Abstract
The B-Raf kinase is a Ras pathway effector activated by mutation in numerous human cancers and certain developmental disorders. Here we report that normal and oncogenic B-Raf proteins are subject to a regulatory cycle of extracellular signal-regulated kinase (ERK)-dependent feedback phosphorylation, followed by PP2A- and Pin1-dependent dephosphorylation/recycling. We identify four S/TP sites of B-Raf phosphorylated by activated ERK and find that feedback phosphorylation of B-Raf inhibits binding to activated Ras and disrupts heterodimerization with C-Raf, which is dependent on the B-Raf pS729/14-3-3 binding site. Moreover, we find that events influencing Raf heterodimerization can alter the transforming potential of oncogenic B-Raf proteins possessing intermediate or impaired kinase activity but have no significant effect on proteins with high kinase activity, such as V600E B-Raf. Mutation of the feedback sites or overexpression of the Pin1 prolyl-isomerase, which facilitates B-Raf dephosphorylation/recycling, resulted in increased transformation, whereas mutation of the S729/14-3-3 binding site or expression of dominant negative Pin1 reduced transformation. Mutation of each feedback site caused increased transformation and correlated with enhanced heterodimerization and activation of C-Raf. Finally, we find that B-Raf and C-Raf proteins containing mutations identified in certain developmental disorders constitutively heterodimerize and that their signaling activity can also be modulated by feedback phosphorylation.
The Ras, Raf, MEK, and extracellular signal-regulated kinase (ERK) proteins are core components of one of the major signaling cascades regulating normal cell proliferation—the Ras pathway. Not surprising, deregulation of Ras pathway signaling is a major contributor to human cancer and has recently been linked with several developmental disorders, such as Noonan's, LEOPARD, and cardiofaciocutaneous (CFC) syndromes (28). Given its importance to both normal and disease states, much effort has been directed toward elucidating the mechanisms that modulate Ras pathway signaling. Of all the pathway components, regulation of the Raf proteins has proved to be the most complex, involving inter- and intramolecular interactions, a change in subcellular localization, and phosphorylation and dephosphorylation events (6, 32).
In mammalian cells, there are three Raf family members: A-Raf, B-Raf, and C-Raf (12). In their inactive state, all Raf proteins are found in the cytosol, with the N-terminal regulatory domain acting as an autoinhibitor of the C-terminal kinase domain (4, 5, 13). 14-3-3 dimers bind to phosphorylation sites present in both the N- and C-terminal regions and stabilize the autoinhibited state (22). To activate the Raf proteins, autoinhibition mediated by the N terminus must be relieved and the kinase domain must adopt the active catalytic conformation (6, 31, 32). Under normal signaling conditions, Ras activation helps mediate these events by recruiting the Raf proteins to the plasma membrane, which induces the release of 14-3-3 from the N-terminal binding site and facilitates phosphorylation of the Raf kinase domain (19). For the C-Raf and A-Raf proteins, phosphorylation occurs in two regions of the kinase domain, the negative-charge regulatory region (N-region) and the activation segment (4). In contrast, the N-region of B-Raf exhibits a constitutive negative charge due to increased basal phosphorylation of an activating serine site and the presence of two aspartic acid residues (18); thus, only phosphorylation of the activation segment is required. Phosphorylation of the activation segment serves both to destabilize the “inactive” catalytic conformation maintained by hydrophobic interactions between the glycine-rich loop and the activation segment and to stabilize the “active” catalytic conformation, whereas the negative charge of the N-region helps to disrupt the autoinhibitory activity of the N-terminal domain (5, 30, 31).
Because the N-region of B-Raf exhibits a constitutive negative charge, B-Raf possesses higher basal kinase activity than other family members and is more susceptible to mutational activation (9, 11, 17). In particular, B-Raf is a major contributor to human cancer: somatic mutations in the B-Raf gene are detected in ∼50% of malignant melanomas and many colorectal, ovarian, and papillary thyroid carcinomas (7). Of the oncogenic mutations identified in B-Raf, the vast majority cluster to the two regions of the kinase domain responsible for maintaining the inactive catalytic conformation—the glycine-rich loop and the activation segment (31). Based on enzymatic activity, the oncogenic B-Raf proteins have been divided into three groups: those with high activity (130- to 700-fold more active than wild-type [WT] B-Raf), those with intermediate activity (64- to 1.3-fold more active), and surprisingly, those with impaired catalytic activity (0.8 to 0.3 of WT B-Raf activity) (31). Further analysis has revealed that all oncogenic B-Raf proteins heterodimerize constitutively with C-Raf and activate C-Raf in a Ras-independent manner that requires an intact C-Raf activation segment as well as the binding of 14-3-3 to the C-terminal pS621 binding site on C-Raf (11). Importantly, for the oncogenic B-Raf proteins with impaired kinase activity, the binding and activation of C-Raf are required for ERK activation in vivo (31). Interestingly, heterodimerization of B-Raf and C-Raf also occurs under normal signaling conditions; however, in this case, heterodimerization is Ras dependent and occurs at the plasma membrane following mitogen stimulation (11, 27).
