Abstract
Nemo-like kinase (NLK) is known to function as a mitogen-activated protein kinase (MAPK)-like kinase. However, the upstream molecules and molecular mechanisms that regulate NLK activity remain unclear. In the present study, we identified p38 MAPK as an upstream kinase and activator of NLK. p38 regulates the function of NLK via phosphorylation, and this modification can be abrogated by depletion of endogenous p38. In Xenopus laevis embryos, depletion of either p38β or NLK by antisense morpholino oligonucleotides results in a severe defect in anterior development and impaired expression of endogenous anterior markers. It is notable that morphants of Xenopus p38α, another isoform of the p38 MAPK family, exhibited no obvious defects in anterior development. Defects in head formation or in the expression of anterior marker genes caused by suppression of endogenous p38β expression could be rescued by expression of wild-type NLK but not by expression of mutant NLK lacking the p38β phosphorylation site. In contrast, defects in head formation or in the expression of anterior marker genes caused by suppression of endogenous NLK expression could not be rescued by expression of p38. These results provide the first evidence that p38 specifically regulates NLK function, which is required for anterior formation in Xenopus development.
Nemo-like kinase (NLK) is an evolutionarily conserved serine-threonine protein kinase that was originally isolated as a murine orthologue of Drosophila melanogaster Nemo, which is involved in diverse signaling processes (3). Studies of Nemo-null mutants in Drosophila revealed that Nemo plays a role in head development and in the pathway governing epithelial planar cell polarity during eye development by controlling programmed cell death (19). In our previous studies, we demonstrated that NLK is involved in the suppression of the Wnt/β-catenin signaling pathways. NLK inactivates a transcriptional unit composed of β-catenin/T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) by phosphorylation of TCF/LEF, which inhibits the binding of this complex to its target gene sequences (10, 28). NLK functions downstream of transforming growth factor β-activated kinase 1 (TAK1), a member of the mitogen-activated protein kinase kinase kinase (MAPKKK or MAP3K) family (10, 22), Wnt1 (9), and Wnt5a (8). Loss of NLK/Nemo function results in an embryonic lethal phenotype in Drosophila (19), Caenorhabditis elegans (24), and mice (15), strongly implicating NLK/Nemo as a very important regulator of cell growth, patterning, and death. We previously demonstrated that in Xenopus laevis embryos, expression of NLK is restricted to the central nervous system, eye field, and anterior neural crest cell populations. Xenopus NLK is involved in anterior formation and the expression of anterior neural marker genes (6). Our recent data indicate that, in addition to TCF/LEF, NLK associates with and modulates the activities of other transcription factors, including xSox11, STAT3 (22), HMG2L1 (27), and MEF2A (26). This suggests that NLK contributes to various signaling pathways via its ability to interact with a diverse collection of transcription factors.
The activation of p38 in response to a wide range of extracellular stimuli is reflected in the diverse range of MAP3Ks (TAK1, ASK1, DLK, and MEKK4, etc.) that participate in p38 activation, illustrating the complexity of this signaling pathway (16, 17). The MAP3Ks phosphorylate and activate the MAPK kinases (MAP2Ks) MKK6 and MKK3, which in turn phosphorylate the p38 MAPKs. In vertebrates, there are four isoforms of p38: p38α, p38β, p38γ, and p38δ. These isoforms are characterized by a Thr-Gly-Tyr (TGY) dual-phosphorylation motif (11). Once activated, p38s phosphorylate their substrates on serine/threonine residues. The list of reported downstream substrates of p38 continues to expand and includes other protein kinases and many transcription factors, suggesting its possible role in regulating gene expression at the transcriptional level. Analysis of several of the downstream targets of p38 that are lineage specific or that play an essential role in development have indicated a central role of the p38 pathway in various developmental and differentiation processes (21).
In the present study, we report the novel finding that the p38β isoform is a functional partner of NLK. NLK was found to associate with, and to be specifically phosphorylated by, p38β. Depletion of either Xenopus p38β (xp38β) or xNLK resulted in defects in anterior neural development in Xenopus embryos, including the loss of eye and head structures. The phenotypes induced by depletion of endogenous xp38β were rescued by overexpression of wild-type xNLK but not by a nonphosphorylatable mutant of xNLK. These results reveal a new role of p38β in the phosphorylation and regulation of NLK function during anterior formation.
MATERIALS AND METHODS
Plasmid construction.
The Xenopus and human p38α and β MAPK isoforms were amplified by reverse transcription-PCR (RT-PCR) from cDNA templates prepared from Xenopus embryos and 293 cells, respectively, and were subcloned into the pCS2+ and pRK5 vectors. Each kinase-negative (KN) mutant was constructed by replacing the lysine residue with methionine: K53M in xp38β, K155M in murine NLK (mNLK), K89M in xNLK1, and K173M in xNLK2.
Embryo handling and morpholino oligonucleotides.
Capped mRNAs were synthesized from linearized vectors using the mMessage Machine kit (Ambion). The morpholino oligonucleotides (MOs) (Gene Tools, LLC) used here were 5′-GCCCTTCCCTACACGGATGTCCCCC-3′ (xNLK1-MO) (22), 5′-GTAGATGTGCCGCAAAGAGACATTC-3′ (xNLK2-MO), 5′-CGCCCGCTCATCTTGCCCCGACCGG-3′ (xp38β-MO), and 5′-GACGTAAGATTGATTGGATGACATA-3′ (xp38α-MO). MOs and mRNAs were then injected into two animal blastomeres at the 2-cell stage for dissection of animal caps or into two animal dorsal blastomeres at the 8-cell stage for RT-PCR analysis and observation of embryo phenotypes. Animal cap explants or head regions of the injected embryos were dissected at the late blastula stage (stage [st.] 9) or tailbud stage (st. 25), respectively. The specificities of xp38β-MO, xp38α-MO, and xNLK2-MO were confirmed by their abilities to inhibit the translation of FLAG-tagged mRNAs (see Fig. S1 in the supplemental material).
