Abstract
Trypanosoma cruzi is the etiologic agent of Chagas' disease. Acute T. cruzi infection results in polyclonal B-cell activation and delayed specific humoral immunity. T. cruzi proline racemase (TcPRAC), a T. cruzi B-cell mitogen, may contribute to this dysfunctional humoral response. Stimulation of murine splenocytes with recombinant protein (rTcPRAC) induced B-cell proliferation, antibody secretion, interleukin-10 (IL-10) production, and upregulation of CD69 and CD86 on B cells. Marginal zone (MZ) B cells are more responsive to T-cell-independent (TI) rTcPRAC stimulation than are follicular mature (FM) B cells in terms of proliferation, antibody secretion, and IL-10 production. During experimental T. cruzi infection, TcPRAC-specific IgG remained undetectable when responses to other T. cruzi antigens developed. Conversely, intradermal genetic immunization via gene gun (GG) delivered TcPRAC as an immunogen, generating high-titer TcPRAC-specific IgG without B-cell dysfunction. TcPRAC GG immunization led to antigen-specific splenic memory B-cell and bone marrow plasma cell formation. TcPRAC-specific IgG bound mitogenic rTcPRAC, decreasing subsequent B-cell activation. GG immunization with rTcPRAC DNA was nonmitogenic and did not affect the generation of specific IgG to another T. cruzi antigen, complement regulatory protein (CRP). These data demonstrate the utility of genetic immunization for the conversion of a protein mitogen to an effective antigen. Furthermore, coimmunization of TcPRAC with another T. cruzi antigen indicates the usefulness of this approach for multivalent vaccine development.
Polyclonal B-cell activation is triggered by many pathogens and contributes to evasion of host immunity through activation of non-pathogen-specific B-cell clones. This nonspecific response often results in a dilution or delay in the generation of specific immune responses, which may contribute to the development of chronic infection (44, 50, 59). Mitogenic proteins that can contribute to this process have been identified from viruses (22, 48), bacteria (18, 59, 66), fungi (63), and parasites (4, 35, 36, 44-46). Characterizations of these proteins are essential for understanding host-pathogen interaction and are instrumental in the development of rational strategies for vaccination. Classical approaches to vaccine development focus on the induction of a robust secondary response to microbial epitopes, and the consequences of pathogen immune evasion strategies are not often considered. Despite effective immunization, protection from challenge infection may not be achieved optimally in cases where the pathogen induces a potent polyclonal B-cell response that can delay secondary responses and dilute the existing immune effector mechanisms generated by vaccination (42, 44).
Infection with the protozoan parasite Trypanosoma cruzi results in polyclonal lymphocyte activation during the early acute phase of infection (31, 33). Long-term persistence of the parasitic infection can lead to chronic Chagas' disease, characterized by progressive cardiomyopathy and congestive heart failure (23, 51). During the acute T. cruzi infection, parasite-specific immune responses are delayed, and induction of a polyclonal B-cell response results in hypergammaglobulinemia and lymphoproliferation that occur concomitant with parasitemia and the generation of nonspecific and autoreactive antibodies (7, 15, 31, 44, 64). In the mouse model of T. cruzi infection, reduction of polyclonal B-cell responses leads to decreased disease severity (32), indicating the potential to enhance host immunity to T. cruzi through the depletion of polyclonal B-cell activation.
T. cruzi proline racemase (TcPRAC) has been identified as a T-cell-independent (TI) B-cell mitogen (9, 44, 45). TcPRAC is a dimeric protein encoded by two paralogous genes per haploid genome: TcPRACA and TcPRACB. TcPRACA encodes a secreted or transmembrane anchored protein, although an alternative second initiation site can result in a cytoplasmic protein (8). TcPRACB has been found in the cytoplasm of insect-stage epimastigotes. TcPRACA is expressed and released by infectious trypomastigotes and differs from TcPRACB by several point mutations and an amino-terminal secretion signal (8, 9). TcPRACA isolated from the culture supernatant of infectious trypomastigotes and recombinant TcPRAC (rTcPRAC) were shown to induce nonspecific in vitro proliferation of T-cell-depleted or athymic murine splenocytes (45), but the effect of TcPRACA on the activation and function of specific B-cell subsets has not been determined.
Marginal zone (MZ) and follicular mature (FM) B cells constitute two functionally and anatomically distinct B-cell subsets within the spleen (3). MZ B cells are located at the marginal sinus of the spleen. MZ B cells are considered first-line responders to pathogens in the blood. MZ B cells are more responsive to TI antigens and generate short-term plasma cells (69). FM B cells circulate through the lymph and are found in B-cell follicles of the spleen. FM B cells respond to T-cell-dependent (TD) antigen and can become long-term plasma cells or memory B cells (17). The different contributions of these two B-cell populations to immunity during infectious disease are still under investigation (3, 29, 39, 42, 60). While differences in MZ and FM cell responsiveness to lipopolysaccharide (LPS) and other Toll-like receptor (TLR) ligands have been reported (21, 30), the responses of these B-cell subsets to pathogen-derived B-cell mitogenic proteins have remained largely unexplored. Due to their different locations and functions, these two B-cell subsets may play different roles during pathogen-induced polyclonal B-cell activation.
In this study, we investigated the mitogenic and immunogenic potential of rTcPRAC. Our results suggest that TcPRAC may contribute to delayed host immune effector mechanisms by nonspecifically activating B cells, especially MZ B cells, causing hypergammaglobulinemia and inducing production of interleukin-10 (IL-10), a cytokine known to increase host susceptibility to T. cruzi (43). Specific TcPRAC antibody responses were not detected during experimental murine infection; however, genetic immunization with TcPRAC induced a robust, antigen-specific humoral response. Coimmunization of mice with TcPRAC and another T. cruzi antigen, complement regulatory protein (CRP), did not interfere with the immune response to CRP, resulting in comparable antibody titers to both immunogens, and improved challenge outcomes. These results identify specific B-cell subset alterations induced by exposure to TcPRAC and demonstrate the utility of genetic immunization as a means for delivery of a pathogen-encoded mitogen in an immunogenic context.
MATERIALS AND METHODS
Parasites and mice.
BALB/c, C57/BL6, and C3H/HeJ mice were obtained from Jackson Laboratories and maintained in specific-pathogen-free housing. Mouse husbandry and procedure protocols were reviewed and performed in accordance with the University of Pittsburgh IACUC. Strain Y parasites were grown in NIH 3T3 cells and harvested by standard techniques (53). Two isolates of strain Y parasite were used, namely, a highly virulent isolate, Y-Br, and a more attenuated isolate, Y-US. BALB/c mice were experimentally infected by intraperitoneal injection with a 0.25× 50% lethal dose (LD50) of Y-US (2.5 × 105 parasites). C57BL/6 mice were experimentally infected by intraperitoneal injection with 0.25× LD50 (5 × 105 parasites) of Y-Br parasites. Blood was collected at multiple points postinfection for analysis by enzyme-linked immunosorbent assay (ELISA).
rTcPRAC and rCRP cloning.
TcPRACA (without a secretion signal) was cloned from T. cruzi strain Y genomic DNA, using the same primers as those previously used to generate rTcPRAC from strain CL parasites (Bg45 and Hi45) (45). The amplified TcPRACA open reading frame (ORF) was cloned into pCRII-Blunt-TOPO vector (Invitrogen). The insert was then cut out with SacI and ligated in frame with a histidine tag in the pET28b (EMD, Novagen) vector. pET28B_TcPRACA was transformed into Escherichia coli BL21(DE3) cells (EMD, Novagen). For eukaryotic expression, TcPRACA was amplified with a 5′ Kozak sequence, inserted into the pcDNA3.1/VF-his-TOPO vector (Invitrogen), and transformed into BL21(DE3) (EMD, Novagen). A full-length cDNA encoding T. cruzi CRP was isolated by reverse transcription-PCR as previously described (37). The T. cruzi CRP cDNA encoding the mature protein (starting at nucleotide 303) was subcloned into the pTrcHis expression vector (Invitrogen). E. coli strain SURE (Stratagene) was transformed with pTrcHis-CRP DNA for recombinant protein production with a histidine tag, as previously described (53). For eukaryotic expression, CRP was cloned into pCDNA3 with the glycosylphosphatidylinositol (GPI) anchor signal sequence from human DAF; a full description of this cloning procedure (pcDNA3-CRP.daf) was previously published (5).
Protein purification.
Expression of recombinant protein was induced by isopropyl-β-d-thiogalactopyranoside (IPTG) (IBI Scientific, Peosta, IA) in transformed E. coli cells, and the cells were harvested by centrifugation (6,000 relative centrifugal force [RCF], 10 min, 4°C). The resulting cleared lysate was prepared under native or denaturing conditions and bound to cobalt metal-affinity resin according to the Talon instruction manual, with slight modifications (Clontech, Mountain View, CA). During binding of lysate to resin, 5 to 10 mM imidazole (Sigma) was added to the binding buffer (50 mM Tris, 300 mM NaCl, pH 7.2). Protein was bound to the resin in batches for 2 h. The bound protein was further washed and packed into a disposable column. An imidazole step gradient (25 to 150 mM) was used to elute bound protein. The protein concentration in eluted fractions was determined by Bradford assay. Elution fractions containing rTcPRAC protein were further selected for active dimers, concentrated, and buffer exchanged into 1× phosphate-buffered saline (PBS) with microconcentrators with a molecular mass cutoff of 50 kDa (Microcon YM-50; Pierce). After purification, the concentration of rTcPRAC was calculated by subtracting the amount of protein present in the equivalent eluted negative control fractions from the protein amount in eluted rTcPRAC fractions.