Once activated, either by upstream signaling or by mutational events, all Raf proteins are capable of initiating the phosphorylation cascade that results in the sequential activation of MEK and ERK. ERK then phosphorylates targets in both the cytoplasm and the nucleus that are required for cell proliferation. Strikingly, the Raf proteins themselves are also substrates of activated ERK. In regard to C-Raf, ERK-dependent feedback phosphorylation has been shown to instigate a regulatory cycle whereby phosphorylation of the feedback sites down-modulates C-Raf signaling, after which the hyperphosphorylated C-Raf protein is dephosphorylated and returned to a signaling-competent state through dephosphorylation events involving protein phosphatase 2A (PP2A) and the Pin1 prolyl-isomerase (8). For B-Raf, two ERK-dependent feedback sites, S750 and T753, have been identified, and phosphorylation of these sites has been reported to have a negative regulatory effect (3).
In this study, we have further investigated the impact of feedback phosphorylation and heterodimerization on B-Raf signaling. Here we find that both normal and oncogenic B-Raf proteins are phosphorylated on four S/TP sites (S151, T401, S750, and T753) by activated ERK. Through mutational analysis, we find that phosphorylation of B-Raf at S151 inhibits binding to activated Ras, whereas phosphorylation of each of the feedback sites contributes to the disruption of B-Raf/C-Raf heterodimers. Moreover, we find that events influencing B-Raf/C-Raf heterodimerization, such as feedback phosphorylation and 14-3-3 binding, can alter the signaling activity of oncogenic B-Raf proteins possessing intermediate or impaired kinase activity as well as that of B-Raf and C-Raf proteins containing mutations identified in CFC and Noonan's syndromes, respectively.
MATERIALS AND METHODS
Antibodies, reagents, and cell culture.
Antibodies to B-Raf (H-145) and Pin1 (G-8) were from Santa Cruz Biotechnology; the anti-C-Raf antibody was from BD Biosciences; and glutathione S-transferase (GST)-14-3-3 and GST-RasV12 resin were from Calbiochem. U0126, okadaic acid (OA), platelet-derived growth factor (PDGF), recombinant activated ERK, and antibodies recognizing phospho-MEK, phospho-ERK, and phospho-S/TP sites were purchased from Cell Signaling. The antibody to the Flag M2 epitope was from Sigma-Aldrich, and the antibody recognizing the Pyo epitope has been described previously (29). NIH 3T3 cells and mouse embryo fibroblasts lacking Pin1 (Pin1−/− MEFs) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C under 5% CO2. In some experiments, cells were treated with 0.5 μM okadaic acid or 10 μM U0126 for 30 to 60 min prior to and during stimulation with 100 ng/ml PDGF or epidermal growth factor (EGF).
Generation of recombinant retroviruses and stable cell lines.
Sequences encoding Flag-tagged B-Raf, Flag-tagged C-Raf, or Pyo-tagged C-Raf were isolated from pcDNA3 constructs and inserted into the pBabe-puro retroviral vector. Point mutations were introduced by site-directed mutagenesis, and all mutations were confirmed by DNA sequencing. pBabe retroviral constructs expressing WT Pin1 or dominant negative (S16A/K63A) Pin1 were also produced. Recombinant retroviruses were generated as previously described (20) and were used to infect NIH 3T3 cells or Pin1−/− MEFs. Stable cell lines were established by puromycin selection (5 μg/ml puromycin).
Cell lysis, coimmunoprecipitation assays, and GST pulldown assays.
