Abstract
Toll-like receptors (TLRs) are crucial pattern recognition receptors in innate immunity that are expressed in microglia, the resident macrophages of the brain. TLR2, -4, and -9 are important in the responses against Streptococcus pneumoniae, the most common agent causing bacterial meningitis beyond the neonatal period. Murine microglial cultures were stimulated with agonists for TLR1/2 (Pam3CSK4), TLR4 (lipopolysaccharide), and TLR9 (CpG oligodeoxynucleotide) for 24 h and then exposed to either the encapsulated D39 (serotype 2) or the nonencapsulated R6 strain of S. pneumoniae. After stimulation, the levels of interleukin-6 and CCL5 (RANTES [regulated upon activation normal T-cell expressed and secreted]) were increased, confirming microglial activation. The TLR1/2, -4, and -9 agonist-stimulated microglia ingested significantly more bacteria than unstimulated cells (P < 0.05). The presence of cytochalasin D, an inhibitor of actin polymerizaton, blocked >90% of phagocytosis. Along with an increased phagocytic activity, the intracellular bacterial killing was also increased in TLR-stimulated cells compared to unstimulated cells. Together, our data suggest that microglial stimulation by these TLRs may increase the resistance of the brain against pneumococcal infections.
Immunocompromised patients have a higher risk of developing bacterial infections in the central nervous system (CNS) (34, 37, 42). The list of the pathogens includes many organisms with low pathogenicity in the immunocompetent host (34, 37). Moreover, the distribution of the pathogens also differs from the immunocompetent host and depends on the nature of the immune defect. Patients with a decrease in B-lymphocyte function or with a loss of splenic function have an increased risk of meningitis caused by encapsulated bacteria, while patients with an impaired T-lymphocyte-macrophage system are more susceptible to CNS infections caused by intracellular pathogens (7, 42). One additional cause of this increased susceptibility to CNS infections probably is a decreased local immune defense (33).
CNS infections not only are more frequent but also are associated with higher mortality rates and more severe long-term sequelae in immunocompromised than in immunocompetent individuals (9, 17, 34, 44). Polymicrobial infections, multiple organ system presentation, and the absence of typical clinical manifestations subsequent to the host's diminished inflammatory response are challenging aspects in the management of these infections (34, 37, 42).
The brain tissue shows a well-organized innate immune reaction in response to bacteria in the cerebrospinal fluid (CSF) (3, 21). Microglial cells, the resident phagocytes of the CNS, express Toll-like receptors (TLRs) that identify pathogen-associated molecular patterns (PAMPs) (41). The receptor-ligand interactions activate microglia to undergo morphological transformation as well as functional changes, such as the production of proinflammatory cytokines, chemokines, and reactive oxygen species, enhanced phagocytic activity, and antigen presentation (15, 39). This immune reaction cannot eliminate high amounts of pneumococci from the CSF but does prevent or minimize the invasion of these pathogens into the brain tissue, thereby limiting tissue destruction and neuronal injury.
TLR2, -4, and -9 contribute to the recognition and response to Streptococcus pneumoniae in the CNS (31). A deficiency of TLR2, -4, or -9 or of the coreceptor CD14, which is necessary for TLR4 signaling increases the susceptibility of mice to S. pneumoniae (1, 11, 12, 40).
Here, we hypothesized that activation of the innate immune response in microglia could increase the resistance of the brain tissue against CNS pneumococcal infections (14). This may be of particular interest in immunocompromised patients, whose outcome after S. pneumoniae meningitis is worse than that of immunocompetent individuals (9, 44). The aim of the present study was to investigate whether the stimulation of microglia by respective PAMPs can increase their ability to phagocytose and kill intracellular nonencapsulated and encapsulated S. pneumoniae strains, thereby protecting the brain during meningitis. Moreover, by using an encapsulated and a nonencapsulated pneumococcal strain, we assessed the protective effect of the capsule against phagocytosis by microglial cells.
MATERIALS AND METHODS
Primary mouse microglial cell cultures.
Primary cultures of microglial cells were prepared from the brains of newborn C57/BL6N mice (1 to 3 days) as previously described (10, 36). Microglial cells were isolated by shaking at 200 times/min for 30 min, and the cells in the supernatant were replated in 96-well plates (for phagocytosis assay) and in 24-well plates (for intracellular survival assay) at a density of 50,000 to 65,000 cells/well. In addition, microglia were plated on poly-l-lysine-coated coverslips in 12-well plates for subsequent staining and confocal microscopy at the same number of cells/well.
Microglial stimulation with TLR agonists.
