blade as well. The knee tissue and joint were separated from the femur and the tibia-fibula by cutting each bone just above or below the joint. The knee tissue and bones were macerated with forceps and minced with scissors prior to DNA extraction or viability testing. Several sets of instruments were employed for the dissections, and each set was either replaced or rinsed at least three times with 70% ethanol before moving to the next tissue sample. Instruments were always changed between experimental groups, and extreme caution was taken to minimize the possibility of contamination between tissue samples and among mice. Mock-inoculated animals were dissected in parallel to serve as indicators of any contamination.
M. genitalium viability was determined by inoculation of reproductive tract or knee tissue homogenates into Friis FB medium for growth at 37°C (for up to 21 days). All tested specimens showing a change in medium color, indicating microbial growth, were observed for attached microcolony formation and then sampled for molecular identification as described below. The Friis FB growth medium contained penicillin and streptomycin to prevent the growth of commensal flora from vaginal swab and tissue samples or environmental contaminants. M. genitalium strains G37 and M2300 were resistant to penicillin G (100 U/ml) and streptomycin (100 μg/ml); to maintain consistency among studies, these antibiotics were included in the Friis FB medium for growth of the M. genitalium inoculum as well.
PCR quantification and molecular typing of M. genitalium.
DNA from all specimens was extracted using a DNEasy 96 or DNEasy blood and tissue spin column kit (Qiagen, Valencia, CA) and eluted into a 0.1-ml volume. To quantify M. genitalium, a real-time PCR assay targeting the single-copy MG309 gene was performed. The sense primer (5′-TTTACACCGAGTAAAATGACCATT-3′), antisense primer (5′-AGATTTTCAATGGTTCTGGAGTTT-3′), and TaqMan probe [5′-(6-carboxyfluorescein)ACAGGCAACAGCAACAATTCCCAA(Black Hole Quencher-1A)-3′] amplified a conserved region specific to M. genitalium. Cycling parameters were 95°C for 5 min, followed by 50 cycles of 95°C for 15 s, 50°C for 32 s, and 72°C for 15 s. A standard curve of a prequantified, MG309-containing plasmid was run in duplicate on each 96-well plate and used to extrapolate M. genitalium DNA loads. Samples from mock-inoculated animals and PCRs that lacked template served as negative controls. Real-time PCR assays were completed using a 7500 real-time PCR system (Applied Biosystems, Foster City, CA).
Multiple molecular techniques were used to confirm the isolation and identity of M. genitalium from animal specimens. First, the presence of M. genitalium DNA in animal swabs or tissues was established using the MG309 PCR assay described above. Next, five MG309 PCR-positive tissue samples were selected at random for verification of M. genitalium identity by the use of PCR and DNA sequencing of the bacterial 16S rRNA gene and the 27F and 518R primers described previously (11). As a final confirmatory identification, a nested PCR was completed that first targeted an approximately 600-bp region of the MG191 gene (16) and then a 281-bp hypervariable subfragment (20) for sequencing and nucleotide alignment. Nucleotide sequences of the resultant PCR products were analyzed using the BLAST algorithm and then compared to sequences generated from the M. genitalium inocula, confirming a lack of cross-contamination between the strains.
Evaluation of hydrosalpinx in M. genitalium-inoculated mice.
In two independent studies (n = 28), M. genitalium G37 or M2300 was inoculated intravaginally into estradiol-conditioned mice as described above. Mice were humanely euthanized at 14 d.p.i. (n = 3 each for G37 and M2300), 21 to 30 d.p.i. (n = 5), or 10 weeks p.i. (n = 6) and carefully dissected to evaluate the presence of gross unilateral or bilateral hydrosalpinx fluid accumulation at the oviduct. Oviduct tissues were then homogenized into Friis FB mycoplasmal growth medium and incubated at 37°C for viable outgrowth of M. genitalium as described above.
Mouse antibody response to M. genitalium G37.
To investigate the host response to M. genitalium infection and determine the most immunodominant proteins, serum was collected from M. genitalium G37-infected or mock-inoculated mice (at the termination date [77 d.p.i.]) for immunoblot experiments using M. genitalium G37 lysates, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and standard methods. Mouse antibodies bound to M. genitalium proteins were detected with a goat anti-mouse immunoglobulin horseradish peroxidase conjugate (Imgenex, San Diego, CA). Each of the immunogenic M. genitalium proteins that were recognized by infected mouse sera was carefully excised from separate Coomassie-stained gels, digested with trypsin, and then identified using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (UTMB Biomolecular Resource Facility) and a 4700 proteomics analyzer (Applied Biosystems). To evaluate antibody responses from individual mice, we tested serum samples collected upon euthanasia from the 12 estradiol-treated animals 10 weeks p.i. in the hydrosalpinx study described above. Whole M. genitalium G37 lysates on SDS-PAGE gels were probed with serum (diluted 1:1,000 or 1:5,000) from animals inoculated with M. genitalium G37 (n = 6) or M2300 (n = 6).
RESULTS
Viable M. genitalium infection of the lower and upper reproductive tract.
We sought first to determine whether M. genitalium could colonize lower and upper reproductive tract tissues of outbred mice and whether the viable organism could be isolated by the use of axenic culture. Following vaginal inoculation of progesterone-treated mice (n = 15), M. genitalium G37 was recovered from vaginal swabs (at 3 d.p.i. [100% of swabs]; Table 1) and the upper genital tract (21 d.p.i. [40% of swabs]; Table 1) of inoculated mice. In three independent studies using estradiol-treated mice (n = 24), viable M. genitalium was detected in 25% of vaginal swabs collected 3 d.p.i. (Table 1) and 12.5% of upper genital tract tissues (30 d.p.i.; Table 1). Viable M. genitalium was recovered from tissues of infected animals after thorough mincing and 21 days of incubation of tissue in Friis medium at 37°C. Viability was determined by a dramatic color change of the Friis FB medium from red to yellow and formation of adherent microcolonies characteristic of M. genitalium growth.
TABLE 1.
Viability of M. genitalium G37 in vaginal swabs and upper genital tract tissues from progesterone- or estradiol-treated mice
| Hormonal treatment | No. (%) of mice positive for viable M. genitalium G37/no. of mice in tested groupa |
|
|---|---|---|
| Vaginal swabb | Upper GTc | |
| Progesteroned | 15/15 (100) | 6/15 (40) |
| Estradiole | 6/24 (25) | 3/24 (12.5) |
Proportions indicate the number of mice per group that were verified to be positive for viable M. genitalium from the indicated specimen type following 21 days of incubation in Friis FB medium.
Vaginal swabs were collected 3 d.p.i.
Upper genital tract (GT) tissues were collected aseptically 3 to 4 weeks p.i.
Single study; n = 15.
Three independent studies; n = 24.