In vitro stimulation of B cells.
Freshly isolated splenocytes from BALB/c mice or C3H/HeJ mice were stained with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (Sigma) and washed with PBS, and the cell number was adjusted to 106 cells per ml in cRPMI (Gibco RPMI+Glutamax, 10% fetal calf serum [FCS], 1× HEPES, penicillin-streptomycin) (Invitrogen) for stimulation. Cells were stimulated with rTcPRAC, immobilized metal-affinity chromatography (IMAC)-purified empty vector protein as a negative control, or 0.001 to 10 μg/ml LPS (Sigma) as a positive control. Cells were maintained at 37°C and 5% CO2 until harvest. Induced empty vector IMAC-purified protein was chosen as the appropriate negative control for rTcPRAC stimulation because it preserves any copurifying heat-labile and heat-stable stimulatory components. LPS in 25 μg/ml rTcPRAC was measured at <100 endotoxin units (EU)/ml (10 ng/ml) by Limulus amebocyte lysate (LAL) assay (Lonza, Basel, Switzerland). To negate any potential LPS contamination, 5 to 10 μg/ml polymyxin B (PMB) was added to the culture containing rTcPRAC, except where indicated (5 μg/ml PMB decreased B-cell proliferation in cultures stimulated with 1 μg/ml LPS to background levels).
Cell sorting for MZ and FM B cells.
Total BALB/c splenocytes were isolated as described above. Cells were incubated with magnetic bead-conjugated anti-CD43 antibodies (Abs) according to the manufacturer's instructions (Miltenyi Biotec). Immediately after anti-CD43 staining, cells were stained with specific Abs for sorting by FACSAria, followed by CD43 depletion using Automacs columns (Miltenyi Biotec) to collect the untouched CD43-negative cells. Pre- and post-CD43 depletion fractions were analyzed for CD19 expression, with 95% CD19+ cells in the CD43-negative population and 33% CD19+ cells in the CD43+ population. The CD43-negative cells were further sorted by a FACSAria sorter with Diva software (BD Biosciences). FM B cells were sorted into CD21int, CD23+, and CD24int cells, and MZ B cells were sorted into CD21high and CD24high cells (30). B-cell numbers were adjusted to approximately 106 cells per ml in cRPMI for stimulation.
Flow cytometry.
For analysis poststimulation, cells were collected by centrifugation (500 × g, 5 min, 4°C) and washed with fluorescence-activated cell sorting (FACS) staining buffer (1× PBS with 2.5% FCS, 1% goat serum, and 1% human AB serum). A total of 106 cells per ml were incubated with fluorescently labeled Abs diluted in FACS buffer for 20 min on ice or 5 min at 4°C. Whole blood cells were directly stained with anti-CD19 and -CD3 antibodies, treated with lysing solution (BD Biosciences), and washed, and data were collected by flow cytometry. Abs used for staining included CD19 (MB19-1), CD3 (17A2) (eBioscience), CD69 (H1.2F3), CD80 (16-10A1), CD86 (GL1), CD21 (eBio4E3), CD23 (B3B4), and CD24 (M1/69) Abs. All antibodies were purchased from BD Biosciences or eBioscience. Data were collected on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star). The data were analyzed using bi-exponential transformation for complete data visualization.
Quantitative IgM, IgG, and IL-10 ELISA.
Four HBX Immulon ELISA plates (Thermo Scientific) were coated with 100 ng of goat anti-mouse Ig antibody (Southern Biotech) overnight at 4°C, washed and blocked with 1% milk in T-PBS (0.05% Tween 20, 1× PBS), washed again, and stored at −20°C until use. Upon harvest of cells, culture supernatant was collected and stored at <−20°C. Culture supernatants were thawed, diluted, and applied to coated plates. A standard curve was generated with mouse IgM or IgG (Southern Biotech, Birmingham, AL). Goat anti-mouse IgM or IgG conjugated with horseradish peroxidase (HRP) was used as the secondary antibody (Southern Biotech). IL-10 ELISA was performed according to the manufacturer's instructions (BD Biosciences). Briefly, ELISA plates were coated overnight with IL-10 capture antibody, washed (with T-PBS), and blocked with 10% FCS in PBS. Culture supernatant or standards were applied to the plates and incubated for 2 h at room temperature. Plates were then washed and incubated with anti-IL-10 HRP-conjugated secondary antibody. After incubation with secondary antibody, plates were washed and developed with OPtEIA (BD) and analyzed for color change (optical density at 450 nm [OD450]). Fitting of the standard curve and calculation of unknowns were performed using Prism software (GraphPad).
GG immunization.
DNA was purified from E. coli cells by use of endotoxin-free Mega prep kits (Qiagen). The CRP.daf gene (53) was subcloned into pcDNA3. DNA was used to coat 1.0-μm gold particles (Bio-Rad) and loaded into Tefzel tubing. DNA (8 μg) was administered by a Helios gene gun (GG; Bio-Rad) at 400 lb/in2, in two shots per mouse on the shaved abdomen per time point. Immunized mice were bled after boosting, and the blood was processed to obtain serum. For combination experiments, mice received four shots per time point, with two of each immunogen. For the single-immunogen controls, mice received two shots of DNA containing the immunogen and two shots of vector DNA to maintain the same amount of DNA delivery in all groups.
rTcPRAC, rCRP, and whole-parasite ELISA.
For rTcPRAC and rCRP analysis, four HBX Immulon ELISA plates (Thermo Scientific) were coated with 100 ng of purified protein and incubated overnight at 4°C. Plates were washed with T-PBS, blocked, and stored at −20°C until use. For whole-parasite ELISA, plates were coated with 2 × 105 heat-inactivated parasites per well and incubated overnight at 4°C. Plates were washed, blocked, and stored at −20°C until use. Mouse serum was diluted in blocking buffer and applied to ELISA plates overnight at 4°C. Plates were washed and developed with the appropriate secondary antibody. The estimated reciprocal end-point titer (RET) was determined graphically based on the OD450 values for an equivalent dilution of pooled mouse preimmunization serum samples. RET was defined as the first dilution with a value below the preimmune OD450 plus 1 standard deviation (SD) (two or three replicates).
Plasma cell and memory B-cell rTcPRAC-specific ELISPOT assay.
Immunized mice were sacrificed after the third booster. Bone marrow (BM) was collected from the femurs and tibiae of mice and processed for single cells. BM was flushed from bones by use of a 32-gauge needle and was mashed through a 40-μm cell strainer (BD Biosciences) with the flat head of a 3-ml syringe. The resulting cell suspension was washed, and the cells were resuspended in cRPMI. Spleens were processed for single cells by gentle mashing in a 40-μm cell strainer, treated with RBC lysis buffer (150 mM NH4Cl, 10 mM NaHCO3, 115 μM EDTA), washed with 1× PBS, and suspended in cRPMI. Multiscreen HTS 96-well enzyme-linked immunospot (ELISPOT) plates (BD Biosciences) were coated with 2.5 μg/ml of rTcPRAC and incubated overnight. ELISPOT plates were washed with T-PBS and blocked with cRPMI for 2 h. Blocking medium was removed, and cells (BM or splenocytes) were plated into the ELISPOT plates (6 to 12 wells per sample) at several dilutions (106, 105, 5 × 104, 2.5 × 104, 1.25 × 104, and 1.0 × 103 cells/well). After 5 to 6 h, the cells were washed off with PBS (three times) followed by T-PBS (three times). Secreted antibodies were detected by incubating cells with anti-mouse IgG conjugated to biotin (16 h, 4°C), washing them with T-PBS (three or four times), incubating them with avidin-peroxidase complex (30 min, room temperature [RT]) (Vector Laboratories, Burlingame, CA), and washing them with T-PBS (three times) and PBS (three times), followed by incubation with AEC ELISPOT substrate (8 min, RT) (BD Biosciences). The reaction was stopped by washing cells with PBS. Spots were analyzed using ImmunoSpot 4.5 image acquisition software and ImmunoSpot 5.0 Professional DC software (ImmunoSpot). BM plasma cells are reported as the number of spots per 106 splenocytes. For analysis of memory B cells, splenocytes were stimulated with 0.4 μg of R595 lipopolysaccharide (Alexis Biochemicals, Plymouth Meeting, PA) and pokeweed mitogen (PWM; Emory stock [gift from Shane Crotty]) for 6 days at 5 × 106 cells/ml (37°C, 5% CO2) to induce differentiation into plasma cells. After stimulation, cells were washed with cRPMI, plated on ELISPOT plates (106, 5 × 105, 2.5 × 105, and 1.25 × 105 cells/well), and incubated for 5 to 6 h (37°C, 5% CO2). Cells were washed off the plates with PBS (three times) followed by T-PBS (three times). Expanded memory B cells were recorded as the number of spots per 106 cells stimulated. This protocol was adapted from previously published studies (14, 55, 56).
Binding of rTcPRAC to IgG from TcPRAC-immune mice.