Cells seeded into 24- and 96-well plates were exposed to one of the different TLR agonists for 24 h. Tripalmitoyl-S-glyceryl-cysteine (Pam3CSK4; molecular mass, 910.5 Da; EMC Microcollections, Tübingen, Germany), endotoxin (lipopolysaccharide [LPS] from Escherichia coli serotype O26:B6; Sigma, Taufkirchen, Germany), and CpG oligodesoxynucleotide (ODN) 1668 (TCC ATG ACG TTC CTG ATG CT; molecular mass, 6,383 Da; TIB Molbiol, Berlin, Germany) were used as specific ligands of TLR1/2, -4, and -9. A control group with unstimulated microglial cells was included in all experiments. TLR agonists were used at the lowest concentrations inducing maximum stimulation of microglial cells in terms of NO release (10): Pam3CSK4 was tested at 0.1 μg/ml (0.1 μM), LPS was tested at 0.01 μg/ml (1 nM), and CpG was tested at 1 μg/ml (150 nM).
Supernatants from stimulated microglial cultures and unstimulated controls were collected after 24 h of incubation and stored frozen at −80°C until measurement of the cytokine and chemokine levels. Microglial cells were assayed for phagocytosis or intracellular survival by quantitative plating of intracellular bacteria or used for staining and subsequent confocal microscopy.
Cytokine and chemokine release.
Interleukin-6 (IL-6) and CCL5 (RANTES [regulated upon activation normal T-cell expressed and secreted]) were chosen as representatives of the inducible spectrum of microglial cytokines and chemokines (15). DuoSet ELISA development kits (R&D Systems, Wiesbaden, Germany) were used for their measurement. The color reaction was measured at 450 nm on a microplate reader (Bio-Rad, Munich, Germany). The total protein content was determined by using the MicroBCA protein assay (Pierce, Rockford, IL).
Bacterial strains, culture conditions, and protein purification.
Streptococcus pneumoniae strains D39 (encapsulated, serotype 2) and its nonencapsulated derivative R6 were used in phagocytosis and intracellular survival assays. Pneumococcal strains were grown in a medium consisting of Dulbecco modified Eagle medium with Glutamax I (DMEM; Gibco, Karlsruhe, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS).
The green fluorescent protein (GFP)-expressing strains D39gfp and its nonencapsulated derivative D39gfpΔcps were used for confocal microscopy to confirm the intracellular location of bacteria in microglial cells. The D39gfp strain was grown in a medium consisting of DMEM supplemented with 10% heat-inactivated FCS and 0.5 μg of tetracycline/ml. The D39gfpΔcps strain was grown in DMEM supplemented with 10% heat-inactivated FCS, 0.5 μg of tetracycline/ml, and 50 μg of kanamycin/ml. GFP-expressing D39 and D39Δcps (35) were generated by transformation of pneumococci with plasmid pMV158GFP (29).
The bacterial inoculum was determined for each assay by quantitative plating on sheep blood agar plates.
Phagocytosis and intracellular survival assay.
After 24 h of stimulation with one TLR agonist, microglial cells were exposed to either S. pneumoniae D39 or R6 (with a ratio of approximately 50 bacteria per phagocyte). Phagocytosis was left to proceed for 30 or 90 min at 37°C and 5% CO2. For phagocytosis inhibition studies cytochalasin D (final concentration, 10 μM; Sigma-Aldrich, St. Louis, MO) was added to the cell monolayers 30 min prior to the addition of bacteria and remained present throughout the experiment (36). After bacterial exposure, cells were incubated for 1 h in culture medium containing gentamicin (final concentration, 200 μg/ml; Sigma-Aldrich). After gentamicin incubation, the cell monolayers were washed and lysed with distilled water. The intracellular bacteria were enumerated by quantitative plating of serial dilutions of the lysates on sheep blood agar plates. The limit of detection was 10 CFU/well. Each protocol was performed at least three times in independent experiments. During the phagocytosis assay, extracellular bacterial replication and gentamicin activity were checked (36).
To monitor intracellular survival and replication inside microglia, cells were allowed to phagocytose bacteria for 30 min. Thereafter, cells were washed and incubated in culture medium containing gentamicin (200 μg/ml) for 2 h. At various times (30, 60, 90, and 120 min), the monolayers were washed and lysed with distilled water, and the amounts of intracellular viable bacteria were quantitatively determined.
Staining and confocal laser imaging of microglia.
Scanning laser confocal microscopy was used to confirm intracellular localization of the encapsulated D39gfp and the nonencapsulated D39gfpΔcps pneumococcal strain after coincubation with microglia. Cells plated on coverslips in 12-well plates were exposed to one of the different TLR agonists for 24 h. Thereafter, the cell monolayers were washed and then incubated with a Vybrant DiI cell-labeling solution (VybrantCell labeling solution kit; Molecular Probes, Leiden, The Netherlands) for 3 min at 37°C according to the manufacturer's instructions. Subsequently, cells were washed twice with warm phosphate-buffered saline (PBS), and bacteria were added for 30 min. For phagocytosis inhibition studies cytochalasin D was added (see above). After 1 h of incubation with gentamicin, cells were washed and fixed in 4% formaldehyde in PBS. The cells were imaged by using a laser-scanning confocal microscope (Zeiss LSM 510 Meta). DiI and GFP S. pneumoniae strains were sequentially excited at 488 and 543 nm. Series of optical sections in Z-plane were acquired at intervals of 0.6 μm. Stacks of images were processed by using ImageJ (version 1.43f). In order to illustrate the intracellular localization of fluorescent bacteria, the z-planes (XZ and YZ) of the images were depicted as orthogonal views. For better visualization of the fluorescent bacteria, three-dimensional (3D) videos were generated by using the ImageJ plugin 3D Viewer (by Benjamin Schmid) and are presented in the supplemental material (Fig. S1 to S6).