After determining the color change of the Friis medium and formation of adherent microcolonies, the outgrowth was verified to be M. genitalium by PCR and sequencing of the M. genitalium 16S rRNA gene (11) and of a 281-bp hypervariable region of MG191 (20). After use of the BLAST (NCBI) algorithm and manual sequence alignment, purified PCR products showed >97% sequence identity to M. genitalium G37 (reference strain GenBank accession no. L43967; data not shown). No color changes were observed with any tissues of mock-inoculated mice (n = 9).
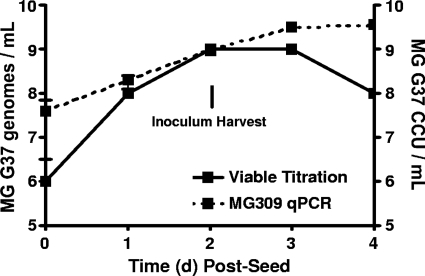
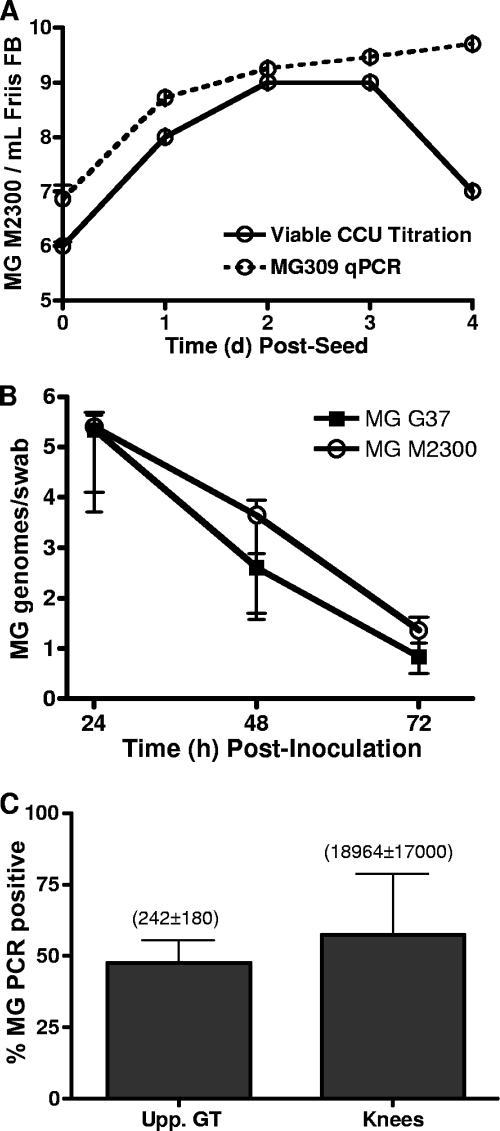
The prevalence of M. genitalium viability was relatively low for each tissue type (Table 1), suggesting that the employed method for viable outgrowth might have been inefficient. In addition, culturing M. genitalium from tissue homogenates did not allow quantification of the bacterial load within each tissue. Therefore, we developed and utilized a highly sensitive and specific quantitative PCR assay for most of the studies presented in this paper. By the use of M. genitalium grown in Friis FB medium, the quantitative PCR assay was compared to a viable titration assay to approximate the ratio of viable M. genitalium organisms to genomes. Following inoculation of M. genitalium into Friis FB medium, PCR titers were very similar to viable titers at 1 to 3 d.p.i. (Fig. 1). Although tested under optimal axenic culture conditions, these results indicated that the PCR assay provided accurate quantification of M. genitalium organisms during exponential growth (Fig. 1).
FIG. 1.
Quantitative comparison of M. genitalium G37 genomes to viable titration. In order to approximate the ratio of M. genitalium genomes to viable organisms, M. genitalium strain G37 (MG 37) was seeded into Friis FB medium and incubated at 37°C for 4 days. Individual wells of a 48-well plate were collected once daily and split for viable titration using a CCU assay (solid line) and for PCR quantification (qPCR; dashed line). Triplicate wells were collected at each time point. PCR quantification was completed using an MG309 gene-targeted real-time PCR assay as described in Materials and Methods. Inocula for animal studies were harvested 3 days after seeding, and a visible color change was observed. Values on the y axis are expressed as log10 genomes/swab ± standard errors of the means of data from a representative experiment.
Long-term detection of M. genitalium in the lower genital tract.
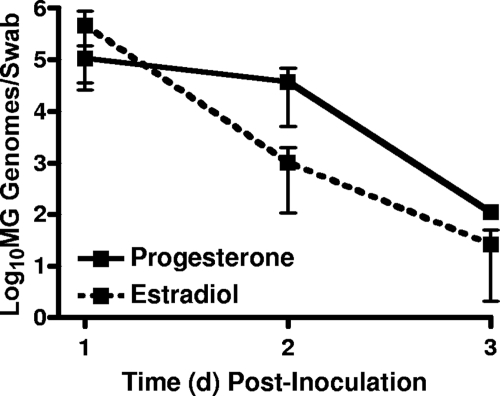
M. genitalium G37 was first delivered to progesterone- or estrogen-treated mice (n = 5/group) to evaluate whether M. genitalium was cleared from the vaginal lumen after inoculation. Using a quantitative MG309 PCR assay, M. genitalium titers in vaginal swabs from progesterone-treated mice were determined. The M. genitalium burden was highest on day 1 p.i. (5/5 mice; an average of 1 × 105 genomes/swab) and then decreased on day 2 (5/5; an average of 3.7 × 104 genomes/swab) and day 3 p.i. (5/5; an average of 2 × 103 genomes/swab; Fig. 2). For estradiol-treated mice, a similar trend was observed, with 5/5 animals PCR positive at 1 d.p.i. (an average of 4.6 × 105 genomes/swab), 5/5 animals PCR positive at 2 d.p.i. (an average of 1 × 103 genomes/swab), and 2/5 animals PCR positive at 3 d.p.i. (an average of 26 genomes/swab) (Fig. 2). Importantly, delivery of an equivalent titer of UV-killed M. genitalium G37 resulted in detectable DNA at 6 and 12 h p.i. but not at 24, 48, or 72 h p.i. (data not shown). Similarly, instillation of an equivalent amount of purified DNA from M. genitalium was detectable only up to 12 h p.i. but not at 24, 48, or 72 h p.i., indicating that the PCR titers observed after inoculation with viable M. genitalium represented active colonization of the vaginal tissues.
FIG. 2.
Colonization and clearance of M. genitalium from the murine vagina. Female Swiss Webster mice were treated with progesterone or estradiol and then inoculated intravaginally with M. genitalium G37 (MG). The early dynamics of vaginal M. genitalium infection was determined by PCR quantification of M. genitalium DNA from vaginal swabs collected at 1, 2, and 3 d.p.i. Values on the y axis are expressed as log10 genomes/swab ± standard errors of the means.