IgG from TcPRAC- or CRP-immunized mice was purified from sera via a Melon column (Pierce, Thermo Scientific). Seventy-five micrograms of purified IgG was bound to protein A/G Plus resin (Pierce, Thermo Scientific) for 1 h at 4°C with rotation. The resin was washed with binding buffer (0.25 M Tris, 0.15 M NaCl) to remove unbound material. rTcPRAC protein (1 to 30 μg) was added to the IgG-protein A/G resin and incubated overnight at 4°C in either 100 or 500 μl of binding buffer. Nonbound protein was spun out of the columns and concentrated to a final volume of 100 μl. Nonbound protein was stored at 4°C until used for splenocyte stimulation or SDS-PAGE/Western blot analysis. The protein bound to the IgG-protein A/G resin was washed with conditioning buffer (Pierce), eluted with low-pH elution buffer (Pierce, Thermo Scientific), neutralized with 1 M Tris, pH 9.5, and stored at 4°C until used for splenocyte stimulation or SDS-PAGE/Western blot analysis.
Western blot analysis.
Proteins diluted in sample buffer (8% SDS, 40% glycerol, 300 mM Tris, 0.04% bromophenol blue) were separated at 110 V for 1.5 h in 10 to 12% SDS-PAGE gels. Gels were transferred to nitrocellulose using an iBlot system (Invitrogen). Memcode Blue protein stain (Pierce, Thermo Scientific) was used to visually confirm protein transfer. Membranes were blocked with blocking solution (3% milk, 1% bovine serum albumin [BSA], 1× PBS, 0.05% Tween 20) and washed with wash buffer (1× PBS, 0.05% Tween 20). Sera were diluted in blocking buffer and incubated at RT for 1 h with rotation. Membranes were washed, and diluted HRP-conjugated secondary antibodies were applied in blocking buffer. Membranes were washed and developed with a Super Signal Pico kit (Pierce, Thermo Scientific).
Statistical analysis.
Two-way analysis of variance (ANOVA) was used to compare two treatments over a range of doses or after passage of time. Bonferroni posttest analysis, Student's t test, or Mann-Whitney tests were used for comparison of individual doses or time points. For analysis of the correlation between two treatments, the Pearson test was applied.
RESULTS
Y strain-derived rTcPRAC-induced B-cell proliferation.
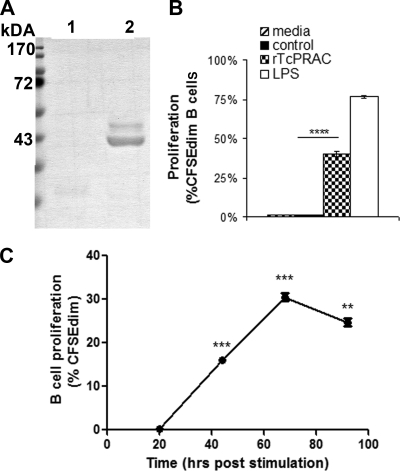
Full-length TcPRACA was cloned from T. cruzi strain Y parasites, and the predicted amino acid sequence was >99% identical to the reported TcPRACA primary protein sequence from the CL strain (9). TcPRACA (rTcPRAC) was subcloned into a prokaryotic expression vector, and the purified rTcPRAC yielded the expected 45-kDa monomeric protein with minimal background protein contamination (Fig. 1A). To confirm that this protein retained the functional activity of the mitogenic dimeric form of rTcPRAC, BALB/c splenocytes were stimulated for 72 h with rTcPRAC and analyzed for B-cell proliferation by CFSE dilution. Purified rTcPRAC was compared to a negative control consisting of an equal-volume addition of mock-induced empty vector IMAC elution fraction (Student's t test; P < 0.0005) (Fig. 1B). To evaluate the kinetics of rTcPRAC-induced B-cell proliferation, splenocytes were stimulated with 10 μg/ml of rTcPRAC. Proliferation was determined by CFSE dilution at 20, 44, 68, and 72 h post-rTcPRAC stimulation (Fig. 1C). rTcPRAC-induced B-cell proliferation was detected at 44 h and was declining by 92 h poststimulation. These results confirm the mitogenic capacity of Y strain-derived rTcPRAC and indicate that 3 days was the optimal stimulation time for analysis of rTcPRAC-induced B-cell proliferation, which was used throughout the rest of this study.
FIG. 1.
Y strain-derived rTcPRAC-induced B-cell proliferation. (A) Empty vector IMAC-purified protein (lane 1) and IMAC-purified rTcPRAC (lane 2). (B) rTcPRAC-induced B-cell proliferation compared with that for negative control in the absence of PMB-treated medium or that for 10 μg/ml LPS as a positive control. (C) Time course analysis of rTcPRAC-induced B-cell proliferation. Statistics compare each time point with the previous one. Data represent the mean percentages for triplicate repeats ± SD. ****, P < 0.00001; ***, P < 0.0001; **, P < 0.001 (Student's t test).
Dose-dependent rTcPRAC-induced B-cell CD69 and CD86 expression and IgG secretion.
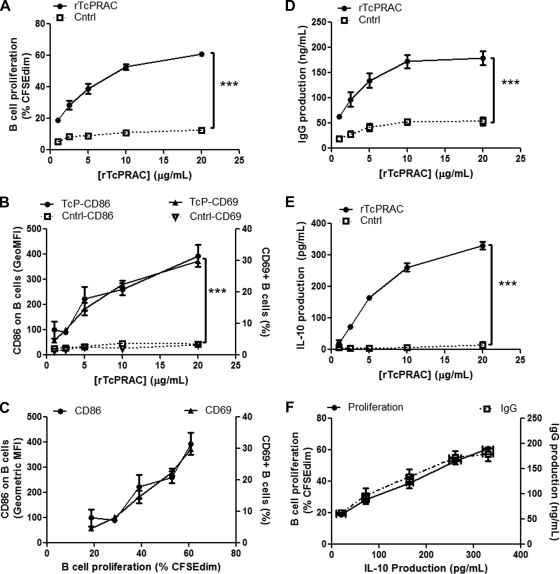
Upregulation of surface activation markers and costimulatory markers is a hallmark of lymphocyte activation and has been reported in the context of polyclonal B-cell mitogenicity (35). Therefore, we assessed the correlation between rTcPRAC-induced B-cell proliferation and the expression of activation and costimulatory surface proteins. To assess whether rTcPRAC-induced B-cell activation increased surface expression of the early activation marker CD69 and the costimulatory molecules CD80 and CD86, BALB/c splenocytes were stimulated with 1 to 20 μg/ml of rTcPRAC or an equal volume of negative control. rTcPRAC stimulation resulted in B-cell proliferation that was significantly greater than that with negative control stimulation (P < 0.0001) and correlated with the rTcPRAC dose (P = 0.02; R2 = 0.86) (Fig. 2A). rTcPRAC treatment increased the number of CD69-positive B cells and increased the geometric mean fluorescence intensity (GeoMFI) of CD86 on B cells compared to that on negative controls (P < 0.0001 for both) (Fig. 2B). The expression of these proteins correlated with the rTcPRAC dose (P = 0.008 and R2 = 0.93 for CD69 and P = 0.009 and R2 = 0.92 for CD86). CD80 expression on B cells was not altered after rTcPRAC stimulation (data not shown). The percentage of CD69-positive B cells and the GeoMFI of CD86 on B cells were plotted against rTcPRAC-induced B-cell proliferation (Fig. 2C). Both the level of CD69-positive B cells and the level of CD86 after rTcPRAC stimulation correlated with rTcPRAC-induced B-cell proliferation (P < 0.001 and R2 = 0.98 for CD69 and P = 0.01 and R2 = 0.90 for CD86).
FIG. 2.
rTcPRAC-induced B-cell proliferation, surface activation phenotype, IgG secretion, and IL-10 secretion. BALB/c splenocytes were stimulated for 72 h with rTcPRAC. (A) Increased B-cell proliferation compared to that of controls (P < 0.0001) was dose dependent (P = 0.02; R2 = 0.86; Pearson test). (B) Increased expression of CD69 and CD86 on B cells compared to that on negative controls (P < 0.0001) was dose dependent (P = 0.008 and R2 = 0.93 for CD69 and P = 0.009 and R2 = 0.92 for CD86). (C) CD69 and CD86 expression on B cells correlated with TcPRAC-induced B-cell proliferation (P < 0.001 and R2 = 0.98 for CD69 and P = 0.01 and R2 = 0.90 for CD86). (D) Increased IgG secretion compared to that in controls (P < 0.0001) was dose dependent (P = 0.03; R2 = 0.94; Pearson test). (E) Increased IL-10 production compared to that in controls (P < 0.0001) was dose dependent (P = 0.02; R2 = 0.88). (F) IL-10 production correlated with both B-cell proliferation and IgG production (P = 0.0001 and R2 = 0.996 for B-cell proliferation and P = 0.0032 and R2 = 0.962 for IgG production). Data are presented as means for duplicate or triplicate repeats ± SEM. Two-way ANOVA was used to compare treatment groups, and the Pearson test was used for correlation.
Hypergammaglobulinemia is a common attribute of B-cell mitogenic responses during infectious disease and is a hallmark of acute-phase Chagas' disease. To assess rTcPRAC-induced IgG secretion, poststimulation culture supernatants were analyzed by ELISA to determine the IgG concentration. rTcPRAC B-cell stimulation resulted in a significant increase in IgG production compared to negative control stimulation (P < 0.0001) and correlated with a rTcPRAC dose between 1 and 10 μg/ml (P = 0.03; R2 = 0.94) (Fig. 2D). Isotype analysis of secreted IgG revealed that rTcPRAC stimulation induced the production of IgG1 and IgG2 (see Fig. S1A in the supplemental material). TcPRAC stimulation also resulted in dose-dependent IgG antibody-secreting-cell (ASC) formation, as measured by ELISPOT assay (see Fig. S1B in the supplemental material).
rTcPRAC-induced B-cell proliferation and IgG secretion correlate with IL-10 secretion.