Statistical analysis.
Prism software (GraphPad Software, San Diego, CA) was used to perform statistical analyses and graphical presentation. Analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test, was used to compare enzyme-linked immunosorbent assay (ELISA) data among all groups. The data from the phagocytosis and intracellular survival assays were not normally distributed and were analyzed by using the Kruskal-Wallis test, followed by Dunn's multiple comparison test to correct for repeated testing. A P value of < 0.05 was considered significant.
RESULTS
TLR agonists stimulated microglia and induced cytokine and chemokine release.
In order to confirm effective microglial stimulation by the different TLR agonists, we determined the induction of representative cytokines and chemokines such as IL-6 and CCL5 (Fig. 1). Microglial cells remained viable after 24 h of exposure to these agonists (35). In all experiments, a group of unstimulated cells was included for comparison.
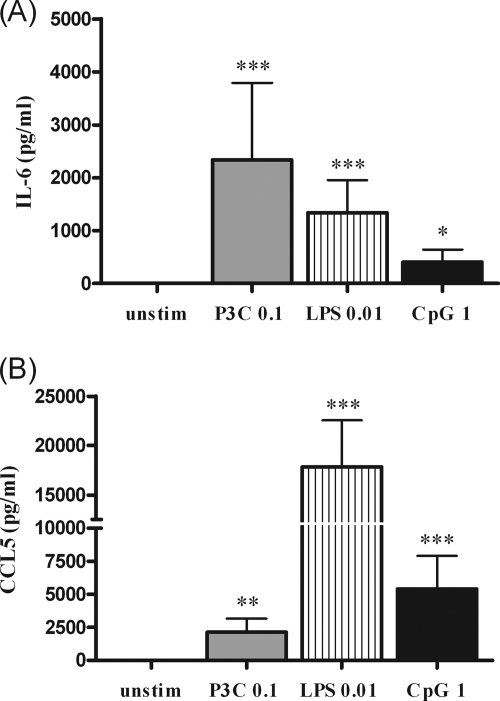
FIG. 1.
(A) IL-6 and (B) CCL5 (RANTES) concentrations in the supernatants of microglia after 24 h of stimulation with 0.1 μg of Pam3CSK4/ml (P3C), 0.01 μg of LPS/ml, 1 μg of bacterial CpG DNA/ml, or DMEM plus 10% FCS (unstim). The data are shown as means ± the standard deviation (SD) (n ≥ 13 wells/group from three independent experiments). The data were analyzed by using ANOVA, followed by Bonferroni's multiple comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The supernatants of unstimulated microglia were devoid of measurable amounts of IL-6 and CCL5. Microglial cells incubated with the individual TLR agonists released much higher amounts of IL-6 and CCL5 than did unstimulated cells (P < 0.05).
Confocal laser imaging confirmed the intracellular localization of encapsulated and nonencapsulated pneumococci.
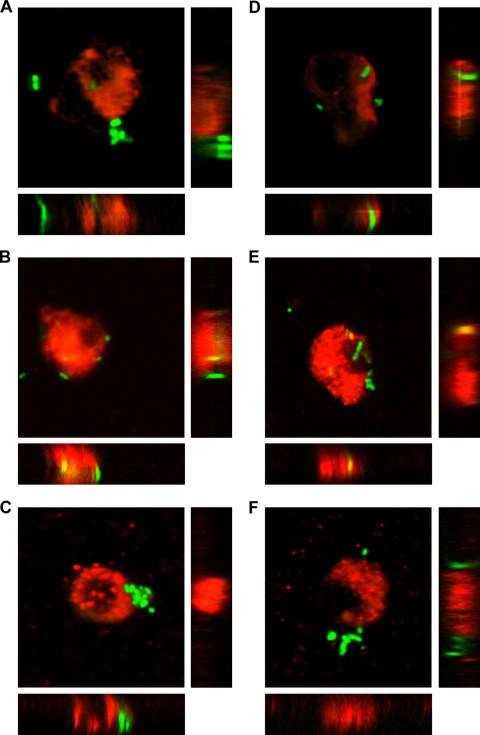
Confocal microscopy confirmed the intracellular localization of the encapsulated D39gfp and the nonencapsulated D39gfpΔcps S. pneumoniae strains within microglial cells. Bacteria expressing GFP and microglia with their cell membrane labeled by red Vybrant DiI were simultaneously visualized in two fluorescent channels, as depicted in the reconstructed images of the z-sections (Fig. 2). The animated 3D isosurface reconstructions are provided as separate figures in the supplemental material. The addition of cytochalasin D prior to the exposure to bacteria inhibited the internalization of pneumococcal strains (Fig. 2C and F).