After establishing that progesterone- and estradiol-treated animals were susceptible to vaginal infection by M. genitalium G37, we next evaluated whether vaginal exposure resulted in a long-term infection of the vagina. In two independent studies using estradiol-conditioned animals, M. genitalium G37 was delivered intravaginally to female Swiss Webster mice to evaluate long-term shedding as determined by PCR using vaginal swabs (see Table 2). In similarity to the vaginal clearance study represented in Fig. 2, a large proportion of the M. genitalium inoculum was cleared from the vagina from 1 to 3 d.p.i. in both independent studies of long-term colonization. Vaginal M. genitalium titers were maintained at low levels (average from all positive samples = 50 genomes/swab) for up to 5 d.p.i. in the first study and up to 77 d.p.i. in the second study (Table 2).
TABLE 2.
Long-term PCR detection of M. genitalium G37 in vaginal swabs of estradiol-treated mice
| Study | No. of PCR-positive mice at indicated d.p.i./total no. of mice in tested groupa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 12 | 24 | 31 | 55 | 77 | |
| 1 | 5/5 | 5/5 | 5/5 | 2/5 | 2/5 | NTb | NT | NT | NT | NT |
| 2 | 6/6 | 6/6 | 2/6 | 3/6 | 2/6 | 2/6 | 2/6 | 3/6 | 3/6 | 3/6 |
Data represent numbers of gene MG309 PCR-positive mice per group at each sampling time.
NT, not tested at this time point.
In the second, longer-duration study, every mouse was PCR positive at least once from 12 to 77 d.p.i.; on average, 41% of the collected swabs were PCR positive at each sampling time (Table 2). M. genitalium DNA was not detected in any swabs from mock-infected mice collected at each time point in parallel (n = 10). A study of equivalent duration was not done using progesterone-treated mice. In a single experiment, however, vaginal M. genitalium titers were maintained at levels similar to those seen with estradiol-treated mice up to 35 d.p.i. (data not shown), when the experiment was terminated. Collectively, these data indicate that, despite the significant reduction of vaginal titers at 1 to 3 d.p.i., M. genitalium was detected from vaginal swabs of both estradiol- and progesterone-treated animals for extended time periods. These results suggested the presence either of an active lower tract infection or of shedding from infected upper genital tract tissues into the vaginal lumen.
M. genitalium colonized mouse upper genital tract tissues.
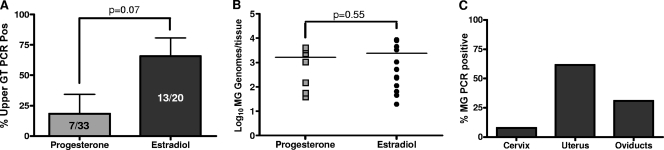
Based on the observation that M. genitalium was present at low levels in the vagina for at least 11 weeks after inoculation, we next evaluated whether M. genitalium colonizes upper reproductive tract tissues. In four independent studies, progesterone-treated (n = 33) or estradiol-treated (n = 20) Swiss Webster mice were inoculated intravaginally with M. genitalium G37 and then humanely euthanized at selected time points for collection of upper genital tract tissues. Dissected tissues were processed for PCR-based quantification of M. genitalium loads. For progesterone-treated animals, the cumulative rate of ascending infection was 21% (7/33 mice; Fig. 3A) in four independent studies and ranged from 0% in two studies to as high as 66% in one experiment. For estradiol-treated mice, ascending M. genitalium infection was observed in 65% of inoculated mice (13/20; Fig. 3A), with rates ranging from 33 to 100% among the four studies. A strong but statistically insignificant trend indicated that estradiol treatment resulted in enhanced upper tract colonization relative to progesterone treatment. However, the levels of M. genitalium burden in upper genital tract tissues from PCR-positive animals were not significantly different between estradiol- and progesterone-treated mice (Fig. 3B), averaging 2.8 × 103 ± 1 × 103 and 1.6 × 103 ± 6 × 102 genomes/tissue, respectively. Among all of the PCR-positive upper genital tract samples from estradiol-treated mice, the majority of isolates were from uterine tissues (8/13), with fewer from the oviduct (4/13) or cervix (1/13) (Fig. 3C).
FIG. 3.
Upper genital tract colonization and localization. Upper genital tract (GT) tissues were collected from estradiol-treated (n = 20) or progesterone-treated (n = 33) Swiss Webster mice at 3 to 4 weeks following inoculation with M. genitalium G37 (MG). Tissues were processed for PCR quantification of M. genitalium DNA loads. (A and B) From four independent studies, the rate of ascending infection (percent PCR-positive mice ± standard errors of the means) (A) and the M. genitalium burden (expressed as M. genitalium genomes/tissue sample) (B) were calculated following either progesterone or estradiol treatment. Pos, positive. (C) For the PCR-positive upper tract tissue samples from estradiol-treated mice, specific localization of M. genitalium within upper reproductive tract tissues was also evaluated. Comparisons between progesterone- and estradiol-treated mice of the levels of mean prevalence and M. genitalium burden of dissemination to the upper genital tract were performed using Student's t test.
To evaluate the kinetics of dissemination in estradiol-treated mice, M. genitalium G37 was inoculated intravaginally and then upper genital tract and knee tissues were collected from each humanely euthanized mouse on days 1, 3, and 7 p.i. for PCR quantification of bacterial load. No M. genitalium was detected in any tissue at 1 d.p.i., but M. genitalium DNA was detected in upper genital tract tissues from one of three mice at 3 d.p.i. (460 genomes/tissue) and then in two of three mice (30 genomes/tissue) by day 7 p.i. (Table 3). No M. genitalium DNA was detected from mock-infected animals dissected in parallel (n = 3 at each time point).
TABLE 3.
Kinetics of dissemination and PCR quantification of M. genitalium G37 in the upper reproductive tract and in knee tissues following estradiol treatment and intravaginal inoculation
| h p.i. | No. of PCR-positive mice (no. of M. genitalium genomes)/no. of mice in tested group for indicated sample categorya |
|||||
|---|---|---|---|---|---|---|
|
M. genitalium G37-inoculated group |
Mock-inoculated group |
|||||
| Vagina | Upper GT | Knees | Vagina | Upper GT | Knees | |
| 24 | 3/3b (3.5 × 104 ± 3,000)c | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| 72 | 1/3 (240) | 1/3 (462) | 0/3 | 0/3 | 0/3 | 0/3 |
| 168 | 2/3 (166 ± 66) | 2/3 (29 ± 25) | 3/3 (127 ± 59) | 0/3 | 0/3 | 0/3 |
The sources of material evaluated by PCR for M. genitalium were vaginal swabs, upper genital tract tissues (upper GT), or knee joint tissues. Proportions indicate the number of PCR-positive mice/group for each sampling time. The numbers of M. genitalium genomes (quantified by PCR) are expressed as means ± standard errors of the means.