Recent studies have highlighted non-antibody-mediated B-cell effector functions, such as cytokine secretion (26). Several other investigators reported IL-10 secretion from murine splenocytes after stimulation with mitogenic proteins (16, 18, 28). To evaluate rTcPRAC-induced IL-10 production, BALB/c splenocytes were stimulated with rTcPRAC or negative control, and culture supernatants were analyzed. IL-10 production correlated with the rTcPRAC dose (P = 0.02; R2 = 0.88) and was virtually undetectable for negative control stimulation (Fig. 2E). IL-10 production also correlated with both B-cell proliferation and IgG production (P = 0.0001 and R2 = 0.996 for B-cell proliferation and P = 0.0032 and R2 = 0.962 for IgG production) (Fig. 2F).
MZ B cells are more responsive to TI rTcPRAC stimulation than are FM B cells.
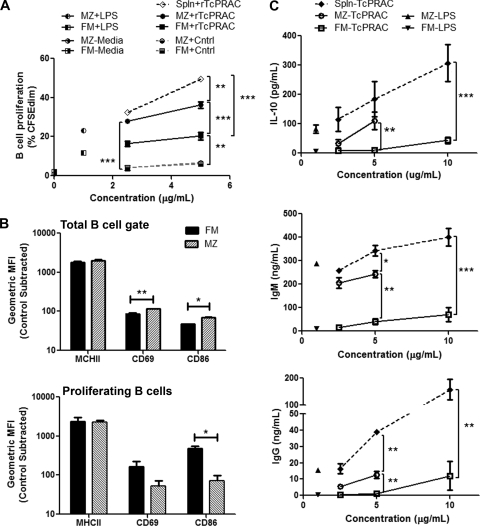
MZ and FM B cells represent functionally and spatially distinct populations in the spleen and have been reported to differentially respond to other TI type 1 antigens (30). Therefore, we determined whether MZ and FM B cells differentially respond to rTcPRAC. To test rTcPRAC-induced MZ and FM B-cell activation, splenocytes were negatively sorted to isolate an untouched mature B-cell population, which was then sorted into MZ and FM mature B cells based on surface phenotype. Sorted MZ B cells, FM B cells, and B cells in a mixed splenocyte population were stimulated with rTcPRAC and analyzed for proliferation (Fig. 3A) and surface marker expression (CD69, CD86, and major histocompatibility complex class II [MHCII]) (Fig. 3B). After stimulation, culture supernatants were analyzed for antibody and IL-10 secretion (Fig. 3C). MZ and FM B cells proliferated in response to rTcPRAC, in contrast to negative controls (P < 0.0001 and P = 0.0004, respectively) (Fig. 3A). MZ B cells proliferated to a greater extent than did FM B cells (P = 0.0007). rTcPRAC-induced B-cell proliferation was greater in the mixed splenocyte population than in either isolated B-cell population (P < 0.001 for both). MZ B cells proliferated more in response to LPS than did FM B cells, and neither subset proliferated without stimulation (medium alone). The difference between rTcPRAC-induced B-cell proliferation of MZ and FM B cells was greatest at 5 μg/ml rTcPRAC, and therefore the activation phenotype was assessed at this concentration. Expression of MHCII, CD69, and CD86 increased compared to that with control stimulation for both subset populations (P < 0.01 for each). Analysis of the total B-cell gate indicated that CD69 and CD86 levels were increased on MZ B cells compared to those on FM B cells (P = 0.009 and P = 0.02, respectively) (Fig. 3B, top panel). Within the proliferating cells, FM B cells displayed higher levels of CD86 (P = 0.02) (Fig. 3B, bottom panel). MZ B cells secreted significantly more IL-10 than did FM B cells (P < 0.01) (Fig. 3C, top panel). MZ B cells secreted sixfold more IgM in response to rTcPRAC than did FM B cells (P < 0.05) (Fig. 3C, middle panel). Neither FM nor MZ B cells produced significant IgG in response to rTcPRAC, whereas B cells in mixed splenocyte populations had robust IgG production (Fig. 3C, bottom panel). These results indicate that rTcPRAC induced TI activation of MZ and FM B cells, although these two subsets produced different patterns of response. MZ B cells were more sensitive than FM B cells to rTcPRAC-induced B-cell proliferation, IL-10 production, and antibody secretion. However, proliferating FM B cells had an activated phenotype that was comparable to or increased compared to that of proliferating MZ B cells. These data support the hypothesis that pathogen-encoded mitogenic proteins may differentially influence MZ and FM B cells during infection.
FIG. 3.
Differential rTcPRAC-induced TI MZ and FM B-cell stimulation. Splenocytes (spln), sorting-purified MZ B cells, or sorting-purified FM B cells were stimulated with 2.5 to 10 μg/ml rTcPRAC, negative control (cntrl), or 1 μg/ml LPS or were left unstimulated (medium control). For flow cytometry data, the CD19+ live cell gates were analyzed. (A) B-cell proliferation was measured by CFSE diminution after stimulation. (B) B-cell activation by surface phenotype was assessed after stimulation with 5 μg/ml rTcPRAC. (C) rTcPRAC-induced antibody and IL-10 secretion was assessed in culture supernatants from splenocyte, MZ B-cell, and FM B-cell stimulations. Data are presented as means for duplicate or triplicate repeats ± SEM. ***, P < 0.0001; **, P < 0.001; *, P < 0.05 (two-way ANOVA and Bonferroni posttest analysis).
Undetectable TcPRAC-specific IgG during experimental infection.
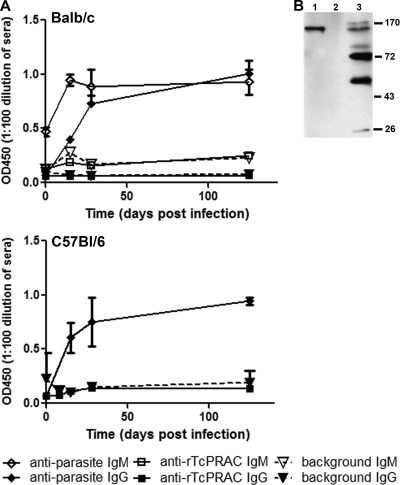
A defining characteristic of a mitogen is the lack of a specific response to the mitogen itself, unless it is present at a very low concentration (12). Thus, a pathogen-encoded mitogen that is expressed during early infection at high enough levels to contribute to polyclonal B-cell proliferation would not be expected to generate a specific immune response. To assess whether a specific response to T. cruzi-expressed TcPRAC develops during infection, relatively susceptible (BALB/c) and resistant (C57BL/6) mouse strains were infected with T. cruzi. Serial serum samples were collected and analyzed for a specific IgG and/or IgM response to rTcPRAC and a specific IgG or IgM response to T. cruzi whole parasites. Analysis of sera over the course of the 125 days of infection indicated that a specific IgM response did not develop in response to rTcPRAC in BALB/c mice and an rTcPRAC-specific IgG response did not develop in either model system when a specific T. cruzi response occurred (Fig. 4A). Negative control-coated wells were used to confirm that there was no rTcPRAC-specific IgM or IgG binding (Fig. 4A). rTcPRAC-specific IgG responses remained undetectable at day 125 postinfection, when specific antibodies to CRP and several other T. cruzi membrane proteins were detected (Fig. 4B). These data support the hypothesis that during experimental infection, TcPRAC protein is not presented in an immunogenic context.
FIG. 4.
rTcPRAC-specific IgG is undetectable during experimental T. cruzi infection. Susceptible BALB/c or resistant C57BL/6 mice were infected with T. cruzi. (A) Serial serum samples were analyzed for responses to whole parasites, rTcPRAC, or negative control to establish background binding. Data represent triplicate repeats with pooled sera from five mice in each experiment. (B) Western blot analysis of IgG reactivity to rCRP (500 ng) (lane 1), rTcPRAC (500 ng) (lane 2), and parasite membrane proteins (10 μg) (lane 3) in BALB/c sera collected at 125 days postinfection (diluted 1:500).
TcPRACA DNA immunization via GG presents rTcPRAC as an immunogen.