FIG. 2.
Phagocytosis of the encapsulated D39gfp (A to C) and the nonencapsulated D39gfpΔcps (D to F) S. pneumoniae strains by murine microglial cells after 30 min of bacterial exposure. Internal and external cell membranes were stained with red Vybrant DiI prior to the addition of bacteria. Confocal images of microglial cells ingesting green fluorescent S. pneumoniae are shown in the x-y plane, as well as two z-axis (x-z and y-z) cuts through (A and D) unstimulated cells and through microglia stimulated for 24 h with (B and E) 1 μg of bacterial CpG DNA/ml. (C and F) The addition of cytochalasin D (final concentration, 10 μM) blocked the phagocytosis of S. pneumoniae strains by CpG-stimulated microglial cells. Scale bars are shown in panel A, 5 μm (x-y plane) and 2 μm (x-z and y-z projected planes).
TLR stimulation increased the phagocytosis of S. pneumoniae D39 and R6 by microglia.
The phagocytosis of D39 and R6 pneumococcal strains was compared quantitatively after 30 and 90 min of incubation with bacteria in unstimulated cultures (control group) and in microglia that were previously stimulated with the TLR1/2, TLR4, or TLR9 agonist (Fig. 3).
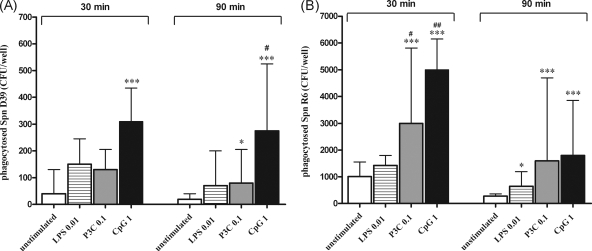
FIG. 3.
Phagocytosis of the encapsulated D39 (A) and the nonencapsulated R6 (B) Streptococcus pneumoniae (Spn) strains by murine microglial cells after 24 h of stimulation with TLR agonists: Pam3CSK4 (P3C, 0.1 μg/ml), LPS (0.01 μg/ml), or CpG DNA (1 μg/ml). A control group of unstimulated cells was included in all experiments. After stimulation, cells were washed and bacteria were added for different times (30 and 90 min). After addition of gentamicin (200 μg/ml), the number of ingested bacteria was determined by quantitative plating of the cell lysates. The data are shown as CFU of recovered bacteria per well (median, 75% interquartile range) (n ≥ 10 wells/group obtained from four independent experiments). Statistical analysis was performed by using the Kruskal-Wallis test, followed by Dunn's multiple-comparison test (*, P < 0.05; and ***, P < 0.001 versus the control group; #, P < 0.05; and ##, P < 0.01 versus the LPS-treated group).
Although unstimulated cells ingested bacteria at a low rate, stimulation with one TLR agonist increased the phagocytic activity of microglia. Treatment with 1 μg of CpG/ml resulted in an increased uptake of both D39 and R6 strains at 30 and 90 min of exposure (P < 0.001). After stimulation with 0.1 μg of Pam3CSK4/ml, the ingestion of the encapsulated D39 strain was increased at 90 min (P < 0.05), while phagocytosis of the nonencapsulated R6 strain was enhanced at 30 and 90 min (P < 0.001). Treatment with 0.01 μg of LPS/ml enhanced the ingestion of the R6 strain at 90 min (P < 0.05).
When we compared the amounts of phagocytosed pneumococci among the different TLR-stimulated groups, we found that TLR1/2- and TLR9-stimulated cells phagocytosed comparable numbers of bacteria (P > 0.05 at 30 and 90 min). In contrast, LPS-stimulated cells ingested lower numbers of both encapsulated D39 (P < 0.05 at 90 min versus TLR9-treated cells) and nonencapsulated R6 strains (P < 0.05 at 30 min versus TLR1/2- and TLR9-treated cells).
The phagocytic rates were different for both strains: the uptake of the nonencapsulated R6 strain was approximately 10 times more rapid than the internalization of the encapsulated D39 strain.
The internalization of both pneumococcal strains by microglia occurred via phagocytosis. Cytochalasin D blocked the uptake of S. pneumoniae D39 and R6 strains by >90% in unstimulated and TLR-stimulated cells, as it was revealed in 30-min phagocytosis inhibition studies.
The extracellular concentration of both pneumococcal strains did not significantly differ throughout 90 min of incubation either in experiments studying phagocytosis or in experiments with phagocytosis inhibitors. After 1 h of gentamicin treatment, the number of extracellular bacteria was below the level of detection in all experiments.
TLR stimulation increased the intracellular killing of S. pneumoniae D39 and R6 by microglia.
Next, we studied whether in TLR-stimulated microglial cells the increase of the phagocytic activity was accompanied by a higher intracellular killing of the ingested bacteria (Fig. 4).