RT-PCR analysis.
Total RNA was prepared using TRIzol (Invitrogen). cDNA synthesis was carried out using Moloney murine leukemia virus reverse transcriptase (Invitrogen). The sequences of the primer pairs have been described previously (6, 26) and are as follows: xp38β, 5′-GACAGCAGCATCACCCTCCTCA-3′ and 5′-TATCTGCTATGTATCCCGTGCCTTTTC-3′; xp38α, 5′-CATGCGACTGACGGGGACTC-3′ and 5′-GCTATCGGCTCATCATCAGG-3′; xNLK1, 5′-ATGCTGCTGTTTGACCCGCTGAAGCG-3′ and 5′-AGGGCACTCATGGCAGAAGGTT-3′; xNLK2, 5′-GCTGTGCAGGTTGGCGAGGGATTG-3′ and 5′-GCGGCGGCAGCTGAAGAGGAA-3′. Xenopus embryonic ornithine decarboxylase (ODC) or histone H4 (His) was used for normalization of cDNA samples.
Antibodies and cell lines.
The following antibodies were used for immunoprecipitation and/or Western blot analysis: horseradish peroxidase conjugated anti-mouse IgG (GE), horseradish peroxidase conjugated anti-rabbit IgG (GE), horseradish peroxidase conjugated anti-rat IgG (GE), anti-T7 (Novagen), anti-hemagglutinin (anti-HA) (3F10; Roche), anti-FLAG (M2; Sigma), anti-p38β2 (Zymed), anti-p38α (Cell Signaling), anti-NLK (8), anti-p38 (Cell Signaling), anti-ph-p38 (Cell Signaling), and anti-ph-mNLK-S510 (Sigma). Also, we used following cell lines: HEK 293 cells, Neuro2A cells, PC12 cells, COS1 cells, and C2C12 cells. The growth medium of each cells is described by the American Type Culture Collection and Evangelopoulos et al. (4).
Protein identification by LC-MS/MS analysis.
FLAG-mouse NLK was expressed in 293 cells, and NLK and associated proteins were recovered from cell extracts by immunoprecipitation with an anti-FLAG antibody. The NLK-associated complexes were digested with Achromobacter protease I (Takara), and the resulting peptides were analyzed using a nanoscale liquid chromatography-tandem mass spectrometry (LC-MS/MS) system, as described previously (20).
In vitro kinase assay.
293 cells, COS-1 cells, or PC12 cells were transfected with the FLAG-NLK or FLAG-p38 expression plasmid. The lysates were prepared from the transfected cells using lysis buffer and were immunoprecipitated with anti-FLAG antibody M2. Immunoprecipitates were incubated with bacterially expressed glutathione S-transferase (GST) fusion proteins in 30 μl kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM dithiothreitol (DTT), 5 mM MgCl2, and 5 μCi of [γ-32P]ATP at 30°C for 15 to 30 min. Phosphorylated substrates were subjected to SDS-PAGE and quantitated using a BAS 2500 image analyzer (Fujifilm).
RNA interference.
For differentiation experiments, PC12 cells were subjected to 50 ng/ml nerve growth factor (NGF) treatment with fresh serum-free Dulbecco's modified Eagle medium (DMEM) and were further incubated for 20 min. We designed small interfering RNAs (siRNAs) against mouse p38α (sense, 5′-GAAUAUCCGCUAAGGAUGC-3′) and p38β (sense, 5′-GCACGAGAACGUCAUAGGA-3′) mRNAs along with their corresponding antisense RNA oligonucleotides with two thymidine residues (dTdT) at the 3′ end of the sequence (Dharmacon). A commercial control siRNA (siCONTROL Non-Targeting siRNA #2; Dharmacon) was used for the negative-control siRNA. These siRNAs were transfected into PC12 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 48 h posttransfection, the medium was replaced with fresh serum-free DMEM to induce the differentiation of PC12 cells.
Whole-mount in situ hybridization.
pBluescript vectors containing a cDNA fragment of xNLK1 encoding the C-terminal region (nucleotides 780 to 1344; GenBank accession no. AB071285), xNLK2 encoding the C-terminal region (nucleotides 2437 to 3280; GenBank accession no. AB490416), xp38β encoding the 3′ untranslated region (nucleotides 2275 to 2864; GenBank accession no. AB490414), or xp38α encoding the 3′ untranslated region (nucleotides 1257 to 1816; GenBank accession no. AB490415) were used as templates to generate digoxigenin (DIG)-labeled RNA probes using a DIG RNA labeling kit (Roche) according to the manufacturer's protocol. Whole-mount in situ hybridization with digoxigenin-labeled RNA probes was performed on staged embryos essentially as described by Hemmati-Brivanlou et al. (5) with the following modifications. After manual removal of the vitelline membranes, embryos were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), followed by dehydration by gradual methanol washing. Embryos were rehydrated with PBS containing 0.01% Triton X-100 and were then treated with proteinase K (2 μg/ml) for 10 min at ambient temperature, followed by postfixation with 4% PFA for 20 min. Hybridization was performed at 68°C with 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 2× Denhardt's solution, 200 μg/ml tRNA, 0.01% Triton X-100, and 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1- propanesulfonate (CHAPS), containing 200 ng/ml of the digoxigenin-labeled RNA probe. Color detection was carried out with BM purple (Roche).
RESULTS
p38β MAPK associates with NLK.