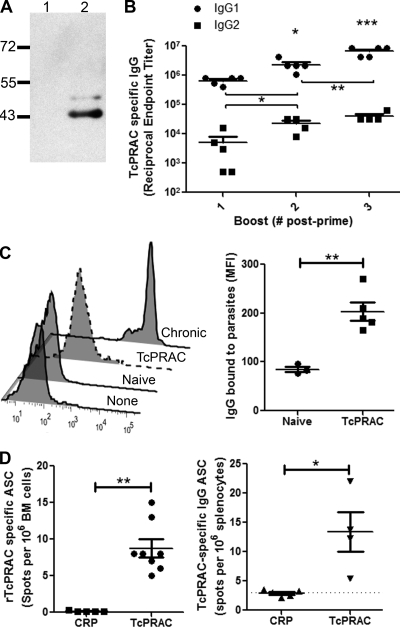
GG delivery of antigen has been shown to be an effective method for generating immunity to target proteins by DNA inoculation into cells within the dermis (40, 47, 65). To evaluate the potential of intradermal genetic immunization to deliver rTcPRAC in an immunogenic context, BALB/c mice were primed and boosted with TcPRACA DNA via GG inoculation. After each immunization, blood was collected and sera analyzed for generation of rTcPRAC-specific IgG responses. Western blot analysis demonstrated that TcPRAC-specific IgG bound to monomeric rTcPRAC protein (Fig. 5A). Specific rTcPRAC IgG responses were evident by boost one and increased between boost one and each subsequent boost (Fig. 5B). While both IgG2 and IgG1 were generated in response to TcPRAC immunization, specific IgG1 predominated, with an RET of 7.6 × 106 ± 2.5 × 106 versus an IgG2 RET of 7.0 × 104 ± 7.8 × 103 at boost 3 (average ± standard error of the mean [SEM]; P = 0.04) (Fig. 5B). Mice receiving empty vector did not mount a TcPRAC-specific IgG response that differed from that of naïve mice (data not shown). Furthermore, TcPRACA immune sera bound to whole parasites by ELISA, with a TcPRAC-specific IgG RET of 320 ± 192 (mean ± SEM for 5 mice) and with a significant increase in binding of IgG to parasites for TcPRAC immune sera compared to naïve control sera (P < 0.01) (Fig. 5C). These data indicate that GG immunization with TcPRAC generates a robust, specific humoral IgG response that is capable of binding TcPRAC on the surface of the whole parasite.
FIG. 5.
GG immunization with rTcPRAC DNA elicits an immunogenic response. BALB/c mice were primed and boosted at 1-month intervals. Ten days after each boost, serum was collected. (A) TcPRAC-specific IgG (diluted 1:2,000) binds to rTcPRAC (7 μg of protein loaded) (lane 2) without binding the negative control (lane 1). (B) Analysis of anti-rTcPRAC IgG1 and IgG2 responses by ELISA. Horizontal lines represent the mean RET (±SEM) for five mice. (C) (Left) Representative histograms showing IgG bound to the surfaces of live parasites, representing mice after 125 days of infection (chronic), TcPRAC-immune mice, naïve mice, or mice with fetal bovine serum as a negative control (no mouse antibody). (Right) Mean florescence intensity (MFI) after treatment of parasites with naïve or TcPRAC immune sera from five separate mice. (D) Bone marrow (BM) cells were isolated after boost 3 and analyzed by rTcPRAC-specific ELISPOT assay for antibody-secreting plasma cells. Data represent the mean number of ASC (±SEM) in 8 mice. Splenocytes were isolated after boost 3 and analyzed for rTcPRAC-specific memory B cells. Data represent the mean number of ASC (±SEM) in 4 mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test).
rTcPRAC immunization generated rTcPRAC-specific bone marrow plasma and splenic memory B cells.
In general, TI B-cell activation leads to the development of short-term plasma cells that reside in the spleen (57). This response is distinct from TD antigens that induce memory B cells or long-lived plasma cells (38). To determine if rTcPRAC DNA immunization induces specific bone marrow plasma cells and memory B cells, mice were immunized and TcPRAC-specific IgG ASC were enumerated (13, 14). rTcPRAC-specific bone marrow plasma cells and memory cells were detected after GG immunization but not following immunization with control antigen (Fig. 5D) (P = 0.004; Mann-Whitney test).
TcPRACA immunization did not induce mitogenic response or B-cell dysfunction.
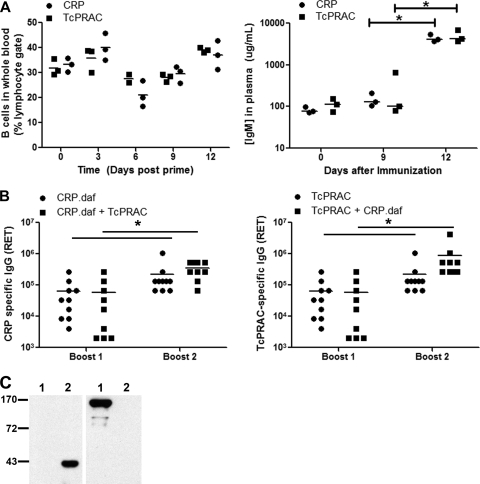
A concern in using B-cell mitogens as immunogens is the potential induction of polyclonal B-cell activation or proliferation. To determine whether intradermal delivery of TcPRACA DNA led to mitogenic activation of B cells, BALB/c mice were primed with either TcPRACA or CRP DNA via GG immunization. Blood was collected prior to inoculation and for up to 12 days postimmunization. Blood was stained for B and T cells and analyzed by flow cytometry to determine the percentages of B and T cells within the lymphocyte gate. During the first 6 days after priming with TcPRACA versus CRP, there was no difference in the percentages of B cells within the peripheral blood (P = 0.18; two-way ANOVA) (Fig. 6A). Between days 9 and 12 postinoculation, there was a small increase in the percentage of B cells in rTcPRAC-immunized mice compared to CRP-immunized mice (P = 0.017; two-way ANOVA). To test for early IgM secretion, which would indicate a mitogenic response, mouse plasma samples were analyzed for total IgM levels postpriming (Fig. 6A). There were no significant differences in the levels of IgM response in mice primed with TcPRACA versus CRP (P = 0.54; two-way ANOVA), although mice primed with either immunogen resulted in increased total IgM by day 12 postpriming (P < 0.0001). These data indicate that the delivery of rTcPRAC via GG did not result in an early, nonspecific polyclonal B-cell mitogenic effect. Rather, the expansion of B cells and secretion of IgM after initial priming with TcPRACA via GG were consistent with the generation of a specific humoral response.
FIG. 6.
GG immunization with rTcPRAC DNA was nonmitogenic and led to successful coimmunization. (A) BALB/c mice were primed by GG immunization with rTcPRAC or CRP DNA. Serum samples were collected prior to priming and every 3 days for 12 days postpriming. Staining of whole blood for B cells (CD19+) and T cells (CD3+) indicated that there was no premature expansion of peripheral B cells after rTcPRAC priming compared to CRP priming. Data represent repeat measures on three mice (right). Analysis of the IgM concentrations in the sera of rTcPRAC-immunized mice showed comparable levels and timing of the postprime increase compared to those for CRP-immunized mice. Data represent serum samples from three mice, with the mean indicated by a line. (B) BALB/c mice were immunized with CRP, rTcPRAC, or both immunogens in combination and then analyzed for specific responses via ELISA. Data represent the mean RET for five mice for each experimental group. *, P < 0.05 (Student's t test or Bonferroni posttest from two-way ANOVA). (C) Western blot analysis demonstrates a lack of cross-reactivity between TcPRAC and CRP in immunized mice. Lane 1 contains rCRP (500 ng), and lane 2 contains rTcPRAC (500 ng). Blots were probed with three pooled booster sera, diluted 1:500, from rTcPRAC-immune (left) or CRP-immune (right) mice.
rTcPRAC codelivered with another T. cruzi antigen.
To investigate the effect of rTcPRAC immunization on the generation of humoral immunity to a codelivered antigen, mice were immunized in combination with the T. cruzi antigen CRP (53). CRP GG immunization was evaluated with or without rTcPRAC DNA coimmunization (Fig. 6B). Administration of CRP with or without rTcPRAC resulted in comparable anti-CRP IgG RET (P = 0.345). The boost response for CRP as a single antigen versus that as a combined antigen was significant (P = 0.014 for both, by Wilcoxon signed rank test and two-way ANOVA; P = 0.0007 for change due to time). Coimmunization resulted in an rTcPRAC-specific IgG response that was comparable to that for GG delivery of rTcPRAC as a single immunogen (P = 0.133). Western blot data confirmed that TcPRAC immunization did not result in the generation of a CRP-specific response or vise versa (Fig. 6C). The total level of IgG in serum after immunization with rTcPRAC was not elevated compared to that for sera from CRP-immunized mice, confirming that a polyclonal immunoglobulin response was not induced by TcPRAC GG immunization (P = 0.4; Student's t test). These data demonstrate that genetic immunization with rTcPRAC does not interfere with the immune response to a codelivered immunogen.
IgG from TcPRAC-immune mice binds to mitogenic rTcPRAC.
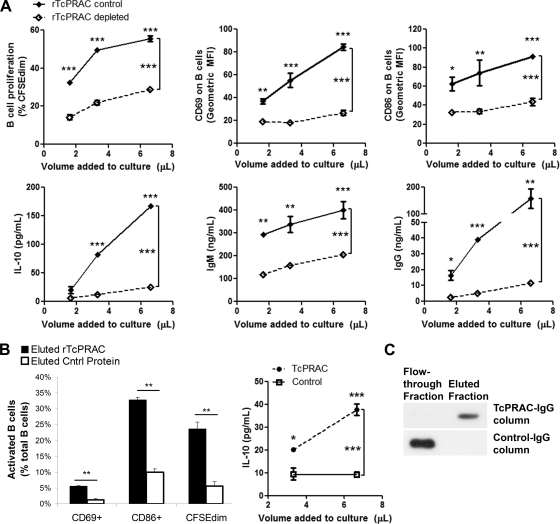
For an antibody response against a protein mitogen to be effective in the host, it must bind to the mitogenic form of the protein. Therefore, we tested whether IgG from the sera of immunized mice could bind to mitogenic rTcPRAC, depleting mitogenic activity. To test for binding, rTcPRAC-immune (TcP-imm) IgG or control CRP-immune (CRP-imm) IgG was bound to protein A/G resin and then incubated with equivalent amounts of rTcPRAC. To test for residual mitogenic activity after IgG column depletion, an equal volume of unbound protein was recovered from each column and added to splenocyte cultures. Mitogenic rTcPRAC was depleted following treatment with TcP-Imm IgG, as indicated by decreased B-cell CD69 and CD86 expression, IL-10 secretion, and IgM and IgG secretion compared to those with the CRP-imm IgG control (P ≤ 0.0001 for each measure) (Fig. 7A). rTcPRAC eluted from TcP-imm IgG was compared to nonspecifically bound protein eluted from control CRP-imm IgG. Eluted rTcPRAC had significant mitogenic activity compared to the control, inducing B-cell proliferation, surface marker expression, and IL-10 secretion (P ≤ 0.0001 for each measure) (Fig. 7B). Western blot analysis confirmed that rTcPRAC bound to the TcP-imm IgG, whereas it did not bind to CRP-imm IgG (Fig. 7C). These data demonstrate that TcP-imm IgG binds to functionally active, mitogenic rTcPRAC.