M. genitalium disseminated to the knees.
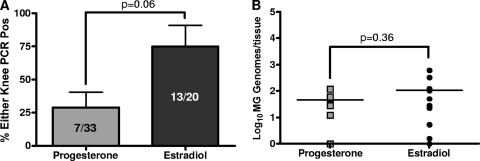
M. genitalium has been detected in human synovial samples of arthritic knees (22, 45, 47) and in synovial fluid of temporomandibular joints (23). To test whether M. genitalium disseminates to the knees of female mice following vaginal inoculation, knee joints were collected from animals humanely euthanized at 3 to 4 weeks p.i. in four independent experiments for PCR quantification. For progesterone-treated animals, dissemination to one or both knees was observed in 21% (7/33) of inoculated animals (Fig. 4A). Among independent studies, rates of knee colonization ranged from 8 to 60%. For estradiol-treated mice, M. genitalium dissemination to knee tissues was observed in 65% (13/20) of inoculated mice, with rates ranging from 33 to 100% among the studies (Fig. 4A). Again, a strong trend toward increased dissemination following estradiol treatment was observed, but the differences, compared to progesterone results, were not significant. The average M. genitalium DNA loads from a single knee were 110 ± 40 genomes/tissue for estradiol-treated animals and 50 ± 10 genomes/tissue for progesterone-treated animals; however, in similarity to the results obtained for the upper genital tract, the mean DNA loads were not significantly different (P = 0.36; Student's t test). In a separate investigation of dissemination kinetics, M. genitalium DNA was isolated from both knee joints of three of three estradiol-treated mice as early as 7 d.p.i. (average of 150 genomes/tissue; Table 3). On the basis of these results, we next used the lower-passage-number Danish M2300 strain to establish whether infection with a more contemporary isolate resulted in differential vaginal colonization, ascending reproductive tract infection, or dissemination to the knees.
FIG. 4.
Dissemination of M. genitalium G37 to the knee tissues. Knee tissue samples were taken from estradiol (n = 20) or progesterone-treated (n = 33) Swiss Webster mice 3 to 4 weeks following inoculation with M. genitalium G37. DNA loads were determined using an MG309 gene quantitative PCR assay. (A and B) From four independent studies, the proportion of ascending infection (percent PCR-positive mice ± standard errors of the means) (A) and the M. genitalium (MG) burden (expressed as M. genitalium genomes/tissue) (B) were calculated following either progesterone or estradiol treatment. Pos, positive. Comparisons of the mean prevalence and M. genitalium burden of dissemination to the knees between estradiol- and progesterone-treated groups were accomplished using Student's t test.
M. genitalium strain M2300 and strain G37 infection dynamics were similar.
On the basis of enhanced dissemination of G37 following estradiol treatment, we first evaluated M2300 viability in vaginal swabs (3 d.p.i.) and upper genital tract tissues (3 to 4 weeks p.i.). In two independent studies (n = 9) where estradiol-treated, Swiss Webster mice were inoculated intravaginally, viable M. genitalium strain M2300 was isolated from vaginal swabs (3/9 [33% viable]), from upper genital tract tissues (1/9 [11%]), and from knees (1/9 [11%]) of inoculated mice. Because PCR afforded enhanced detection of M. genitalium G37 compared to culture, we next tested whether viable titrations and quantitative PCR titers were similar for M2300 as well. Throughout the exponential-growth phase, PCR titers were slightly increased relative to viable CCU titration (Fig. 5A), indicating that the PCR assay was a more sensitive detection tool. Using quantitative PCR, we next compared vaginal clearance and dissemination prevalence results for G37 and the low-passage-number M2300 strain (n = 5 mice/group). Vaginal clearance results for the G37 strain and the M2300 strain were similar (Fig. 5B) and confirmed the findings from the previous G37 studies. Among three independent studies using M. genitalium M2300, the rate of ascending infection was 53% (7/13), averaging 2.4 × 103 genomes/tissue (Fig. 5C). Collectively, 61% (8/13) of mice had M. genitalium PCR-positive knee tissue samples, with an average DNA load of 1.9 × 104 genomes/tissue (Fig. 5C), indicating that the observed results were not specific to G37 and that strain M2300 inoculation resulted in significantly higher DNA loads in the knee compared to the G37 strain (P < 0.05; Student's t test).
FIG. 5.
Comparison of M. genitalium strains G37 and M2300. Swiss Webster mice were treated with estradiol and then inoculated with M. genitalium strain G37 or M2300. (A) The ratio of M. genitalium M2300 genomes to viable organisms was determined using in vitro growth kinetics and MG309 gene quantitative PCR (qPCR) compared to a CCU viable titration assay. (B) Vaginal infection by M. genitalium G37 and M2300 was monitored by PCR quantification using vaginal swabs obtained 24 to 72 h following inoculation (n = 5 mice/group; values are expressed as genomes/swab ± standard errors of the means). (C) For three separate studies (n = 13), ascending infection by M. genitalium M2300 to the upper genital tract (Upp. GT) tissues or dissemination to the knees was quantified by PCR following dissection of mice 3 to 4 weeks p.i. The proportion of mice with upper tract or knee infections is expressed as percent PCR positive ± standard errors of the means. Values in parentheses indicate the means ± standard errors of the means of PCR titers (M. genitalium genomes/tissue) among all PCR-positive samples.
To ensure the specificity of the MG309 PCR assay for detection of M. genitalium, five randomly selected tissue samples were verified to contain M. genitalium DNA by PCR and sequencing of the complete M. genitalium 16S rRNA gene and a hypervariable region of MG191. When the BLAST (NCBI) algorithm and manual sequence alignment were used, purified PCR products showed >97% sequence identity to M. genitalium (reference strain GenBank accession no. L43967; data not shown). Detection of the single nucleotide polymorphism within the MG191 gene of M2300 compared to that of G37 indicated that the DNA detected in the mouse tissues indeed represented M. genitalium M2300 and that no cross-contamination among samples had occurred.
Preliminary evidence for M. genitalium-induced hydrosalpinx.
Considering the observed rates of oviduct colonization, we next evaluated whether inoculation of M. genitalium resulted in hydrosalpinx formation, a surrogate marker for infertility (6, 35). Careful dissection of the reproductive tract is required to preserve hydrosalpinx fluid, so two additional independent studies were performed to specifically address this outcome. In G37-inoculated mice pretreated with estradiol and then dissected 14 days, 21 to 30 days, or 10 weeks p.i., 8/14 (57%) of mice developed either unilateral or bilateral hydrosalpinx (Table 4). The proportions of mice with hydrosalpinx were 0% at 14 d.p.i., 80% at 21 to 30 d.p.i., and 67% at 10 weeks p.i. For mice inoculated with the Danish M2300 strain of M. genitalium, the overall trend for hydrosalpinx development was similar, with 0% at 14 d.p.i. but 80% at 21 to 30 d.p.i. and 50% at 10 weeks p.i. (Table 4). Based on these results, it is evident that hydrosalpinx requires more than 14 days to develop, but whether hydrosalpinx was a persistent condition or resolved and reappeared at later time points is unclear. No hydrosalpinx was observed in samples from mock-inoculated animals (n = 15) collected at parallel time points (Table 4). Among mice that developed hydrosalpinx, the overall prevalence of viable M. genitalium isolation from oviduct tissues was 14% for G37-inoculated mice and 21% for M2300-inoculated mice (Table 4).