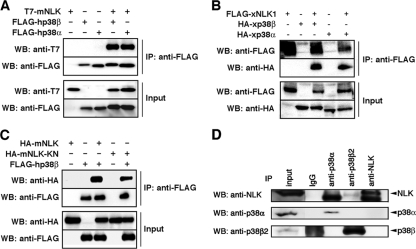
Our previous studies have shown that NLK is involved in forebrain development and neural differentiation in Xenopus (6, 26). However, there is no information about direct upstream regulators of NLK that may function in these processes. To explore potential regulators of NLK function, we initially performed a high-throughput analysis of proteins that coimmunoprecipitated with FLAG-tagged NLK in 293 cells using direct nanoflow liquid chromatography-coupled tandem MS (20). We identified the p38β MAPK isoform as a candidate protein that may physically interact with NLK (data not shown). Both p38β and NLK belong to the MAPK family; however, we have found no reports of direct regulation of a MAPK by another MAPK family member. The interaction between ectopically expressed p38β and NLK was confirmed in 293 cells (Fig. 1A and B). p38β could also be coimmunoprecipitated with a kinase-negative mutant of NLK, NLK-KN (Fig. 1C). This indicates that the association between NLK and p38β does not require NLK kinase activity. Immunoprecipitation analysis using an anti-NLK antibody confirmed the existence of an endogenous NLK and p38β complex in the mouse neuroblastoma cell line Neuro2A (Fig. 1D). Moreover, analysis of both ectopically expressed and endogenous molecules showed that p38α also associates with NLK (Fig. 1A, B, and D). Thus, these results suggest that p38α and β indistinguishably associate with NLK.
FIG. 1.
p38 MAPK associates with NLK. (A to C) Interactions among ectopically expressed genes. 293 cells were transfected as indicated. Immunoprecipitates (IP) obtained using an anti-FLAG antibody were subjected to Western blotting (WB) with the indicated antibodies. +, present; −, absent. (A) Interaction between mNLK and human p38 (hp38β or hp38α). (B) Interaction between xNLK1 and xp38 (xp38β or xp38α). (C) Interaction between xp38 and kinase-negative mNLK (mNLK-KN). (D) Interactions among endogenous genes. Immunoprecipitates obtained from mouse neuroblastoma Neuro2A cells using the indicated antibodies were subjected to Western blotting with the indicated antibodies. Each specific antibody detected the respective endogenous protein, and the anti-NLK antibody detected NLK protein in immunoprecipitates using anti-p38β and anti-p38α.
p38 phosphorylates a specific site in NLK.
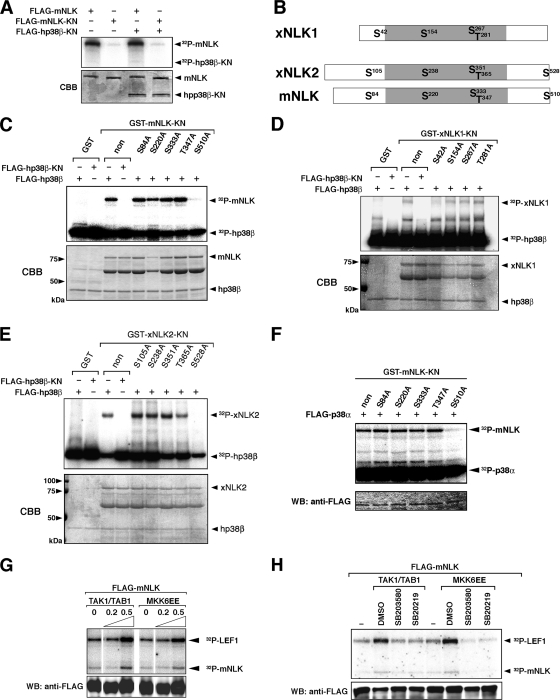
To examine whether p38 phosphorylates NLK or vice versa, we performed in vitro kinase assays using a catalytically inactive mutant of NLK-KN as a substrate. Figure 2A shows that p38β was barely phosphorylated by NLK. On the other hand, good phosphorylation of NLK by p38β was obtained in a kinase activity-dependent manner (Fig. 2C, lanes 1 to 4). There are two NLK species in Xenopus and zebrafish: NLK1 and NLK2 (6). Three of four putative p38 phosphorylation motifs, Ser-Pro or Thr-Pro, are conserved between these two NLKs. The putative phosphorylation site of NLK2 in the C terminus is conserved among many different species, including mice and humans (Fig. 2B; see also Fig. S2 in the supplemental material). We thus tested whether p38β phosphorylates either of these putative phosphorylation residues using NLK S/A or T/A mutants, in which the serine or threonine residues are replaced with alanine. A single amino acid replacement of Ser42 in xNLK1 with alanine (S42A) significantly abrogated phosphorylation of xNLK1 (Fig. 2D, lanes 5 to 8) in vitro, indicating that Ser42 is the specific site of xNLK1 phosphorylation by p38β. On the other hand, replacement of Ser510 of mNLK with alanine (GST-mNLK S510A) also caused a reduction in NLK phosphorylation by p38β (Fig. 2C). Moreover, Ser528 of xNLK2 was also specifically phosphorylated by p38β. (Fig. 2E). We confirmed that the same Ser residues were phosphorylated by p38α (Fig. 2F). These results indicate that the phosphorylation of the conserved Ser residue in different species is involved in the p38-mediated reaction.