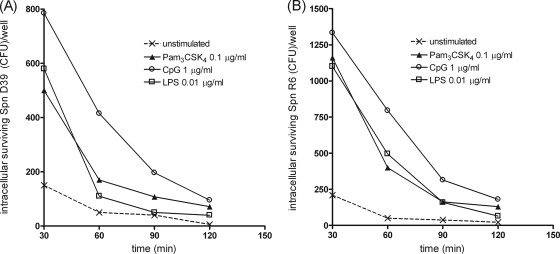
FIG. 4.
Time course of the number of live intracellular pneumococci (encapsulated D39 [A] and nonencapsulated R6 [B] Streptococcus pneumoniae [Spn]) detected within microglial cells after 24 h of stimulation with the TLR agonists Pam3CSK4 (P3C, 0.1 μg/ml), LPS (0.01 μg/ml), or CpG DNA (1 μg/ml). Monolayers were washed and allowed to ingest bacteria for 30 min. Then, gentamicin was added, and the amount of intracellular bacteria was quantified by plating at several postinfection times for up to 120 min. For each group, intracellular killing is expressed as the number of recovered bacteria (median) at the different time points (n ≥ 6 wells/group obtained from three independent experiments).
The absolute amounts of killed S. pneumoniae D39 (calculated as the difference between the medians of intracellular bacteria at 30 and 120 min) were higher in TLR-stimulated microglia than in unstimulated cells (Fig. 4A). The time course of intracellular killing of S. pneumoniae R6 strain was similar to that of the encapsulated strain (Fig. 4B).
DISCUSSION
Streptococcus pneumoniae is an important cause of bacterial meningitis causing death in ca. 25% of the cases and long-term neurological sequelae in up to one-third of the survivors (9, 17, 38, 44). Proinflammatory and directly cytotoxic pneumococcal products (such as pneumococcal cell wall products, pneumolysin, and bacterial DNA) contribute to neuronal injury in S. pneumoniae meningitis.
Microglial cells are the major constituents of innate immunity within the CNS (20). Parenchymal microglia, as well as meningeal and perivascular macrophages, which become activated by bacterial products are critically involved in protecting the brain from infection (30, 33). On the one hand, microglial cells can exert protective effects by phagocytosis of both pathogens and injured cells, and by mediating repair mechanisms (20, 28). When MyD88 bone marrow chimeric mice were studied after intracerebral injection of Staphylococcus aureus, lack of MyD88 expression in the CNS compartment led to elevated intracerebral S. aureus burdens despite the presence of immunocompetent bone marrow-derived cells (14). On the other hand, activated microglial cells can be toxic to surrounding neurons by releasing, e.g., nitric oxide, glutamate, TNF-α, and IL-1β. The diminished inflammatory response decreased hearing loss in pneumococcal meningitis in MyD88-deficient mice, and neuronal injury caused by group B streptococci depended on the presence of TLR2 and MyD88 (18, 22). Thus, activation of microglia during infections seems to be a double-edged sword. The innate immune response can protect neurons by preventing the entry of pathogens into the brain, but its dysregulation can also be harmful for neuronal integrity and can cause neuronal injury (6, 16, 20, 22, 28). Deeper understanding of the roles for TLRs in resident CNS glia and infiltrating immune cells will provide insights into how the immune response to bacterial infection can be tailored to achieve effective pathogen destruction without inducing excessive bystander damage of surrounding brain parenchyma (13, 26).
In this context, we focused our research on the phagocytosis of microglia activated by TLR stimulation. We hypothesized that the activation of the TLR system in microglial cells by agonist stimulation may enhance their phagocytic activity, thereby enabling them to protect the brain in pneumococcal CNS infections in patients with an impaired immune system.
The release of cytokines and chemokines in the CSF during pneumococcal meningitis has been analyzed. IL-6 is one of the major early response cytokines that can trigger an inflammatory cascade in pneumococcal meningitis (15). In many resident cells, such as microglial cells and astrocytes, chemokine production is rapidly upregulated upon activation by stimuli such as bacteria or inflammatory mediators (24, 32). An upregulation of the expression of CCL2, CCL5, and CXCL2 chemokines was observed in lungs, blood, and brain tissue after intranasal inoculation of S. pneumoniae strains (serotypes 2, 4, and 6A) in mice (25). In the present study, when microglia were exposed to a TLR1/2, -4, or -9 ligand for 24 h, the release of IL-6 and CCL5 was strongly increased, confirming microglial activation.
Upon TLR stimulation, reactive microglia develop a phagocytic phenotype to engulf and kill microbes. In contrast to cytokine and chemokine induction, the phagocytic and bactericidal profiles of activated microglia have been explored less thoroughly. Our group has recently reported that TLR1/2, -4, and -9 agonists can increase the ability of murine microglial cells to phagocytose and kill intracellularly located Escherichia coli strains (36). The present data demonstrate that microglia can also phagocytose and kill Gram-positive bacteria which have a thicker cell wall and that stimulation of TLRs can increase their phagocytic and bactericidal activity. This applies for both nonencapsulated apathogenic and encapsulated pathogenic pneumococci. Stimulation with either a TLR1/2, -4, or -9 agonist significantly increased the ability of microglia to phagocytose pneumococci. From our data, the effect of the stimulation through the TLR9 system was clearly greater than the effect caused via TLR1/2 or TLR4. Similarly, phagocytosis and killing of live S. pneumoniae were found to be impaired in alveolar and bone marrow-derived macrophages from TLR9-deficient mice (1) and in blood-derived polymorphonuclear leukocytes from TLR2-deficient mice (23).