FIG. 7.
GG rTcPRAC immunization generates specific IgG that binds mitogenic rTcPRAC. rTcPRAC-immune (TcP-imm) IgG or control CRP-immune (CRP-imm) IgG was bound to protein A/G resin and then incubated with rTcPRAC. (A) Equal volumes of nonbound protein after incubation of 30 μg rTcPRAC with TcP-imm IgG (rTcPRAC depleted) or CRP-imm IgG (TcPRACA control) were added to splenocyte cultures. After stimulation, live cells were assessed for B-cell activation in terms of B-cell proliferation and surface phenotype (CD69 and CD86) by flow cytometry. IL-10 and antibody (IgM and IgG) secretion was assessed by ELISA. Data represent the means for triplicate repeats (±SEM) for each measure. (B) rTcPRAC eluted from TcP-imm IgG was compared to nonspecifically bound protein eluted from control CRP-imm IgG for the ability to stimulate B cells. (Left) Cells were stimulated with eluted protein. After stimulation, cells were analyzed for B-cell proliferation and activation (CD69 and CD86) by flow cytometry. Data represent the means for triplicate repeats (±SD) for each measure. (Right) Cells were stimulated with eluted protein. After stimulation, the culture supernatant was analyzed for IL-10 secretion by ELISA. Eluted rTcPRAC induced dose-dependent IL-10 secretion compared to control eluted protein. Data represent the means for triplicate repeats (±SEM). *, P < 0.05; **, P < 0.001; ***, P < 0.0001 (Bonferroni posttests after two-way ANOVA or Student's t test). (C) Application of rTcPRAC to TcPRAC-specific and control IgG resulted in rTcPRAC in the flowthrough fraction from the control IgG column and in the elution fraction from the TcPRAC-specific IgG column. TcPRAC was visualized by use of anti-TcPRAC IgG from polyclonal sera from protein-immunized rabbits.
DISCUSSION
This study provides a comprehensive characterization of rTcPRAC-stimulated polyclonal B-cell responses in vitro, showing that B-cell proliferation correlates with activation phenotype, secretion of antibodies, and cytokine production and that rTcPRAC differentially stimulates splenic B-cell subsets. Previous reports of the TI mitogenic activity of TcPRAC were based on proliferation of T-cell-depleted or athymic splenocytes (45). In this study, the activation of B cells by TcPRAC was directly measured both in mixed splenocyte populations and after purification of specific B-cell subsets. In addition, this study provides the first evidence that TcPRAC is not immunogenic during experimental infection but that TcPRAC-specific IgG can be induced via intradermal genetic immunization.
Analysis of B-cell surface markers showed that rTcPRAC stimulation increased B-cell surface expression of an early activation marker, CD69, as well as CD86, in a dose-dependent manner that correlated with B-cell proliferation. TcPRAC-induced, dose-dependent IgG secretion correlated with B-cell proliferation as well. These data emphasize the potential of TcPRAC to contribute to B-cell polyclonal activation during acute Chagas' disease, which is associated with increased B-cell activation (M. A. Bryan et al., unpublished data) and is characterized by hypergammaglobulinemia as well as nonspecific lymphoproliferation, both of which are thought to contribute to parasite evasion of host immunity (31, 44).
Recent research into the function of B cells has highlighted multifunctional effector attributes of these cells (24), especially the role of IL-10 cytokine-secreting B cells (26, 27, 34). IL-10 can be secreted from a variety of B-cell subsets, including plasma cells and plasmablasts (27), regulatory B1 cells (B10) (71), and potentially MZ and FM B cells (26). We found that IL-10 was secreted by splenocytes upon stimulation with rTcPRAC. Several other B-cell mitogenic proteins are known to stimulate production of IL-10, including a similar proline racemase, PrpA, from Brucella abortus (16, 18, 28, 59). rTcPRAC-induced IL-10 secretion correlated with B-cell proliferation and IgG secretion, suggesting that this cytokine was produced by rTcPRAC-activated B cells. IL-10 is considered a master regulator of immunity to infection and influences the fate of many cell types, depending on the timing and context of IL-10 production (11). IL-10 production from rTcPRAC-stimulated B cells could contribute to increased plasma cell differentiation from memory B cells (2), as well as influence other immune effector mechanisms, such as macrophage responses to parasites (1).
Marginal zone and follicular B cells constitute two functionally and anatomically distinct mature B-cell subsets within the spleen (3). MZ B cells are located at the marginal sinus of the spleen and are considered first responders to blood-stage pathogens. In addition, MZ B cells are more responsive to LPS, suggesting a greater responsiveness to TI antigens in general (30, 39). FM B cells are the predominant responders to TD antigenic stimulation (3). MZ B cells were activated and proliferated more in response to rTcPRAC than did FM B cells. The in vitro stimulation of MZ B cells by rTcPRAC is similar to the early and sustained expansion of MZ B cells that is associated with polyclonal B-cell activation during experimental infection (Bryan et al., unpublished data). Furthermore, analysis of purified MZ and FM B cells indicated that upregulation of CD69 and CD86 was a TI response to rTcPRAC.
MZ B cells produce robust IgM in response to rTcPRAC stimulation, but FM B cells produce minimal IgM. These data agree with evidence from other studies showing that MZ B cells have enhanced secretory ability (21, 39). IgG secretion was minimal from both MZ and FM B cells compared to the level of IgG produced by B cells in mixed splenocytes, suggesting that accessory cells, probably T cells, are necessary for driving the isotype switch to IgG after rTcPRAC stimulation. These data fit with previous studies showing that polyclonal B-cell activation during experimental T. cruzi infection can largely be abrogated by depletion of CD4 T cells (49). TcPRAC may be a TI mitogen in vitro but may rely more on T-cell activation for a full B-cell mitogenic effect in vivo.
The correlation of rTcPRAC-induced IL-10 production with B-cell proliferation and antibody secretion suggested that B cells produced IL-10 in response to rTcPRAC. rTcPRAC-induced IL-10 secretion from purified B cells confirmed this hypothesis and indicated that MZ B cells were more sensitive to rTcPRAC-induced IL-10 secretion than were FM B cells. IL-10 is secreted from splenocytes during experimental infection and has a negative impact on the development of effective antiparasite immune responses during T. cruzi infection (43, 54). Since splenic MZ B cells are poised to be first responders to blood-borne pathogens (39), the ability of TcPRAC to induce IL-10 secretion from these cells may significantly contribute to immunopathology during early T. cruzi infection.
Mitogens induce a nonspecific immune response and do not generally induce specific immune responses, unless modified or delivered in low doses (12). Therefore, we investigated whether experimental infection with T. cruzi led to TcPRAC-specific IgG responses in two mouse models of T. cruzi infection. In both instances, TcPRAC-specific IgG was not detected. This was not due to a lack of antigen availability, as TcPRAC is expressed on the surfaces of and secreted by trypomastigote parasites (45). TcPRAC has also been shown to be necessary for parasite differentiation and infectivity. Thus, a lack of TcPRAC antigen-specific immunogenicity may help to preserve this essential parasite function (8). Many microbes produce mitogenic molecules that elicit polyclonal B-cell activation in combination with poor specific host responses during experimental infection (25, 28, 63). Delivery of mitogenic moieties in an alternative context can diminish the mitogenic effect, producing antigen-specific responses (16, 58, 62). For example, the Candida albicans mitogenic protein p43, experimentally inoculated at submitogenic doses, is able to partially neutralize the biological effects of this protein (61, 62).
To test whether immunization with TcPRAC could induce an antigen-specific response without mitogenic activation of B cells, we utilized intradermal genetic immunization, with the TcPRAC gene delivered in a eukaryotic expression vector. Intradermal genetic immunization via GG uses small amounts of DNA as starting material and has a highly reproducible efficacy for generation of humoral and cellular responses (40, 47). Delivery of rTcPRAC in this context resulted in the generation of high-titer rTcPRAC-specific IgG capable of binding to rTcPRAC as well as to whole parasites. The specific IgG1 RET was 100-fold greater than the IgG2 RET, indicating a predominant type 2 T-cell (Th2) helper response. In addition to providing proof of concept that GG immunization can render a mitogenic protein immunogenic, these experiments further emphasize the ability of rTcPRAC to activate splenic B cells, as removal of rTcPRAC by rTcPRAC-specific IgG significantly diminished B-cell activation, proliferation, and IL-10 production.