FIG. 2.
p38β phosphorylates a specific site in NLK. (A) Phosphorylation of p38 by NLK. 293 cells were transfected with Flag-tagged genes and a constitutively active form of MKK6 (MKK6EE). Immunoprecipitates obtained with an anti-FLAG antibody were incubated with [γ-32P]ATP. (B) Schematic of NLK genes. The putative p38 phosphorylation motifs are indicated as S and T. The kinase domain is shaded. (C to E) Phosphorylation of NLK by p38β. 293 cells were transfected with FLAG-tagged hp38β or hp38β-KN, together with MKK6EE. Immunoprecipitates obtained with an anti-FLAG antibody were incubated with [γ-32P]ATP and bacterially expressed mNLK-KN and its mutants (C), xNLK1-KN and its mutants (D), or xNLK2-KN and its mutants (E). Phosphorylation and Coomassie brilliant blue (CBB) staining of each protein are indicated by arrowheads. (F) Phosphorylation of NLK by p38α. 293 cells were transfected with FLAG-tagged p38α. Immunoprecipitates obtained with an anti-FLAG antibody were incubated with [γ-32P]ATP and bacterially expressed mNLK-KN and its mutants. The phosphorylation of mNLK and p38α is indicated by arrowheads. (G and H) COS1 cells were transfected with FLAG-tagged mNLK, together with TAK1/TAB1 or MKK6EE. Immunoprecipitates obtained with an anti-FLAG antibody were incubated with [γ-32P]ATP and bacterially expressed LEF1 as a substrate. (G) Enhancement of phosphorylation by TAK1/TAB1 or MKK6 (MKK6EE). Numbers indicate amounts of transfected genes (μg). (H) Preincubation with SB203580 and SB20219, specific inhibitors of p38 activity, reduced phosphorylation of LEF1.
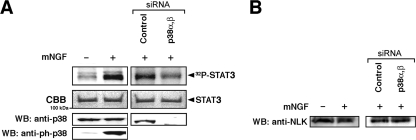
We then asked whether NLK is activated by p38. Flag-mNLK was coexpressed in COS1 cells together with increasing amounts of TAK1/TAB1, an upstream MAPKKK of NLK (10, 18), or together with the constitutively active MKK6 (MKK6EE), an upstream MAPKK of p38 (1, 7). Flag-mNLK protein was immunoprecipitated from the cell lysates with an anti-Flag antibody, and kinase activities in the immunoprecipitates were measured. The NLK immunoprecipitates were assayed using bacterially expressed GST-LEF-1, which is a specific substrate for NLK (9). Both TAK1/TAB1 and MKK6EE enhanced NLK-mediated phosphorylation of GST-LEF-1 in a dose-dependent manner (Fig. 2G). When in vitro kinase assays were performed in the presence of SB203580 or SB20219, specific inhibitors of p38 (7), phosphorylation of GST-Lef1 by NLK was weak (Fig. 2H), suggesting that p38 is required for the observed activation of NLK. To evaluate the physiological relevance of p38 to activate NLK in the signaling pathway, we used siRNA to suppress expression of the endogenous p38 proteins in PC12 cells treated with NGF. We examined the effect of p38 siRNA on the phosphorylation level of STAT3, another substrate of NLK (22). As shown in Fig. 3A (left panels), NGF treatment enhanced the phosphorylation of STAT3. When endogenous p38 expression was suppressed with a p38 siRNA (sip38), NGF-induced phosphorylation of STAT3 was reduced compared with that in the control (siControl) (Fig. 3A, right panels). We also confirmed that endogenous NLK expression was not affected with or without the p38 siRNA pretreatment (Fig. 3B). These data suggest that p38 is physiologically required for the activation of NLK.
FIG. 3.
p38 is required for the activation of NLK. (A) PC12 cells were treated with 50 ng/ml mNGF. Immunoprecipitates obtained with an anti-NLK antibody were incubated with [γ-32P]ATP and bacterially expressed STAT3 as a substrate of NLK. anti-ph-p38, anti-phospho-antibody for p38. (Left lanes) Phosphorylation of STAT3 and endogenous p38 following NGF treatment. (Right lanes) PC12 cells were pretreated with the control siRNA or a p38α, β siRNA. Phosphorylation and Coomassie brilliant blue (CBB) staining of STAT3 are indicated by arrowheads. (B) Effects of p38 siRNAs on NLK expression. p38-siRNAs did not alter NLK expression.
p38β functions in anterior neural development.
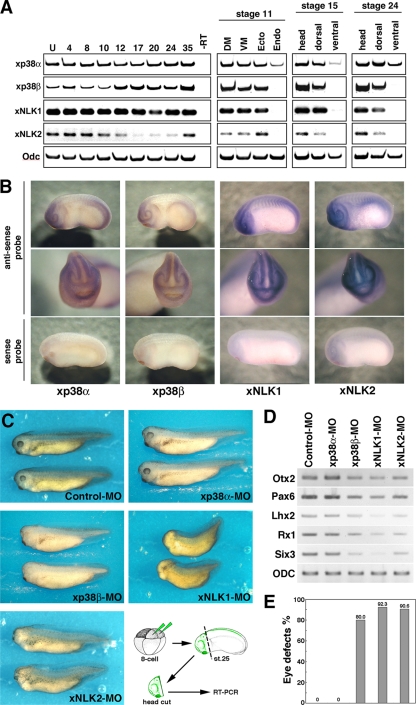
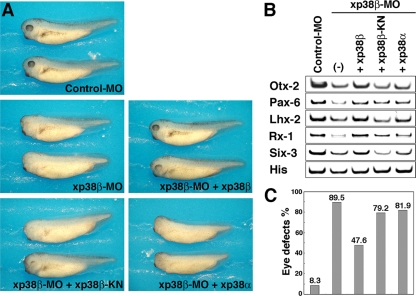
To assess the possible involvement of p38 in Xenopus embryonic development, we first examined the temporal and spatial expression patterns of xp38α, xp38β, xNLK1, and xNLK2 by RT-PCR analysis and whole-mount in situ hybridization (Fig. 4A and B; see also Fig. S3 in the supplemental material). We found that the expression of both xp38α and xNLK1 was relatively constant level throughout embryogenesis. On the other hand, xp38β was expressed from maternally deposited mRNA, and its zygotic expression was induced after the neurula stage, especially in the head region of the embryo. The expression of xNLK2 was relatively weak compared to the expression of xNLK1. As shown in Fig. 4B, symmetrical expression of these genes was detected in both the anterior and posterior neural tubes (two stripes along the lateral neural plate, two eye primordial, forebrain, cement gland, and posterior neural tubes) at the late neural stage (stage 19). To address the physiological relationship between NLK and p38, we synthesized antisense morpholino oligonucleotides (MOs) against xNLK1, xNLK2, xp38β, and xp38α (see Materials and Methods). By Western blot analysis, we confirmed that injection of the xNLK1-, xNLK2-, xp38β- and xp38α-MOs specifically reduced the expression of these proteins (22) (see Fig. S1 in the supplemental material). When xp38β-MO was injected into the anterior region, which develops mainly into neuroectodermal tissues and head structure, the resulting phenotype was similar to that resulting from injection of the xNLK1- or xNLK2-MO, namely, incomplete formation of the eyes (Fig. 4C; see also Fig. S4 and S5 in the supplemental material) and reduced expression of anterior markers such as Otx2, Pax6, Lhx2, Rx, and Six3 (Fig. 4D; see also Fig. S4 and S5 in the supplemental material). On the other hand, no apparent phenotype was detected following injection of as much as 20 ng of xp38α-MO or a control MO. The anterior defects induced by xp38β-MO were rescued by coinjection of wild-type xp38β mRNA, but not by kinase-inactive or wild-type xp38α mRNA (Fig. 5). These results suggest that xp38β is specifically involved in anterior formation in Xenopus embryos.