TABLE 4.
Preliminary evidence for hydrosalpinx caused by M. genitalium in estradiol-treated mice
| M. genitalium strain or inoculation category | No. (%) of mice positive for HS at indicated sampling time/total no. of mice testeda |
No. (%) of results positive for HS for all sampling times combined/total no. of resultsb | No. (%) of results positive for viable isolation for all sampling times combined/total no. of resultsc | ||
|---|---|---|---|---|---|
| 14 d.p.i. | 21-30 d.p.i. | 10 wk p.i. | |||
| G37 | 0/3 | 4/5 (80) | 4/6 (67) | 8/14 (57) | 2/14 (14) |
| M2300 | 0/3 | 4/5 (80) | 3/6 (50) | 7/14 (50) | 3/14 (21) |
| Mock inoculation | 0/5 | 0/5 | 0/5 | 0/15 (0) | 0/15 (0) |
Frequency of observation of M. genitalium-induced hydrosalpinx (HS) at 14 days, 21 to 30 days, or 10 weeks p.i. in groups of animals.
Compilation of the data sets, showing hydrosalpinx prevalence following experimental vaginal inoculation.
Culture isolation results of M. genitalium from oviduct tissues.
Murine antibody responses to M. genitalium infection.
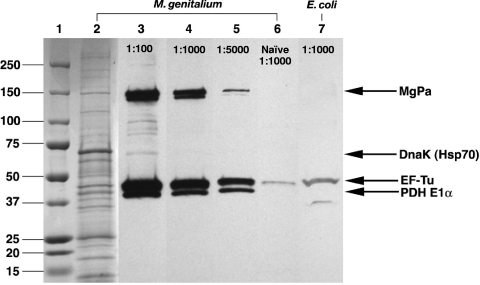
Clinically, urogenital M. genitalium infection of women (4) and men (38) is associated with the development of antibodies to MgPa, the major outer membrane protein. Estradiol-conditioned Swiss Webster mice (n = 6/group) were inoculated intravaginally with M. genitalium G37 or with PBS as a control. Pooled serum samples from M. genitalium-inoculated mice (77 d.p.i.) showed antibody responses to several M. genitalium proteins (Fig. 6, lane 3) at a 1:100 dilution, but the strongest responses observed were to three proteins of approximately 40, 45, and 150 kDa that were recognized by high-titer antibodies (dilutions of 1:1,000 [lane 4] and 1:5,000 [lane 5]). Using MALDI-TOF mass spectrometry, we identified those M. genitalium proteins as pyruvate dehydrogenase component E1α, elongation factor Tu (EF-Tu), and MgPa (MG191), respectively (100% identity confidence for each protein). A weaker response to DnaK (Hsp70), a protein that was observed at high concentrations on SDS-PAGE gels, also was observed on Western blots probed with M. genitalium-exposed serum samples. Mock-infected mice showed only a very weak antibody response to EF-Tu (Fig. 6, lane 6), but this response also was observed when Escherichia coli lysates were probed (lane 7) with serum samples from mock-infected mice. This suggests that the weak antibody responses to the EF-Tu protein from serum of mock-infected mice did not represent specificity for M. genitalium.
FIG. 6.
Host antibody response to M. genitalium G37 infection. Estradiol-conditioned Swiss Webster mice were inoculated with M. genitalium G37 or the PBS vehicle (n = 6/group). Pooled serum samples from each group (77 d.p.i.) were used for immunoblot analysis of purified M. genitalium lysates (Coomassie staining; lane 2). Serum samples from M. genitalium-infected mice were used to probe M. genitalium lysates following a 1:100 (lane 3), 1:1,000 (lane 4), and 1:5000 (lane 5) dilution. Incubation of serum samples from mock-infected animals and M. genitalium G37 (lane 6) or E. coli (lane 7) lysates was performed in parallel to determine the specificity of the antibody response. The indicated immunogenic M. genitalium proteins, namely, MgPa (MG191), DnaK (Hsp70), elongation factor Tu (EF-Tu), and pyruvate dehydrogenase E1α (PDH E1α), were identified using MALDI-TOF mass spectrometry.
Considering the antibody responses of individual animals, MG191 antibody responses were evaluated for the mice inoculated with M. genitalium G37 or M2300 in the study of hydrosalpinx formation described above. A semiquantitative scale was used to grade intensity levels observed on Western blots for whole G37 lysates. Utilizing mice collected at 10 weeks p.i. (n = 6 for each of the G37 and M2300 groups), we observed seroconversion to the MgPa adhesin protein encoded by MG191 for 83% of G37-inoculated and 67% of M2300-inoculated mice. Similar results were obtained at 1:1,000 and 1:5,000 serum dilutions. Antibodies to EF-Tu and pyruvate dehydrogenase component E1α were observed for all inoculated mice. Animals with moderate hydrosalpinx showed robust responses to MG191, while animals with severe hydrosalpinx showed no or very low MG191 antibody responses (Table 5).
TABLE 5.
Comparison of protein MG191 seroconversion to hydrosalpinx formation 10 weeks after M. genitalium inoculationa
| M. genitalium strain or inoculation category | No. (%) of mice positive for MG191 seroconversion/no. of mice testedb | No. of mice positive for MG191 seroconversion at indicated HSc severity level (intensity of Ab responsed)/no. of mice tested |
|
|---|---|---|---|
| Severe HS | HS | ||
| G37 | 5/6 (83) | 1/6 | 3/6 |
| Ne | +, ++, +++ | ||
| M2300 | 4/6 (67) | 1/6 | 2/6 |
| + | +++; N | ||
| Mock inoculation | 0/5 (0) | N/Af | N/A |
Serum samples were diluted 1:1,000 and 1:5,000 for immunoblot analysis with similar results.
Frequency of seroconversion to protein MG191 within groups of animals, as determined by Western blot analysis using whole M. genitalium lysates.
HS, hydrosalpinx.
+, low level of antibody (Ab) response to the MG191 protein; ++, moderate response; +++, high response.
N, no antibodies against MG191 were observed.
NA, not applicable.
DISCUSSION
Despite the growing body of literature that implicates M. genitalium as a cause of genital disease in women, very little evidence indicating that M. genitalium establishes upper reproductive tract infections previously existed. In the current paper, we report that M. genitalium can establish viable infection of lower and upper reproductive tract tissues and disseminate to the knees of female mice after experimental vaginal inoculation. Importantly, upper tract colonization was rapid (as early as 3 d.p.i.) and ultimately resulted in oviduct colonization and hydrosalpinx for approximately 60% of infected animals. In addition, dissemination of M. genitalium to the knee joints (Fig. 4 and 5) was observed by 7 days after vaginal inoculation, providing supportive experimental evidence for reported clinical cases of arthritis (22, 45, 47). This is the first experimental evidence that M. genitalium is capable of persistently colonizing upper reproductive tract tissues, a process that could lead to inflammatory outcomes similar to those observed clinically.