Once bacteria have been phagocytosed, they are incorporated into phagolysosomes and exposed to reactive oxygen species that eventually will result in bacterial lysis. The intracellular killing of S. pneumoniae by microglial cells was more rapid than that of E. coli studied in the same experimental setting (36). For this reason, the number of viable intracellular bacteria determined after 90 min of phagocytosis was lower than the concentration of viable intracellular bacteria detected after 30 min.
The presence of the polysaccharide capsule is an important virulence factor of pneumococci because it decreases bacterial uptake into microglia by more than 10 times (Fig. 3). In addition, we showed that the internalization of pneumococcal strains by murine microglia requires intact actin filaments since this process was blocked by >90% by cytochalasin D (Fig. 2). Not only the phagocytic but also the bactericidal activities of reactive microglia depend on the stimulation of the TLR system. In our study, plotting the intracellular bacterial concentration versus time revealed higher absolute numbers of killed bacteria in TLR-stimulated than in unstimulated microglia, i.e., TLR stimulation clearly increased the efficacy of microglia in neutralizing the internalized S. pneumoniae (Fig. 4).
An intact TLR signaling through the pathway organized by MyD88 appears to be necessary to protect the brain tissue against invading microorganisms. A poor outcome because of high bacterial counts in the CNS and severe bacteremia was observed in MyD88-deficient mice after intracisternal induction of pneumococcal meningitis (19). Similarly, MyD88−/− mice showed an increased susceptibility to pneumococcal colonization within the upper respiratory tract, an enhanced bacterial proliferation in infected lung tissue, precocious bacterial spread into the bloodstream, and increased mortality (2). These findings illustrate the importance of an intact innate immune system to efficiently limit the spread of S. pneumoniae.
Stimulation of the TLR system is a potential target for the development of new therapies in multiple diseases (45). Several TLR agonists are currently at different stages of clinical trials (4). The TLR7 agonist imiquimod has been successfully used and approved for the treatment of warts associated with human papillomavirus and is in a second phase trial as a therapeutic agent for herpes simplex virus (HSV) infections (43). The TLR7/8 ligand resiquimod is also the subject of clinical investigations for the treatment of HSV infections (27). CpG DNA has been tested as a vaccine adjuvant showing good results (8). One of the most interesting clinical trials with CPG 7909 has been recently completed and aimed at comparing the immune responses after TLR9-boostered pneumococcal vaccination in human immunodeficiency virus-infected adults (www.clinicaltrials.gov/ct2/show/NCT00562939?term=TLR9&rank=3).
Therefore, the agonists used in the present study or related compounds could be of value as adjuvants to improve the efficiency of the local immune system of the CNS against bacteria. In the pharmacological administration of TLR agonists as adjuvants, the dose, timing, and duration of the immunotherapy, as well as the route of administration, have to be selected not only to maximize the benefit of the enhancement of the immune response but also to restrict an excessive induced response that might lead to autoimmune diseases or increased neuronal injury (4).
One clear advantage of using TLR agonists as adjuvants for the prophylaxis of bacterial meningitis is the low risk of development of resistance to the compound. For microglial activation, agonists with a low molecular mass would be preferable because of their higher penetration across the BBB (4). The entry of LPS into the central nervous compartments is minimal (5).
In conclusion, stimulation of TLRs increases phagocytosis of Gram-positive S. pneumoniae by microglia. Stimulation of the TLR system may be a therapeutic approach to protect the brain from invading pathogens. Further studies in immunocompromised mice are in progress in order to assess whether the resistance of the brain against infections can be increased by priming microglial cells with TLR agonists.
Supplementary Material
Acknowledgments
This study was supported by the European Union (grant CAREPNEUMO), the Else Kröner-Fresenius-Stiftung (R.N. and A.S.) and the SFB/TR43 (U.-K.H.). S.R. was the recipient of a fellowship from the Departament d'Educació i Universitats de la Generalitat de Catalunya.
This work is dedicated to Viktor Papiol.