Cells were washed twice with phosphate-buffered saline (PBS) and were lysed for 20 min at 4°C in NP-40 lysis buffer (20 mM Tris [pH 8.0], 137 mM NaCl, 10% glycerol, 1% NP-40, 0.15 U/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 20 μM leupeptin, 5 mM sodium vanadate, and 0.1 μM calyculin). Lysates were cleared of insoluble material by centrifugation at 4°C for 20 min at 16,000 × g. For GST pulldown assays, cell lysates were incubated for 2 to 4 h at 4°C with glutathione resins containing the indicated GST fusion protein. For coimmunoprecipitation assays, cell lysates were incubated for 4 h at 4°C with the indicated antibody and protein A/G agarose beads. Protein complexes binding to the bead resins were isolated by centrifugation, washed extensively in NP-40 lysis buffer, and examined by immunoblot analysis.
Metabolic labeling and phosphorylation site mapping.
NIH 3T3 cells expressing Flag-tagged B-Raf proteins were incubated for 4 to 6 h at 37°C in phosphate-free DMEM containing 2.5% dialyzed calf serum and [32P]orthophosphate (1 mCi/ml of medium). Cells were stimulated with the appropriate agent and were washed in Tris-buffered saline (TBS) (20 mM Tris [pH 7.4], 137 mM NaCl) prior to lysis in NP-40 lysis buffer. Labeled Flag-B-Raf proteins were immunoprecipitated from cell lysates, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), eluted from the gel matrix, digested with trypsin, and analyzed by reverse-phase high-pressure liquid chromatography (HPLC), phosphoamino acid analysis, and Edman degradation as previously described (21).
Focus-forming assays.
NIH 3T3 cells were plated at a concentration of 2.5 × 105/60-mm-diameter dish in DMEM containing 4% FBS. Eighteen hours after plating, cells were infected with the designated recombinant retrovirus (∼500 CFU per dish) in 2 ml of DMEM containing 4% FBS and 8 μg/ml Polybrene. Twenty-four hours postinfection, the cells were trypsinized and plated onto two 10-cm-diameter dishes. One plate of cells was cultured for 2 weeks in DMEM containing 4% FBS, and the other plate was cultured in DMEM containing 4% FBS and 5 μg/ml puromycin. Cell morphology was visualized by light microscopy, after which the cells were fixed in 3.7% formaldehyde for 20 min at room temperature and were stained using 1% (wt/vol, in water) methylene blue for 30 min.
In vitro kinase assays.
To determine Raf kinase activity, Flag-tagged B-Raf, endogenous B-Raf, or endogenous C-Raf proteins were immunoprecipitated from cells lysed in radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris [pH 8.0], 137 mM NaCl, 10% glycerol, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 0.15 U/ml aprotinin, 1 mM PMSF, 20 μM leupeptin, 5 mM sodium vanadate, 0.1 μM calyculin). Raf immunoprecipitates were washed extensively with NP-40 lysis buffer and were incubated in 40 ml kinase buffer (30 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 5 mM EGTA, 1 mM dithiothreitol, 1 mM NaVO4, 15 mM ATP, 10 mM MgCl2) containing 20 mCi [γ-32P]ATP and 0.1 mg of purified kinase-inactive MEK. After incubation for 30 min at 25°C, the samples were resolved by SDS-PAGE and transferred to nitrocellulose filters. The level of Raf protein present in the immunoprecipitates was quantified by immunoblot analysis, and the radioactivity incorporated into MEK was determined by scintillation counting. Raf activity levels were normalized for protein levels. In experiments where B-Raf was used as an in vitro substrate for ERK, Flag-tagged kinase-dead B-Raf (KD-B-Raf) was affinity purified from serum-starved NIH 3T3 cells that had been lysed in RIPA buffer. Purified KD-B-Raf was incubated in 40 ml kinase buffer containing 20 mCi [γ-32P]ATP and recombinant activated ERK. After incubation for 30 min at 25°C, the samples were resolved by SDS-PAGE and processed for phosphorylation site mapping.
RESULTS
B-Raf is a target of feedback phosphorylation and recycling.