Terminally differentiated end-stage B-cell plasma cells in the bone marrow and long-lived circulating memory B cells are the cellular basis for enduring antibody-mediated immunity and acquire longevity as a result of antigen-specific, CD40-dependent interactions with helper T cells (38). The generation of TcPRAC-specific memory B cells and bone marrow plasma cells by rTcPRAC GG immunization is consistent with the presentation of rTcPRAC as a TD immunogen.
To address the possibility that delivery of TcPRAC DNA may induce mitogenic effects in mice, we compared delivery of TcPRAC DNA with that of CRP DNA, encoding a T. cruzi antigen (53), and found similar levels of circulating B cells and similar timing for increased circulating IgM postpriming, which was consistent with an antigenic response. Furthermore, rTcPRAC DNA GG coimmunization with CRP DNA had no negative effect on the generation of high-titer CRP-specific IgG. Conversely, delivery of TcPRAC with or without coimmunization with CRP did not alter the generation of TcPRAC-specific IgG. These data further indicate that rTcPRAC was delivered as an immunogen rather than as a mitogen by GG immunization and highlight the feasibility of multivalent vaccine design with a pathogen-encoded B-cell mitogen.
As is the case with many infectious disease immunization strategies, the approach to T. cruzi immunization has been focused primarily on the introduction of immunodominant targets that would initiate a strong secondary response upon primary infection with parasite (6, 10, 19, 20, 41, 52, 67, 68, 70). The novel strategy presented here was to induce an immune response to a parasite-derived immune evasion factor, in this case a B-cell mitogen. This approach has the potential to combine development of immunity against immune evasion factors with traditional immunization strategies in a multicomponent design, leading to improved host responses to the invading pathogen. Preliminary immunization and challenge studies indicate that coimmunization with TcPRAC and CRP, as described here, improved survival and decreased parasitemia in mice infected with a lethal dose of T. cruzi compared to those obtained with either immunogen alone (our unpublished data).
The results presented here demonstrate that the T. cruzi B-cell mitogen TcPRAC differentially stimulates B-cell subsets and primarily affects MZ B cells. B-cell stimulation correlated with production of Ig and IL-10 secretion, indicating that this protein expressed by infectious trypomastigotes may contribute to the early humoral immune dysfunction seen in acute Chagas' disease. While TcPRAC is apparently nonantigenic during experimental T. cruzi infection, it can be delivered as a potent antigen via genetic immunization. Genetic immunization with TcPRAC did not lead to detectable systemic B-cell expansion and did not interfere with the immune response to a different test immunogen. These data offer the basis for further development of this novel strategy of immunoprotection for this and other infectious diseases.
Supplementary Material
Acknowledgments
This work was supported by grant AI072244 (K.A.N.) from the National Institutes of Health. Marianne Bryan was supported by NIH grant 5T32 CA82084-08 (to Olivera Finn).
We give sincere thanks to Siobhan Guyach for excellent technical assistance with immunizations, sample processing, and protein-specific ELISA data.
Editor: J. H. Adams
Footnotes
Published ahead of print on 16 November 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abrahamsohn, I. A., and R. L. Coffman. 1996. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Exp. Parasitol. 84:231-244. [DOI] [PubMed] [Google Scholar]
- 2.Agematsu, K., H. Nagumo, Y. Oguchi, T. Nakazawa, K. Fukushima, K. Yasui, S. Ito, T. Kobata, C. Morimoto, and A. Komiyama. 1998. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood 91:173-180. [PubMed] [Google Scholar]
- 3.Allman, D., and S. Pillai. 2008. Peripheral B cell subsets. Curr. Opin. Immunol. 20:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aosai, F., M. Chen, H. K. Kang, H. S. Mun, K. Norose, L. X. Piao, M. Kobayashi, O. Takeuchi, S. Akira, and A. Yano. 2002. Toxoplasma gondii-derived heat shock protein HSP70 functions as a B cell mitogen. Cell Stress Chaperones 7:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beucher, M., W. S. Meira, V. Zegarra, L. M. Galvao, E. Chiari, and K. A. Norris. 2003. Expression and purification of functional, recombinant Trypanosoma cruzi complement regulatory protein. Protein Expr. Purif. 27:19-26. [DOI] [PubMed] [Google Scholar]
- 6.Boscardin, S. B., S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 71:2744-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brener, Z. 1980. Immunity to Trypanosoma cruzi. Adv. Parasitol. 18:247-292. [DOI] [PubMed] [Google Scholar]
- 8.Chamond, N., M. Goytia, N. Coatnoan, J. C. Barale, A. Cosson, W. M. Degrave, and P. Minoprio. 2005. Trypanosoma cruzi proline racemases are involved in parasite differentiation and infectivity. Mol. Microbiol. 58:46-60. [DOI] [PubMed] [Google Scholar]
- 9.Chamond, N., C. Gregoire, N. Coatnoan, C. Rougeot, L. H. Freitas-Junior, J. F. da Silveira, W. M. Degrave, and P. Minoprio. 2003. Biochemical characterization of proline racemases from the human protozoan parasite Trypanosoma cruzi and definition of putative protein signatures. J. Biol. Chem. 278:15484-15494. [DOI] [PubMed] [Google Scholar]
- 10.Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirao, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. [DOI] [PubMed] [Google Scholar]
- 11.Couper, K. N., D. G. Blount, and E. M. Riley. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771-5777. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho, A., E. Gronowicz, W. W. Bullock, and G. Moller. 1974. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J. Exp. Med. 139:74-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty, S., R. D. Aubert, J. Glidewell, and R. Ahmed. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111-122. [DOI] [PubMed] [Google Scholar]
- 14.Crotty, S., E. N. Kersh, J. Cannons, P. L. Schwartzberg, and R. Ahmed. 2003. SAP is required for generating long-term humoral immunity. Nature 421:282-287. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham, D. S., R. E. Kuhn, and E. C. Rowland. 1978. Suppression of humoral responses during Trypanosoma cruzi infections in mice. Infect. Immun. 22:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinis, M., D. Tavares, A. J. M. M. Fonseca, R. Faria, A. Ribeiro, A. M. Silverio Cabrita, and P. Ferreira. 2004. Therapeutic vaccine against Streptococcus sobrinus-induced caries. J. Dental Res. 83:354-358. [DOI] [PubMed] [Google Scholar]
- 17.Fairfax, K. A., A. Kallies, S. L. Nutt, and D. M. Tarlinton. 2008. Plasma cell development: from B-cell subsets to long-term survival niches. Semin. Immunol. 20:49-58. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira, P., A. Bras, D. Tavares, M. Vilanova, A. Ribeiro, A. Videira, and M. Arala-Chaves. 1997. Purification, and biochemical and biological characterization of an immunosuppressive and lymphocyte mitogenic protein secreted by Streptococcus sobrinus. Int. Immunol. 9:1735-1743. [DOI] [PubMed] [Google Scholar]
- 19.Fralish, B. H., and R. L. Tarleton. 2003. Genetic immunization with LYT1 or a pool of trans-sialidase genes protects mice from lethal Trypanosoma cruzi infection. Vaccine 21:3070-3080. [DOI] [PubMed] [Google Scholar]
- 20.Fujimura, A. E., S. S. Kinoshita, V. L. Pereira-Chioccola, and M. M. Rodrigues. 2001. DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect. Immun. 69:5477-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn, K. E., and J. W. Brewer. 2006. Evidence that marginal zone B cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J. Immunol. 177:3791-3798. [DOI] [PubMed] [Google Scholar]
- 22.He, B., X. Qiao, P. J. Klasse, A. Chiu, A. Chadburn, D. M. Knowles, J. P. Moore, and A. Cerutti. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 176:3931-3941. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhoff, L. V. 2001. American trypanosomiasis (Chagas' disease), p. 335-353. In S. G. R. Pearson (ed.), Principles and practice of clinical parasitology. John Wiley & Sons Ltd., New York, NY.
- 24.LeBien, T. W., and T. F. Tedder. 2008. B lymphocytes: how they develop and function. Blood 112:1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima, M., A. Bandeira, D. Portnoi, A. Ribeiro, and M. A. Chaves. 1992. Protective effect of a T-cell-dependent immunosuppressive, B-cell-mitogenic protein (F3′EP-Si, or P90) produced by Streptococcus intermedius. Infect. Immun. 60:3571-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund, F. E. 2008. Cytokine-producing B lymphocytes—key regulators of immunity. Curr. Opin. Immunol. 20:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madan, R., F. Demircik, S. Surianarayanan, J. L. Allen, S. Divanovic, A. Trompette, N. Yogev, Y. Gu, M. Khodoun, D. Hildeman, N. Boespflug, M. B. Fogolin, L. Grobe, M. Greweling, F. D. Finkelman, R. Cardin, M. Mohrs, W. Muller, A. Waisman, A. Roers, and C. L. Karp. 2009. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J. Immunol. 183:2312-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madureira, P., M. Baptista, M. Vieira, V. Magalhaes, A. Camelo, L. Oliveira, A. Ribeiro, D. Tavares, P. Trieu-Cuot, M. Vilanova, and P. Ferreira. 2007. Streptococcus agalactiae GAPDH is a virulence-associated immunomodulatory protein. J. Immunol. 178:1379-1387. [DOI] [PubMed] [Google Scholar]
- 29.Malkiel, S., C. J. Kuhlow, P. Mena, and J. L. Benach. 2009. The loss and gain of marginal zone and peritoneal B cells is different in response to relapsing fever and Lyme disease Borrelia. J. Immunol. 182:498-506. [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Bahlburg, A., A. D. Bandaranayake, S. F. Andrews, and D. J. Rawlings. 2009. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J. Immunol. 182:4065-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minoprio, P. 2002. Impact of polyclonal lymphocyte responses on parasite evasion and persistence, p. 101-107. In J. M. Kelly (ed.), Molecular mechanisms of pathogenesis in Chagas disease. Landes Bioscience, Georgetown, TX.