FIG. 4.
p38β functions in anterior neural development. (A) RT-PCR revealed temporal expression of xp38α, xp38β, xNLK1, and xNLK2. Numbers above lanes indicate developmental stages; U, unfertilized eggs. Semiquantitative RT-PCR to determine spatial expression was also performed using different dissections of embryos at several stages (stages 11, 15, and 24). DM, dorsal marginal zone; VM, ventral marginal zone; Ecto, ectoderm; Endo, endoderm. (B) Whole-mount in situ hybridization showed that these genes are similarly expressed in the head and on the dorsal side (stage 19). The sense RNA probe for each gene was used as a negative control (bottom panels). Images are lateral views, with the anterior ends toward the left (top and bottom panels), and front views (center panels) of head regions. (C to E) Characterization of each morphant. A control morpholino oligonucleotide (Control-MO) (20 ng), xp38α-MO (20 ng), xp38β-MO (20 ng), xNLK1-MO (10 ng), and xNLK2-MO (40 ng) were injected into two animal dorsal blastomeres of 8-cell-stage embryos. (C) Phenotypes of injected embryos at stage 35. Methods for RT-PCR analysis of anterior neural marker genes are indicated in the bottom right panel. (D) Expression of anterior neural marker genes Otx2, Pax6, Lhx2, Rx1, and Six3 in each morphant. ODC (ornithine decarboxylase) was used as a loading control. Total RNAs from the heads of the injected embryos at stage 25 were prepared. (E) Proportion of embryos with eye defects for each morphant in panel C. Each bar corresponds to the lane in panel D with which it is lined up.
FIG. 5.
p38β and its kinase activity are necessary for anterior development. A control MO (20 ng) or xp38β-MO (20 ng) was coinjected with xp38β (500 pg), xp38β-KN (500 pg), or xp38α (500 pg) mRNA. (A) Phenotypes of injected embryos at stage 35. (B) RT-PCR analysis of anterior neural marker genes. His (histone H4) was used as a loading control. (C) Proportion of embryos with eye defects for each morphant in panel A. Each bar corresponds to the lane in panel B with which it is lined up.
p38 regulates NLK function through Ser phosphorylation.
To further examine the phosphorylation of specific Ser residues in NLK by p38, we generated polyclonal antibodies against mouse NLK phospho-Ser510. Wild-type mNLK or mutant mNLK-S510A was expressed in C2C12 cells together with p38β and the constitutively active MKK6 (MKK6EE), and cell lysates were subjected to immunoblot analysis using anti-phospho-Ser510. A band corresponding to wild-type mNLK could be detected in cells expressing wild-type mNLK, but not in those expressing mNLK-S510A (Fig. 6A). Significantly smaller amounts of phospho-Ser510 mNLK were detected in Xenopus embryos treated with xp38β-MO, whereas phospho-Ser510 mNLK amounts were unaffected in embryos treated with xp38α-MO or a control MO (Fig. 6B). These results demonstrate that endogenous p38β phosphorylates the specific Ser residue of NLK.
FIG. 6.
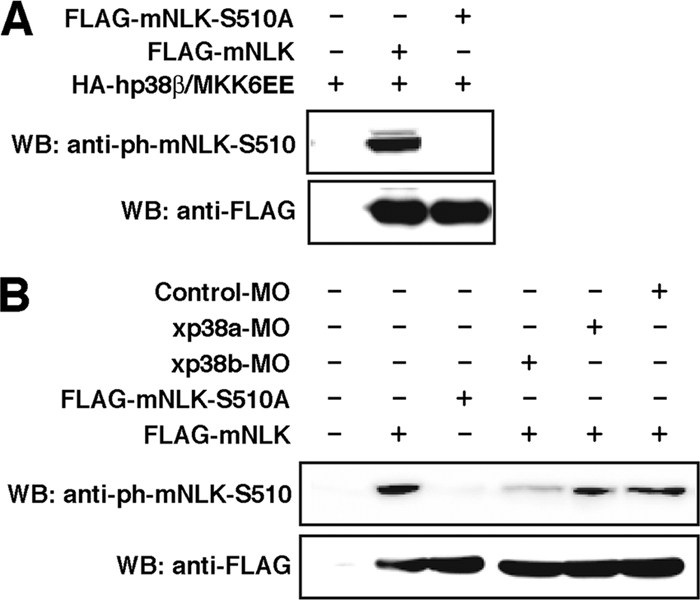
p38 regulates NLK function through Ser phosphorylation. (A) C2C12 cells were transfected as indicated. anti-ph-mNLK-S510 is the anti-phospho-antibody for mNLK-S510. Whole-cell lysates were subjected to Western blotting using the indicated antibodies. (B) A control MO (20 ng), xp38α-MO (20 ng), or xp38β-MO (20 ng) was coinjected with FLAG-tagged mNLK (50 pg) or mNLK-S510A (50 pg) mRNA as described in Materials and Methods. Lysates from the heads of the injected embryos at stage 25 were subjected to Western blotting using the indicated antibodies.