The presence of M. genitalium in the vaginal lumen is a necessary factor for sexual transmission as well as for the potential for ascending infection of upper reproductive tract tissues. Following inoculation of Swiss Webster mice, vaginal titers were detected up to 77 d.p.i. (Table 2), indicating persistent colonization of the lower reproductive tract. In clinical cases of M. genitalium, chronic reproductive tract infections have been previously observed (2, 3, 5, 9, 13, 16) and are often associated with antibiotic treatment failure (2, 3, 9, 13) that results in persistence in the lower tract. However, detection of M. genitalium in the vaginal lumen could be attributable to either active infection of the lower tract or periodic movement of M. genitalium into the vaginal lumen from infected endocervical or upper tract tissues. Among the mouse reproductive tract tissues that were found to be M. genitalium PCR positive in our studies, 92% of the upper tract isolates were from the uterus and oviducts and 8% were from the cervix (Fig. 3C).
Considering these findings and the clinical associations with infertility and upper tract disease, it seems reasonable to suspect that the upper reproductive tract could be the primary site of M. genitalium infection, leading to periodic shedding into the vaginal lumen. This is consistent with intermittent vaginal detection of M. genitalium in mice with an upper genital tract infection in our studies. In addition, more than 25% of PCR-positive tissues in our studies were from the oviducts; interestingly, both the G37 and M2300 strains of M. genitalium induced hydrosalpinx in 64 and 50% of inoculated mice, respectively.
Hydrosalpinx, as defined by gross oviduct dilation and accumulation of a clear, serous fluid (35), is a surrogate marker for tubal occlusion and infertility (6, 35, 39). In mice inoculated with Chlamydia muridarum, a murine pathogen often utilized as a model of C. trachomatis, oviduct occlusion correlates with the presence of hydrosalpinx and is often due to oviduct fibrosis causing the tubes to fill with fluid (35). In our studies, hydrosalpinx was observed late (21 days to 10 weeks) following inoculation, a finding similar to data reported for Chlamydia mouse studies that have previously shown that hydrosalpinx is only rarely observed before 35 d.p.i. (35) and often after an inflammatory event (39). Clearly, additional studies are warranted to confirm tubal occlusion and infertility in mice, but these findings are the first to indicate that M. genitalium infection of upper tract tissues results in gross oviduct abnormalities similar to those seen with chlamydial infection.
When outbred mice were used, estradiol treatment prior to M. genitalium inoculation consistently led to increased rates of ascending reproductive tract infection (Fig. 3) and dissemination to the knees (Fig. 4) relative to data from progesterone-treated animals. The apparent discrepancy between our results and a previous report of estradiol-induced resistance to vaginal infection (10) could be attributed to the regimens, dosages, and formulations of estrogen delivery or outcome measures evaluated. In previous studies by Taylor-Robinson and colleagues, estradiol benzoate was delivered once weekly to BALB/c mice throughout the study (10), whereas our studies utilized a longer-acting estradiol cypionate delivered to Swiss Webster mice immediately prior to inoculation. As with previous studies, progesterone-conditioned animals were susceptible to vaginal infection by M. genitalium, resulting in long-term infection of the lower reproductive tract (10).
It remains unclear, however, why some species (such as M. pneumoniae and M. pulmonis) require progesterone treatment for infection whereas others (such as M. fermentans) colonize the genital tract only after estrogen treatment (10). Estradiol treatment increases the susceptibility of BALB/c mice to Neisseria gonorrhoeae infection (21), whereas progesterone enhances murine susceptibility to C. trachomatis (46) and C. muridarum (1). Despite the similar cell-associated phenotypes of these bacterial species, it is evident that systemic manipulation of sex hormones dramatically affects susceptibility, duration of infection, and pathological outcomes in murine STI models. At this point, it is unclear why estradiol treatment increased the proportion of mice with disseminated infection relative to progesterone-treated animals. Estrogens are known to have both immunostimulatory and immunosuppressive effects (36), but their impact on STI pathogenesis is largely unknown.
One surprising finding in the current study was the infection susceptibility of mice treated with either estradiol or progesterone. M. genitalium is known to interact heavily with and invade vaginal and cervical epithelial cells (29, 49), so it is possible that estradiol-treated mice have a greater reservoir of susceptible epithelial cells in the vagina. Estradiol is known to induce thickening of the vaginal mucosa through increased epithelial proliferation (7), but no studies to date have evaluated M. genitalium shedding or susceptibility on the basis of hormone levels or different stages of the menstrual cycle in mice or humans. Estradiol treatment could allow enhanced primary infection, thus explaining the increased prevalence of ascending and disseminated infections in estradiol-treated animals. The observation that progesterone treatment also renders mice susceptible to infection indicates that specific hormone treatment might not be absolutely necessary for establishment of M. genitalium infection. We did not evaluate mice that received no hormone treatment, because hormone treatment also synchronizes the estrus cycle among members of treatment groups, thereby allowing for more accurate comparisons. Therefore, using the paradigms reported in this paper, future investigations are planned to include the impact of natural hormonal changes or oral contraceptives on susceptibility, transmission, and pathogenesis of M. genitalium.
Despite minimal passage in our laboratory, it is unknown how phenotypically similar the G37 strain used in previous murine studies (10) would be to our ATCC-derived strain obtained almost 15 years later. The G37 strain of M. genitalium was isolated originally from the male urethra (48) and was deposited into the ATCC with an unknown passage history. Genetic heterogeneity among M. genitalium strains has been previously demonstrated clinically and experimentally (16, 17, 25, 26), suggesting that alternative pathogenic phenotypes likely exist. Like most laboratory strains, the G37 and M2300 organisms used in our studies were adapted for efficient axenic growth and may not accurately represent the currently circulating clinical strains. Adaptation to axenic growth might explain the partial M. genitalium clearance from the mouse vaginal lumen at up to 3 d.p.i. (Fig. 2 and 5B), leaving a less axenic culture-adapted subset that establishes long-term infection. To this end, it will be important to compare G37 to additional clinical isolates and clonal derivatives to better understand the mechanisms of primary infection and specific factors influencing long-term inflammation at genital and synovial sites in humans.