- 32.Minoprio, P., A. Coutinho, S. Spinella, and M. Hontebeyrie-Joskowicz. 1991. Xid immunodeficiency imparts increased parasite clearance and resistance to pathology in experimental Chagas' disease. Int. Immunol. 3:427-433. [DOI] [PubMed] [Google Scholar]
- 33.Minoprio, P. M., H. Eisen, L. Forni, M. R. D'Imperio Lima, M. Joskowicz, and A. Coutinho. 1986. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantitation of both T- and B-cell responses. Scand. J. Immunol. 24:661-668. [DOI] [PubMed] [Google Scholar]
- 34.Mizoguchi, A., and A. K. Bhan. 2006. A case for regulatory B cells. J. Immunol. 176:705-710. [DOI] [PubMed] [Google Scholar]
- 35.Montes, C. L., E. V. Acosta-Rodriguez, M. C. Merino, D. A. Bermejo, and A. Gruppi. 2007. Polyclonal B cell activation in infections: infectious agents' devilry or defense mechanism of the host? J. Leukoc. Biol. 82:1027-1032. [DOI] [PubMed] [Google Scholar]
- 36.Montes, C. L., E. I. Zuniga, J. Vazquez, C. Arce, and A. Gruppi. 2002. Trypanosoma cruzi mitochondrial malate dehydrogenase triggers polyclonal B-cell activation. Clin. Exp. Immunol. 127:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norris, K. A., J. E. Schrimpf, and M. J. Szabo. 1997. Identification of the gene family encoding the 160-kilodalton Trypanosoma cruzi complement regulatory protein. Infect. Immun. 65:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Connor, B. P., M. W. Gleeson, R. J. Noelle, and L. D. Erickson. 2003. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol. Rev. 194:61-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliver, A. M., F. Martin, G. L. Gartland, R. H. Carter, and J. F. Kearney. 1997. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 27:2366-2374. [DOI] [PubMed] [Google Scholar]
- 40.Payne, L. G., D. H. Fuller, and J. R. Haynes. 2002. Particle-mediated DNA vaccination of mice, monkeys and men: looking beyond the dogma. Curr. Opin. Mol. Ther. 4:459-466. [PubMed] [Google Scholar]
- 41.Quanquin, N. M., C. Galaviz, D. L. Fouts, R. A. Wrightsman, and J. E. Manning. 1999. Immunization of mice with a TolA-like surface protein of Trypanosoma cruzi generates CD4(+) T-cell-dependent parasiticidal activity. Infect. Immun. 67:4603-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radwanska, M., P. Guirnalda, C. De Trez, B. Ryffel, S. Black, and S. Magez. 2008. Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog. 4:e1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed, S., C. Brownell, D. Russo, J. Silva, K. Grabstein, and P. Morrissey. 1994. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J. Immunol. 153:3135-3140. [PubMed] [Google Scholar]
- 44.Reina-San-Martin, B., A. Cosson, and P. Minoprio. 2000. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol. Today 16:62-67. [DOI] [PubMed] [Google Scholar]
- 45.Reina-San-Martin, B., W. Degrave, C. Rougeot, A. Cosson, N. Chamond, A. Cordeiro-Da-Silva, M. Arala-Chaves, A. Coutinho, and P. Minoprio. 2000. A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat. Med. 6:890-897. [DOI] [PubMed] [Google Scholar]
- 46.Rico, A. I., N. Girones, M. Fresno, C. Alonso, and J. M. Requena. 2002. The heat shock proteins, Hsp70 and Hsp83, of Leishmania infantum are mitogens for mouse B cells. Cell Stress Chaperones 7:339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson, H. L., and C. A. Torres. 1997. DNA vaccines. Semin. Immunol. 9:271-283. [DOI] [PubMed] [Google Scholar]
- 48.Rott, O., J. Charreire, and E. Cash. 1996. Influenza A virus hemagglutinin is a B cell-superstimulatory lectin. Med. Microbiol. Immunol. 184:185-193. [DOI] [PubMed] [Google Scholar]
- 49.Russo, M., P. Minoprio, A. Coutinho, and M. Hontebeyrie-Joskowicz. 1988. Depletion of L3T4+ T lymphocytes during acute Trypanosoma cruzi infection abolish macrophage and B lymphocyte activation but not tissue inflammatory reaction. Mem. Inst. Oswaldo Cruz 83(Suppl. 1):527-538. [DOI] [PubMed] [Google Scholar]
- 50.Sangster, M. Y., D. J. Topham, S. D'Costa, R. D. Cardin, T. N. Marion, L. K. Myers, and P. C. Doherty. 2000. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J. Immunol. 164:1820-1828. [DOI] [PubMed] [Google Scholar]
- 51.Schijman, A. G., C. A. Vigliano, R. J. Viotti, J. M. Burgos, S. Brandariz, B. E. Lococo, M. I. Leze, H. A. Armenti, and M. J. Levin. 2004. Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic Chagas heart disease. Am. J. Trop. Med. Hyg. 70:210-220. [PubMed] [Google Scholar]
- 52.Schnapp, A. R., C. S. Eickhoff, D. Sizemore, R. Curtiss III, and D. F. Hoft. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 70:5065-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sepulveda, P., M. Hontebeyrie, P. Liegeard, A. Mascilli, and K. A. Norris. 2000. DNA-based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect. Immun. 68:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva, J., P. Morrissey, K. Grabstein, K. Mohler, D. Anderson, and S. Reed. 1992. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J. Exp. Med. 175:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slifka, M. K., and R. Ahmed. 1996. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J. Immunol. Methods 199:37-46. [DOI] [PubMed] [Google Scholar]
- 56.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363-372. [DOI] [PubMed] [Google Scholar]
- 57.Slifka, M. K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soares, R., P. Ferreira, M. M. Santarem, M. Teixeira da Silva, and M. Arala-Chaves. 1990. Low T- and B-cell reactivity is an apparently paradoxical request for murine immunoprotection against Streptococcus mutans. Murine protection can be achieved by immunization against a B-cell mitogen produced by these bacteria. Scand. J. Immunol. 31:361-366. [DOI] [PubMed] [Google Scholar]
- 59.Spera, J. M., J. E. Ugalde, J. Mucci, D. J. Comerci, and R. A. Ugalde. 2006. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc. Natl. Acad. Sci. USA 103:16514-16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suarez, F., O. Lortholary, O. Hermine, and M. Lecuit. 2006. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood 107:3034-3044. [DOI] [PubMed] [Google Scholar]
- 61.Tavares, D., P. Ferreira, and M. Arala-Chaves. 2003. Increased resistance in BALB/c mice to reinfection with Candida albicans is due to immunoneutralization of a virulence-associated immunomodulatory protein. Microbiology 149:333-339. [DOI] [PubMed] [Google Scholar]
- 62.Tavares, D., P. Ferreira, M. Vilanova, A. Videira, and M. Arala-Chaves. 1995. Immunoprotection against systemic candidiasis in mice. Int. Immunol. 7:785-796. [DOI] [PubMed] [Google Scholar]
- 63.Tavares, D., A. Salvador, P. Ferreira, and M. Arala-Chaves. 1993. Immunological activities of a Candida albicans protein which plays an important role in the survival of the microorganism in the host. Infect. Immun. 61:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teixeira, A. R., G. Teixeira, V. Macedo, and A. Prata. 1978. Acquired cell-mediated immunodepression in acute Chagas' disease. J. Clin. Invest. 62:1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, S., C. Zhang, L. Zhang, J. Li, Z. Huang, and S. Lu. 2008. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine 26:2100-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe, K., H. Kumada, F. Yoshimura, and T. Umemoto. 1996. The induction of polyclonal B-cell activation and interleukin-1 production by the 75-kDa cell surface protein from Porphyromonas gingivalis in mice. Arch. Oral Biol. 41:725-731. [DOI] [PubMed] [Google Scholar]
- 67.Wizel, B., N. Garg, and R. L. Tarleton. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wizel, B., M. Nunes, and R. L. Tarleton. 1997. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J. Immunol. 159:6120-6130. [PubMed] [Google Scholar]
- 69.Won, W.-J., and J. Kearney. 2002. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J. Immunol. 168:5605-5611. [DOI] [PubMed] [Google Scholar]
- 70.Wrightsman, R. A., K. A. Luhrs, D. Fouts, and J. E. Manning. 2002. Paraflagellar rod protein-specific CD8+ cytotoxic T lymphocytes target Trypanosoma cruzi-infected host cells. Parasite Immunol. 24:401-412. [DOI] [PubMed] [Google Scholar]
- 71.Yanaba, K., J.-D. Bouaziz, T. Matsushita, T. Tsubata, and T. F. Tedder. 2009. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 182:7459-7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.