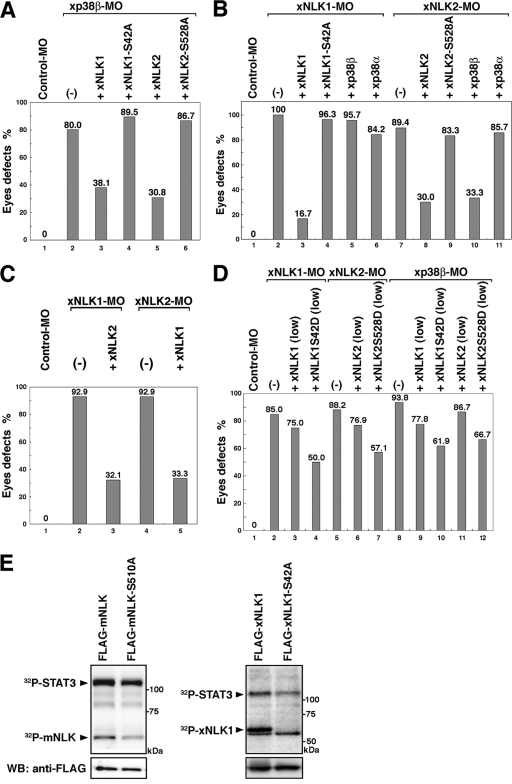
We next examined whether xp38β functions upstream of xNLK. We found that anterior defects induced by xp38β-MO could be rescued by coinjection with wild-type xNLK1 or xNLK2 mRNA (Fig. 7A, lanes 3 and 5), but not with mRNA encoding nonphosphorylatable mutants (xNLK1-S42A or xNLK2-S528A) (Fig. 7A, lanes 4 and 6), indicating that anterior formation depends on the phosphorylation of xNLK. In addition, anterior defects induced by xNLK1- or xNLK2-MO were not rescued by either nonphosphorylatable xNLK-SA mutant (Fig. 7B, lanes 4 and 9). In contrast, coinjection with xp38β mRNA failed to rescue the defects in anterior formation caused by depletion of xNLK1 but could rescue those caused by depletion of xNLK2 (Fig. 7B, lanes 5 and 10). We also confirmed that the anterior defect caused by xNLK1- or xNLK2-MO was redundantly rescued by xNLK2 or xNLK1 mRNA, respectively (Fig. 7C, lanes 3 and 5). These results suggest that xNLK1 is necessary for inducing anterior formation downstream of xp38β signaling in Xenopus. We examined this further using the xNLK1-S42D, xNLK2-S528D, and mNLK-S510D mutants, in which the Ser residues of NLK were replaced with an aspartic acid, mimicking the phosphorylated serine. We found that the anterior defects induced by xNLK1-MO or xNLK2-MO could be rescued by coinjection with small amounts of xNLK1-S42D or xNLK2-S528D mRNA, respectively, but not by coinjection with wild-type NLK mRNA (Fig. 7D, lanes 2 to 7). The anterior defects caused by xp38β-MO were also rescued by coinjection with NLK SD mutant mRNA (Fig. 7D, lanes 8 to 12). It is noteworthy that mutation of each phosphorylation site in mNLK and xNLK1 to Ala resulted in reduced kinase activity in the cultured cells (Fig. 7E). These results suggest that the sites of p38 phosphorylation in NLK are essential for its function and catalytic activity.
FIG. 7.
The Ser phosphorylation of NLK by p38 is essential for anterior formation. (A to D) Phenotypes of injected embryos were observed at stage 35. Each graph shows the proportion of embryos with eye defects for each morphant. Each MO or mRNA was injected as described in Materials and Methods. (A) A control MO (20 ng) or xp38β-MO (20 ng) was coinjected with xNLK1 (62.5 pg), xNLK2 (50 pg), xNLK1-S42A (62.5 pg), or xNLK2-S528A (50 pg) mRNA. (B) A control MO (20 ng), xNLK1-MO (10 ng), or xNLK2-MO (20 ng) was coinjected with xNLK1 (62.5 pg), xNLK2 (50 pg), xNLK1-S42A (62.5 pg), xNLK2-S528A (50 pg), xp38β (500 pg), or xp38α (500 pg) mRNA. (C) A control MO (20 ng), xNLK1-MO (10 ng), or xNLK2-MO (40 ng) was coinjected with xNLK1 (62.5 pg) or xNLK2 (50 pg) mRNA. (D) A control MO (20 ng), xp38β-MO (20 ng), xNLK1-MO (10 ng), or xNLK2-MO (20 ng) was coinjected with low-dose xNLK1 (10 pg), xNLK1-S42D (10 pg), xNLK2 (10 pg), or xNLK2-S528D (10 pg) mRNA. (E) 293 cells were transfected with Flag-tagged NLKs and SA mutants (left panels for mNLK, right panels for xNLK1). Immunoprecipitates obtained with an anti-FLAG antibody were incubated with [γ-32P]ATP and bacterially expressed STAT3 as a substrate of NLK. The phosphorylation of STAT3 and NLK itself is indicated by arrowheads.