Editor: A. Camilli
Footnotes
Published ahead of print on 23 November 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Albiger, B., S. Dahlberg, A. Sandgren, F. Wartha, K. Beiter, H. Katsuragi, S. Akira, S. Normark, and B. Henriques-Normark. 2007. Toll-like receptor 9 acts at an early stage in host defense against pneumococcal infection. Cell Microbiol. 9:633-644. [DOI] [PubMed] [Google Scholar]
- 2.Albiger, B., A. Sandgren, H. Katsuragi, U. Meyer-Hoffert, K. Beiter, F. Wartha, M. Hornef, S. Normark, and B. H. Normark. 2005. Myeloid differentiation factor 88-dependent signaling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 7:1603-1615. [DOI] [PubMed] [Google Scholar]
- 3.Aravalli, R. N., P. K. Peterson, and J. R. Lokensgard. 2007. Toll-like receptors in defense and damage of the central nervous system. J. Neuroimmune Pharmacol. 2:297-312. [DOI] [PubMed] [Google Scholar]
- 4.Averett, D. R., S. P. Fletcher, W. Li, S. E. Webber, and J. R. Appleman. 2007. The pharmacology of endosomal TLR agonists in viral disease. Biochem. Soc. Trans. 35:1468-1472. [DOI] [PubMed] [Google Scholar]
- 5.Banks, W. A., and S. M. Robinson. 2010. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 24:102-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao, C. C., S. Hu, T. W. Molitor, E. G. Shaskan, and P. K. Peterson. 1992. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 149:2736-2741. [PubMed] [Google Scholar]
- 7.Cunha, B. A. 2001. Central nervous system infections in the compromised host: a diagnostic approach. Infect. Dis. Clin. N. Am. 15:567-590. [DOI] [PubMed] [Google Scholar]
- 8.Daubenberger, C. A. 2007. TLR9 agonists as adjuvants for prophylactic and therapeutic vaccines. Curr. Opin. Mol. Ther. 9:45-52. [PubMed] [Google Scholar]
- 9.Durand, M. L., S. B. Calderwood, D. J. Weber, S. I. Miller, F. S. Southwick, V. S. Caviness, Jr., and M. N. Swartz. 1993. Acute bacterial meningitis in adults: a review of 493 episodes. N. Engl. J. Med. 328:21-28. [DOI] [PubMed] [Google Scholar]
- 10.Ebert, S., J. Gerber, S. Bader, F. Muhlhauser, K. Brechtel, T. J. Mitchell, and R. Nau. 2005. Dose-dependent activation of microglial cells by Toll-like receptor agonists alone and in combination. J. Neuroimmunol. 159:87-96. [DOI] [PubMed] [Google Scholar]
- 11.Echchannaoui, H., K. Frei, M. Letiembre, R. M. Strieter, Y. Adachi, and R. Landmann. 2005. CD14 deficiency leads to increased MIP-2 production, CXCR2 expression, neutrophil transmigration, and early death in pneumococcal infection. J. Leukoc. Biol. 78:705-715. [DOI] [PubMed] [Google Scholar]
- 12.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 13.Esen, N., and T. Kielian. 2009. Toll-like receptors in brain abscess. Curr. Top. Microbiol. Immunol. 336:41-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg, S., J. R. Nichols, N. Esen, S. Liu, N. K. Phulwani, M. M. Syed, W. H. Wood, Y. Zhang, K. G. Becker, A. Aldrich, and T. Kielian. 2009. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN Neuro 1 pii:e00007. doi: 10.1042/AN20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanisch, U. K. 2002. Microglia as a source and target of cytokines. Glia 40:140-155. [DOI] [PubMed] [Google Scholar]
- 16.Iliev, A. I., A. K. Stringaris, R. Nau, and H. Neumann. 2004. Neuronal injury mediated via stimulation of microglial Toll-like receptor-9 (TLR9). FASEB J. 18:412-414. [DOI] [PubMed] [Google Scholar]
- 17.Kastenbauer, S., and H. W. Pfister. 2003. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126:1015-1025. [DOI] [PubMed] [Google Scholar]
- 18.Klein, M., C. Schmidt, S. Kastenauer, R. Paul, C. J. Kirschning, H. Wagner, B. Popp, H. W. Pfister, and U. Koedel. 2008. MyD88-dependent immune response contributes to hearing loss in experimental pneumococcal meningitis. J. Infect. Dis. 195:1189-1193. [DOI] [PubMed] [Google Scholar]
- 19.Koedel, U., T. Rupprecht, B. Angele, J. Heesemann, H. Wagner, H. W. Pfister, and C. J. Kirschning. 2004. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain 127:1437-1445. [DOI] [PubMed] [Google Scholar]
- 20.Kreutzberg, G. W. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19:312-318. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme, N., and S. Rivest. 1999. Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor κB alpha within specific cellular populations of the rat brain. J. Neurochem. 73:309-321. [DOI] [PubMed] [Google Scholar]
- 22.Lehnardt, S., P. Henneke, E. Lien, D. L. Kasper, J. J. Volpe, I. Bechmann, R. Nitsch, J. R. Weber, D. T. Golenbock, and T. Vartanian. 2006. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 177:583-592. [DOI] [PubMed] [Google Scholar]