Although the real-time PCR assay was a sensitive and high-throughput detection method, demonstrating M. genitalium viability in mouse reproductive tract and knee specimens was essential (Table 1). Using PCR, however, the dissemination prevalence values were increased and yielded a more informative, quantitative measure of M. genitalium loads compared to viable outgrowth. Importantly, it cannot be ruled out that viable isolation of M. genitalium from mouse tissues might have been enhanced if tissues had been cultured for more than 21 days or cocultured with eukaryotic cells as described previously (14, 19) or if efforts had been made to purify M. genitalium from the tissues prior to culture in Friis FB medium. Despite the approximate 1:1 ratio of genomes to viable organisms observed in pure M. gentalium cultures (Fig. 1), it is possible that this ratio would be different in animal specimens due to dead or noncultivable organisms. In addition, although not well understood, difficulty in viable isolation of M. genitalium from human reproductive tract tissues is widely recognized among clinical and laboratory investigators in the field and has been documented previously (34, 42). Although not yet studied, it is possible that M. genitalium infections could enter a viable but noncultivable state similar to persistent C. trachomatis infections (30), thus accounting for enhanced PCR detection. Taking these findings together, viable M. genitalium infection of the vagina, upper reproductive tract, and knees was observed, but PCR was determined to be a more reliable, high-throughput, and informative measure of infection.
In female patients, M. genitalium can establish a persistent genital tract infection (5, 16) that results in the development of antibodies to MgPa, the major adhesin protein (4). In our studies, MgPa also was observed to be an immunodominant membrane protein in outbred mice and to provide good supportive evidence for the validity of the murine model. Additional immunogenic proteins included EF-Tu, DnaK (Hsp70), and the E1 α subunit of pyruvate dehydrogenase. Each of these proteins had been previously identified as a putative component of the cytoskeleton in M. pneumoniae (33); those proteins could next be evaluated for their importance for M. genitalium replication or human infection or as clinical diagnostic targets. Considering the antibody responses from individual animals, 83% of G37- and 67% of M2300-inoculated mice developed antibodies against the immunogenic MgPa protein encoded by MG191. As with the results obtained using pooled serum samples, mice showed robust MG191 responses even after 1:1,000 to 1:5,000 serum dilution. Although this preliminary study was not designed for reliable comparisons in this respect, animals with severe hydrosalpinx showed no or very low MG191 antibody response resembling the responses seen with animals without hydrosalpinx (Table 5). Interestingly, animals with moderate hydrosalpinx show the most robust responses, indicating that intensity of MG191 antibody response is not positively correlated with severity of disease. Instead, severe hydrosalpinx might be correlated with lower humoral responses, but additional studies are needed to adequately compare these outcomes.
Collectively, our studies outline a reproducible small-animal model to investigate M. genitalium urogenital tract disease in women. Because progesterone- and estrogen-treated animals were susceptible to M. genitalium infection, it should now be possible to model coinfections with other sexually transmitted pathogens that require similar hormonal regulation. The observation that M. genitalium can localize to knee tissues following intravaginal exposure is intriguing and warrants continued investigation into the potential implications for inflammatory arthritides. Most importantly, these results provide experimental evidence for the causal associations of M. genitalium with reproductive tract disease by demonstrating ascending upper tract infection following vaginal exposure. Future studies utilizing this model would help to uncover important factors and mechanisms of female reproductive tract disease, including host responses, and allow evaluation of prospective therapeutic interventions to address the global epidemic of STI.
Acknowledgments
The work was supported by the Gulf South Sexually Transmitted Infections and Topical Microbicide Cooperative Research Center (NIAID U19 AI61972). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank R. J. McLean for providing us with the 16S rRNA gene primers for molecular verification of M. genitalium. We also are grateful to the UTMB Protein Chemistry Core Laboratory for performing all DNA sequencing reactions and the Biomolecular Resource Facility for mass spectrometry analyses. We also thank Jørgen Jensen, Liang Ma, and David H. Martin for informative discussions.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 7 December 2009.
REFERENCES
- 1.Beale, A. S., and P. A. Upshon. 1994. Characteristics of murine model of genital infection with Chlamydia trachomatis and effects of therapy with tetracyclines, amoxicillin-clavulanic acid, or azithromycin. Antimicrob. Agents Chemother. 38:1937-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björnelius, E., C. Anagrius, G. Bojs, H. Carlberg, G. Johannisson, E. Johansson, H. Moi, J. S. Jensen, and P. Lidbrink. 2008. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex. Transm. Infect. 84:72-76. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw, C. S., M. Y. Chen, and C. K. Fairley. 2008. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One 3:e3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866-1874. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, C. R., M. Nosek, A. Meier, S. G. Astete, S. Iverson-Cabral, N. R. Mugo, and P. A. Totten. 2007. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex. Transm. Dis. 34:274-279. [DOI] [PubMed] [Google Scholar]
- 6.de la Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, G. S., D. F. Gibson, S. A. Roberts, T. M. Hind, and C. S. Potten. 1990. Proliferative changes in the genital tissue of female mice during the oestrous cycle. Cell Tissue Kinet. 23:619-635. [DOI] [PubMed] [Google Scholar]
- 8.Falk, L., H. Fredlund, and J. S. Jensen. 2005. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex. Transm. Infect. 81:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk, L., H. Fredlund, and J. S. Jensen. 2003. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex. Transm. Infect. 79:318-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furr, P. M., and D. Taylor-Robinson. 1993. Factors influencing the ability of different mycoplasmas to colonize the genital tract of hormone-treated female mice. Int. J. Exp. Pathol. 74:97-101. [PMC free article] [PubMed] [Google Scholar]
- 11.Gillan, D. C., A. G. Speksnijder, G. Zwart, and C. De Ridder. 1998. Genetic diversity of the biofilm covering Montacuta ferruginosa (Mollusca, bivalvia) as evaluated by denaturing gradient gel electrophoresis analysis and cloning of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 64:3464-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haggerty, C. L. 2008. Evidence for a role of Mycoplasma genitalium in pelvic inflammatory disease. Curr. Opin. Infect. Dis. 21:65-69. [DOI] [PubMed] [Google Scholar]