DISCUSSION
Our previous studies have shown that NLK induces the expression of anterior neural markers in animal pole explants (6). In the present study, we identified the MAPK family member p38 as a novel activator of the serine/threonine kinase NLK, and we show that p38-NLK signaling controls anterior neural development in Xenopus. Depletion of xNLK1, xNLK2, or xp38β resulted in severe defects in anterior neural development, including loss of eye formation. The defects induced by depletion of xp38β were rescued by expression of wild-type xNLK1 or xNLK2, but not by either of two nonphosphorylatable mutants (xNLK1-S42A or xNLK2-S528A). Interestingly, coinjection with xp38β mRNA failed to rescue the defects in anterior formation caused by depletion of xNLK1 but could rescue those caused by depletion of xNLK2 (Fig. 7B). Our studies demonstrated that the expression of xNLK2 was considerably weaker than that of xNLK1 (Fig. 4A). Moreover, xNLK1 and xNLK2 function redundantly in anterior formation, such that when xNLK2 is depleted, the xNLK1 signal mediated by p38β can compensate (Fig. 7C). Thus, these results indicate that xp38β functions upstream of xNLK in the development of anterior neural structures. Our results demonstrate the existence of a new molecular mechanism involving p38-NLK signaling in the regulation of endogenous anterior tissue development, including eye formation, in Xenopus.
We identified the p38β MAPK isoform as a protein that physically interacts with NLK. In Xenopus, three isoforms of the p38 family have been identified: xp38α, xp38β, and xp38γ (23, 25). Studies of mammalian p38α and p38β molecules suggest that these isoforms may overlap in their biological functions (16, 17). On the other hand, it is well known that the function of p38γ is different from those of p38α and p38β (16, 17). Indeed, a previous report indicates that xp38γ, but not xp38α or xp38β, promotes the meiotic G2/M transition in Xenopus (23). Therefore, we focused on the function of xp38α and xp38β in Xenopus development. Interestingly, we found that depletion of xp38α did not affect anterior formation or the expression of any anterior marker genes, whereas depletion of endogenous xp38β blocked both anterior formation and the induction of these markers (Fig. 4). Thus, xp38β appears to be selectively responsible for regulating certain aspects of anterior formation via the activation of xNLK. Recently, Keren et al. reported that p38α regulates the expression of xMyf5 during Xenopus development (13). Depletion of xp38α by an MO was not reported to have any effect on anterior formation, but this may be due to the limited function of xp38α in Xenopus. On the other hand, Beardmore et al. reported that p38β knockout mice are viable, with no obvious health problems (2). They speculate that the reason for this mild phenotype could be compensation between p38α and p38β isoforms. Although there are discrepancies in that p38α could not compensate for the function of p38β in Xenopus anterior formation in contrast to the function in mice, our studies of Xenopus may provide the first indication of p38β function in embryogenesis, and they provide the first evidence of isoform-specific functional differences between p38α and p38β family members. However, the details of how p38β specifically contributes to early developmental processes in Xenopus embryos remain to be determined and will require additional study.
We have previously demonstrated that the MAP3K TAK1 activates the MAPK-like kinase NLK in a signaling pathway (9, 18). Since TAK1 does not directly interact with NLK, it was assumed that a known molecule, such as a MAPKK, may function upstream of NLK in this pathway. Kanei-Ishii et al. reported that HIPK2 could bind to and activate NLK in the TAK1 signaling pathway (12). However, they failed to detect activation of HIPK2 kinase activity by TAK1. In this study, we demonstrated that p38 MAPK directly interacts with NLK and regulates its kinase activity. Moreover, it is well known that p38 MAPK is activated by MAP3K TAK1 (14). Actually, we found that NLK was activated by activated TAK1 and MKK6 (Fig. 2G) and that this activation was inhibited in the presence of p38-specific inhibitors (Fig. 2H). Taken together, these findings provide the first evidence that NLK functions as a downstream kinase of a MAPK rather than as a MAPK-like kinase.
We demonstrated that p38 directly phosphorylates NLKs at specific sites and that this leads to their activation. We found that NLK1, a gene conserved in Xenopus and zebrafish, and NLK2, a gene conserved among many species, are each phosphorylated on different sites by p38. We also found that each p38 phosphorylation site is similarly required for activation of NLK. Interestingly, the p38 phosphorylation sites in the two NLK molecules are localized to the N-terminal or C-terminal region. This suggests that the position of the p38 phosphorylation site in the NLK molecule is structurally critical. It is likely that the specific site in NLK is phosphorylated by p38 and that phosphorylation at the terminal region of the molecule may suffice to induce its activation. However, further study will be required to understand the precise molecular mechanisms by which p38 regulates the activities of NLK1 and NLK2.
Supplementary Material
Acknowledgments
We thank K. Matsumoto for valuable discussions, H. Nishitoh and T. Maruyama for technical advice, and M. Lamphier for critical reading of the manuscript.