- 23.Letiembre, M., H. Echchannaoui, P. Bachmann, F. Ferracin, C. Nieto, M. Espinosa, and R. Landmann. 2005. Toll-like receptor 2 deficiency delays pneumococcal phagocytosis and impairs oxidative killing by granulocytes. Infect. Immun. 73:8397-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lokensgard, J. R., S. Hu, E. M. van Fenema, W. S. Sheng, and P. K. Peterson. 2000. Effect of thalidomide on chemokine production by human microglia. J. Infect. Dis. 182:983-987. [DOI] [PubMed] [Google Scholar]
- 25.Mahdi, L. K., A. D. Ogunniyi, K. S. LeMessurier, and J. C. Paton. 2008. Pneumococcal virulence gene expression and host cytokine profiles during pathogenesis of invasive disease. Infect. Immun. 76:646-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani, M. M., and T. Kielian. 2009. Microglia in infectious diseases of the central nervous system. J. Neuroimmune Pharmacol. 4:448-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark, K. E., L. Corey, T. C. Meng, A. S. Magaret, M. L. Huang, S. Selke, H. B. Slade, S. K. Tyring, T. Warren, S. L. Sacks, P. Leone, V. A. Bergland, and A. Wald. 2007. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J. Infect. Dis. 195:1324-1331. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen, M. D., J. P. Julien, and S. Rivest. 2002. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 3:216-227. [DOI] [PubMed] [Google Scholar]
- 29.Nieto, C., and M. Espinosa. 2003. Construction of the mobilizable plasmid pMV158GFP, a derivative of pMV158 that carries the gene encoding the green fluorescent protein. Plasmid 49:281-285. [DOI] [PubMed] [Google Scholar]
- 30.Nimmerjahn, A., F. Kirchhoff, and F. Helmchen. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314-1318. [DOI] [PubMed] [Google Scholar]
- 31.Paterson, G. K., and T. J. Mitchell. 2006. Innate immunity and the pneumococcus. Microbiology 152:285-293. [DOI] [PubMed] [Google Scholar]
- 32.Peterson, P. K., S. Hu, J. Salak-Johnson, T. W. Molitor, and C. C. Chao. 1997. Differential production of and migratory response to beta chemokines by human microglia and astrocytes. J. Infect. Dis. 175:478-481. [DOI] [PubMed] [Google Scholar]
- 33.Polfliet, M. M., P. J. Zwijnenburg, A. M. van Furth, T. van der Poll, E. A. Dopp, C. Renardel de Lavalette, E. M. van Kesteren-Hendrikx, N. van Rooijen, C. D. Dijkstra, and T. K. van den Berg. 2001. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J. Immunol. 167:4644-4650. [DOI] [PubMed] [Google Scholar]
- 34.Pruitt, A. A. 1991. Central nervous system infections in cancer patients. Neurol. Clin. 9:867-888. [PubMed] [Google Scholar]
- 35.Rennemeier, C., S. Hammerschmidt, S. Niemann, S. Inamura, U. Zahringer, and B. E. Kehrel. 2007. Thrombospondin-1 promotes cellular adherence of gram-positive pathogens via recognition of peptidoglycan. FASEB J. 21:3118-3132. [DOI] [PubMed] [Google Scholar]
- 36.Ribes, S., S. Ebert, D. Czesnik, T. Regen, A. Zeug, S. Bukowski, A. Mildner, H. Eiffert, U. K. Hanisch, S. Hammerschmidt, and R. Nau. 2009. Toll-like receptor prestimulation increases phagocytosis of Escherichia coli DH5alpha and Escherichia coli K1 strains by murine microglial cells. Infect. Immun. 77:557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safdieh, J. E., P. A. Mead, K. A. Sepkowitz, T. E. Kiehn, and L. E. Abrey. 2008. Bacterial and fungal meningitis in patients with cancer. Neurology 70:943-947. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt, H., B. Heimann, M. Djukic, C. Mazurek, C. Fels, C. W. Wallesch, and R. Nau. 2006. Neuropsychological sequelae of bacterial and viral meningitis. Brain 129:333-345. [DOI] [PubMed] [Google Scholar]
- 39.Smith, M. E., K. van der Maesen, and F. P. Somera. 1998. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J. Neurosci. Res. 54:68-78. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 73:6479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 42.Tunkel, A. R., and W. M. Scheld. 2002. Central nervous system infection in the immunocompromised host, p. 163-214. In R. H. Rubin, and L. S. Young (ed.), Clinical approach to infection in the compromised host, 4th ed. Kluwer Academic Press, Inc., New York, NY.
- 43.Tyring, S. K., I. I. Arany, M. A. Stanley, M. H. Stoler, M. A. Tomai, R. L. Miller, M. L. Owens, and M. H. Smith. 1998. Mechanism of action of imiquimod 5% cream in the treatment of anogenital warts. Prim. Care Update Ob. Gyns. 5:151-152. [DOI] [PubMed] [Google Scholar]
- 44.Weisfelt, M., D. van de Beek, L. Spanjaard, J. B. Reitsma, and J. de Gans. 2006. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 5:123-129. [DOI] [PubMed] [Google Scholar]
- 45.Zuany-Amorim, C., J. Hastewell, and C. Walker. 2002. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Drug Discov. 1:797-807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.