- 13.Haggerty, C. L., P. A. Totten, S. G. Astete, S. Lee, S. L. Hoferka, S. F. Kelsey, and R. B. Ness. 2008. Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex. Transm. Infect. 84:338-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamasuna, R., Y. Osada, and J. S. Jensen. 2007. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with Vero cells. J. Clin. Microbiol. 45:847-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara, S., M. Yasuda, S. Ito, S. Maeda, and T. Deguchi. 2004. Mycoplasma genitalium urethritis in men. Int. J. Antimicrob. Agents 24(Suppl. 1):S23-S27. [DOI] [PubMed] [Google Scholar]
- 16.Iverson-Cabral, S. L., S. G. Astete, C. R. Cohen, E. P. Rocha, and P. A. Totten. 2006. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect. Immun. 74:3715-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iverson-Cabral, S. L., S. G. Astete, C. R. Cohen, and P. A. Totten. 2007. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol. Microbiol. 66:55-73. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, J. S., S. A. Uldum, J. Sondergard-Andersen, J. Vuust, and K. Lind. 1991. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J. Clin. Microbiol. 29:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerse, A. E. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 67:5699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, S., and D. Pitcher. 2000. Distribution of ecto 5′-nucleotidase on Mycoplasma species associated with arthritis. FEMS Microbiol. Lett. 192:59-65. [DOI] [PubMed] [Google Scholar]
- 23.Kim, S. J., Y. H. Park, S. P. Hong, B. O. Cho, J. W. Park, and S. G. Kim. 2003. The presence of bacteria in the synovial fluid of the temporomandibular joint and clinical significance: preliminary study. J. Oral Maxillofac. Surg. 61:1156-1161. [DOI] [PubMed] [Google Scholar]
- 24.Korte, J. E., J. B. Baseman, M. P. Cagle, C. Herrera, J. M. Piper, A. E. Holden, S. T. Perdue, J. D. Champion, and R. N. Shain. 2006. Cervicitis and genitourinary symptoms in women culture positive for Mycoplasma genitalium. Am. J. Reprod. Immunol. 55:265-275. [DOI] [PubMed] [Google Scholar]
- 25.Ma, L., J. S. Jensen, L. Myers, J. Burnett, M. Welch, Q. Jia, and D. H. Martin. 2007. Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome. Mol. Microbiol. 66:220-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, L., and D. H. Martin. 2004. Single-nucleotide polymorphisms in the rRNA operon and variable numbers of tandem repeats in the lipoprotein gene among Mycoplasma genitalium strains from clinical specimens. J. Clin. Microbiol. 42:4876-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650-657. [DOI] [PubMed] [Google Scholar]
- 28.McGowin, C. L., L. Ma, D. H. Martin, and R. B. Pyles. 2009. Mycoplasma genitalium-encoded MG309 activates NF-κB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect. Immun. 77:1175-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowin, C. L., V. L. Popov, and R. B. Pyles. 2009. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol. 9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mpiga, P., and M. Ravaoarinoro. 2006. Chlamydia trachomatis persistence: an update. Microbiol. Res. 161:9-19. [DOI] [PubMed] [Google Scholar]
- 31.Napierala Mavedzenge, S., and H. A. Weiss. 2009. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 23:611-620. [DOI] [PubMed] [Google Scholar]
- 32.Pépin, J., A. C. Labbe, N. Khonde, S. Deslandes, M. Alary, A. Dzokoto, C. Asamoah-Adu, H. Meda, and E. Frost. 2005. Mycoplasma genitalium: an organism commonly associated with cervicitis among west African sex workers. Sex. Transm. Infect. 81:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regula, J. T., G. Boguth, A. Gorg, J. Hegermann, F. Mayer, R. Frank, and R. Herrmann. 2001. Defining the mycoplasma ‘cytoskeleton’: the protein composition of the Triton X-100 insoluble fraction of the bacterium Mycoplasma pneumoniae determined by 2-D gel electrophoresis and mass spectrometry. Microbiology 147:1045-1057. [DOI] [PubMed] [Google Scholar]
- 34.Samra, Z., M. Borin, Y. Bukowsky, Y. Lipshitz, and D. Sompolinsky. 1988. Non-occurrence of Mycoplasma genitalium in clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 7:49-51. [DOI] [PubMed] [Google Scholar]
- 35.Shah, A. A., J. H. Schripsema, M. T. Imtiaz, I. M. Sigar, J. Kasimos, P. G. Matos, S. Inouye, and K. H. Ramsey. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex. Transm. Dis. 32:49-56. [DOI] [PubMed] [Google Scholar]
- 36.Straub, R. H. 2007. The complex role of estrogens in inflammation. Endocr. Rev. 28:521-574. [DOI] [PubMed] [Google Scholar]
- 37.Svenstrup, H. F., J. Fedder, S. E. Kristoffersen, B. Trolle, S. Birkelund, and G. Christiansen. 2008. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil. Steril. 90:513-520. [DOI] [PubMed] [Google Scholar]
- 38.Svenstrup, H. F., J. S. Jensen, K. Gevaert, S. Birkelund, and G. Christiansen. 2006. Identification and characterization of immunogenic proteins of Mycoplasma genitalium. Clin. Vaccine Immunol. 13:913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swenson, C. E., E. Donegan, and J. Schachter. 1983. Chlamydia trachomatis-induced salpingitis in mice. J. Infect. Dis. 148:1101-1107. [DOI] [PubMed] [Google Scholar]
- 40.Taylor-Robinson, D., and P. M. Furr. 2001. Failure of Mycoplasma pneumoniae infection to confer protection against Mycoplasma genitalium: observations from a mouse model. J. Med. Microbiol. 50:383-384. [DOI] [PubMed] [Google Scholar]
- 41.Taylor-Robinson, D., and P. M. Furr. 1993. Models of infection due to mycoplasmas, including Mycoplasma fermentans, in the genital tract and other sites in mice. Clin. Infect. Dis. 17(Suppl. 1):S280-S282. [DOI] [PubMed] [Google Scholar]
- 42.Taylor-Robinson, D., P. M. Furr, and N. F. Hanna. 1985. Microbiological and serological study of non-gonococcal urethritis with special reference to Mycoplasma genitalium. Genitourin. Med. 61:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor-Robinson, D., P. M. Furr, and C. M. Hetherington. 1990. Neisseria gonorrhoeae colonises the genital tract of oestradiol-treated germ-free female mice. Microb. Pathog. 9:369-373. [DOI] [PubMed] [Google Scholar]
- 44.Taylor-Robinson, D., P. M. Furr, J. G. Tully, M. F. Barile, and B. R. Moller. 1987. Animal models of Mycoplasma genitalium urogenital infection. Isr. J. Med. Sci. 23:561-564. [PubMed] [Google Scholar]
- 45.Taylor-Robinson, D., C. B. Gilroy, S. Horowitz, and J. Horowitz. 1994. Mycoplasma genitalium in the joints of two patients with arthritis. Eur. J. Clin. Microbiol. Infect. Dis. 13:1066-1069. [DOI] [PubMed] [Google Scholar]
- 46.Tuffrey, M., P. Falder, J. Gale, and D. Taylor-Robinson. 1986. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br. J. Exp. Pathol. 67:605-616. [PMC free article] [PubMed] [Google Scholar]
- 47.Tully, J. G., D. L. Rose, J. B. Baseman, S. F. Dallo, A. L. Lazzell, and C. P. Davis. 1995. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J. Clin. Microbiol. 33:1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 49.Ueno, P. M., J. Timenetsky, V. E. Centonze, J. J. Wewer, M. Cagle, M. A. Stein, M. Krishnan, and J. B. Baseman. 2008. Interaction of Mycoplasma genitalium with host cells: evidence for nuclear localization. Microbiology 154:3033-3041. [DOI] [PubMed] [Google Scholar]
- 50.Uno, M., T. Deguchi, H. Komeda, M. Hayasaki, M. Iida, M. Nagatani, and Y. Kawada. 1997. Mycoplasma genitalium in the cervices of Japanese women. Sex. Transm. Dis. 24:284-286. [DOI] [PubMed] [Google Scholar]