This work was supported by Grants-in-Aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
Published ahead of print on 23 November 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alonso, G., C. Ambrosino, M. Jones, and A. R. Nebreda. 2000. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem. 275:40641-40648. [DOI] [PubMed] [Google Scholar]
- 2.Beardmore, V. A., H. J. Hinton, C. Eftychi, M. Apostolaki, M. Armaka, J. Darragh, J. McIlrath, J. M. Carr, L. J. Armit, C. Clacher, L. Malone, G. Kollias, and J. S. C. Arthur. 2005. Generation and characterization of p38b (MAPK11) gene-targeted mice. Mol. Cell. Biol. 25:10454-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brott, B. K., B. A. Pinsky, and R. L. Erikson. 1998. Nlk is a murine protein kinase related to Erk/MAP kinases and localized in the nucleus. Proc. Natl. Acad. Sci. U. S. A. 95:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evangelopoulos, M. E., J. Weis, and A. Kruttgen. 2005. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene 24:3309-3318. [DOI] [PubMed] [Google Scholar]
- 5.Hemmati-Brivanlou, A., D. Frank, M. E. Bolce, B. D. Brown, H. L. Sive, and R. M. Harland. 1990. Localization of specific mRNAs in Xenopus embryos by whole-mount in situ hybridization. Development 110:325-330. [DOI] [PubMed] [Google Scholar]
- 6.Hyodo-Miura, J., S. Urushiyama, S. Nagai, M. Nishita, N. Ueno, and H. Shibuya. 2002. Involvement of NLK and Sox11 in neural induction in Xenopus development. Genes Cells 7:487-496. [DOI] [PubMed] [Google Scholar]
- 7.Inoue, T., D. Hammaker, D. L. Boyle, and G. S. Firestein. 2005. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J. Immunol. 174:4301-4306. [DOI] [PubMed] [Google Scholar]
- 8.Ishitani, T., S. Kishida, J. Hyodo-Miura, N. Ueno, J. Yasuda, M. Waterman, H. Shibuya, R. T. Moon, J. Ninomiya-Tsuji, and K. Matsumoto. 2003. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 23:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishitani, T., J. Ninomiya-Tsuji, and K. Matsumoto. 2003. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol. Cell. Biol. 23:1379-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishitani, T., J. Ninomiya-Tsuji, S. Nagai, M. Nishita, M. Meneghini, N. Barker, M. Waterman, B. Bowerman, H. Clevers, H. Shibuya, and K. Matsumoto. 1999. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399:798-802. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, Y., H. Gram, M. Zhao, L. New, J. Gu, L. Feng, F. Di Padova, R. J. Ulevitch, and J. Han. 1997. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J. Biol. Chem. 272:30122-30128. [DOI] [PubMed] [Google Scholar]
- 12.Kanei-Ishii, C., J. Ninomiya-Tsuji, J. Tanikawa, T. Nomura, T. Ishitani, S. Kishida, K. Kokura, T. Kurahashi, E. Ichikawa-Iwata, Y. Kim, K. Matsumoto, and S. Ishii. 2004. Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev. 18:816-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keren, A., E. Bengal, and D. Frank. 2005. p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev. Biol. 288:73-86. [DOI] [PubMed] [Google Scholar]
- 14.Keren, A., Y. Tamir, and E. Bengal. 2006. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell Endocrinol. 252:224-230. [DOI] [PubMed] [Google Scholar]
- 15.Kortenjann, M., M. Nehls, A. J. Smith, R. Carsetti, J. Schuler, G. Kohler, and T. Boehm. 2001. Abnormal bone marrow stroma in mice deficient for Nemo-like kinase, Nlk. Eur. J. Immunol. 31:3580-3587. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, S., J. Boehm, and J. C. Lee. 2003. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2:717-726. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 18.Meneghini, M. D., T. Ishitani, J. C. Carter, N. Hisamoto, J. Ninomiya-Tsuji, C. J. Thorpe, D. R. Hamill, K. Matsumoto, and B. Bowerman. 1999. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature 399:793-797. [DOI] [PubMed] [Google Scholar]
- 19.Mirkovic, I., K. Charish, S. M. Gorski, K. McKnight, and E. M. Verheyen. 2002. Drosophila nemo is an essential gene involved in the regulation of programmed cell death. Mech. Dev. 119:9-20. [DOI] [PubMed] [Google Scholar]
- 20.Natsume, T., Y. Yamauchi, H. Nakayama, T. Shinkawa, M. Yanagida, N. Takahashi, and T. Isobe. 2002. A direct nanoflow liquid chromatography-tandem mass spectrometry system for interaction proteomics. Anal. Chem. 74:4725-4733. [DOI] [PubMed] [Google Scholar]
- 21.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawara, B., K. Shirakabe, J. Hyodo-Miura, R. Matsuo, N. Ueno, K. Matsumoto, and H. Shibuya. 2004. Role of the TAK1-NLK-STAT3 pathway in TGF-beta-mediated mesoderm induction. Genes Dev. 18:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perdiguero, E., M. J. Pillaire, J. F. Bodart, F. Hennersdorf, M. Frodin, N. S. Duesbery, G. Alonso, and A. R. Nebreda. 2003. Xp38gamma/SAPK3 promotes meiotic G(2)/M transition in Xenopus oocytes and activates Cdc25C. EMBO J. 22:5746-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocheleau, C. E., J. Yasuda, T. H. Shin, R. Lin, H. Sawa, H. Okano, J. R. Priess, R. J. Davis, and C. C. Mello. 1999. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell 97:717-726. [DOI] [PubMed] [Google Scholar]
- 25.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 26.Satoh, K., J. Ohnishi, A. Sato, M. Takeyama, S. Iemura, T. Natsume, and H. Shibuya. 2007. Nemo-like kinase-myocyte enhancer factor 2A signaling regulates anterior formation in Xenopus development. Mol. Cell. Biol. 27:7623-7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada, M., B. Ohkawara, N. Ichimura, J. Hyodo-Miura, S. Urushiyama, K. Shirakabe, and H. Shibuya. 2003. Negative regulation of Wnt signalling by HMG2L1, a novel NLK-binding protein. Genes Cells 8:677-684. [DOI] [PubMed] [Google Scholar]
- 28.Yamada, M., J. Ohnishi, B. Ohkawara, S. Iemura, K. Satoh, J. Hyodo-Miura, K. Kawachi, T. Natsume, and H. Shibuya. 2006. NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF). J. Biol. Chem. 281:20749-20760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.