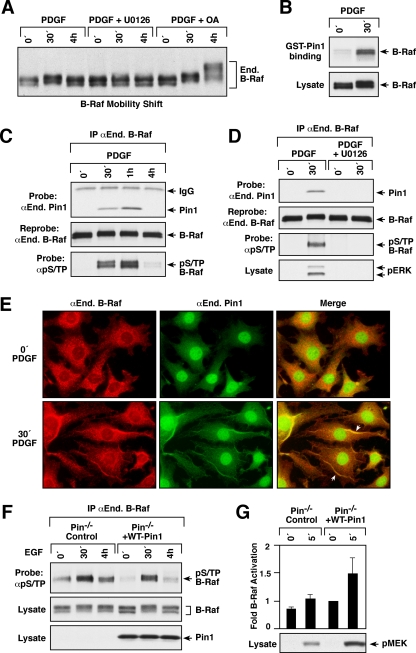
Previously, we found that in response to growth factor treatment, signaling from C-Raf is downregulated by ERK-dependent feedback phosphorylation on S/TP sites and that C-Raf is subsequently dephosphorylated and returned to a signaling-competent state through the activities of PP2A and the Pin1 prolyl-isomerase (8). Given that shifts in the electrophoretic mobility of C-Raf have been shown to be indicative of the C-Raf feedback phosphorylation/dephosphorylation cycle, we examined the electrophoretic mobility of B-Raf under various signaling conditions in order to determine if B-Raf is subject to a similar regulatory cycle. As shown in Fig. 1A, comparison of endogenous B-Raf proteins from quiescent and growth factor-treated NIH 3T3 cells revealed a decrease in B-Raf mobility after 30 min of PDGF treatment, with the mobility of B-Raf returning to that observed in quiescent cells 4 h following treatment. The growth factor-induced shift in B-Raf mobility could be blocked by pretreating cells with the MEK inhibitor U0126, indicating that signaling downstream of MEK was required for the generation of the slower-migrating forms of B-Raf. When cells were treated with OA at concentrations that inhibit PP2A but not other OA-sensitive phosphatases (10), the mobility of B-Raf at the 4-h time point was still retarded, and even slower-migrating forms of B-Raf were observed, indicating that PP2A activity is needed to return B-Raf to its faster-migrating form.
FIG. 1.
B-Raf is a target of feedback phosphorylation and recycling. (A) Serum-starved NIH 3T3 cells were treated either with PDGF alone (100 ng/ml) or with PDGF in the presence of the MEK inhibitor U0126 (10 μM) or the phosphatase inhibitor OA (0.5 μM) for the indicated times. Lysates were prepared, and the electrophoretic mobility of endogenous (End.) B-Raf was examined by immunoblot analysis. (B) Lysates from serum-starved or PDGF-treated NIH 3T3 cells were incubated with glutathione beads containing GST-Pin1, and binding of endogenous B-Raf to GST-Pin1 was determined by immunoblot analysis. (C and D) Serum-starved NIH 3T3 cells were treated with PDGF alone or with both PDGF and U0126, as indicated, prior to lysis. Endogenous B-Raf was immunoprecipitated (IP) and examined for the binding of endogenous Pin1 by immunoblot analysis. The blot was reprobed to confirm equal B-Raf levels. B-Raf proteins were also examined for phosphorylation on S/TP sites (pS/TP) by immunoblot analysis. (E) Localization of endogenous B-Raf and endogenous Pin1 was visualized in serum-starved and PDGF-treated NIH 3T3 cells by immunofluorescent staining. As a control for specificity, PDGF-treated Pin1−/− MEFs were also stained. (F) Serum-starved Pin1−/− or WT Pin1 MEFs were treated as indicated prior to lysis. Endogenous B-Raf was immunoprecipitated and examined for pS/TP levels. Lysates were examined for Pin1 expression and for the shift in B-Raf mobility. (G) Serum-starved Pin1−/− or WT Pin1 MEFs were treated as indicated. Endogenous B-Raf proteins were immunoprecipitated and examined for kinase activity using kinase-dead MEK as an exogenous substrate. B-Raf activity is expressed as fold activation relative to the activity of B-Raf in untreated WT Pin1 MEFs. Data are means ± standard deviations for at least three independent experiments where activity measurements have been normalized for B-Raf protein levels. Lysates were also examined for in vivo MEK activation by probing with antibodies against pMEK.
The Pin1 prolyl-isomerase binds specifically to phosphorylated S/TP (pS/TP) motifs (33), and isomerization of the pS/TP bond is required for PP2A to efficiently dephosphorylate certain proteins, such as cdc25C, Myc, and C-Raf (16). Using a GST fusion protein to detect potential Pin1-interacting molecules, we found that the slower-migrating forms of B-Raf present in PDGF-treated cells strongly associated with GST-Pin1, whereas little interaction was detected for the faster-migrating B-Raf forms observed in quiescent cells (Fig. 1B). To confirm that B-Raf does interact with Pin1, coimmunoprecipitation and colocalization assays were performed using NIH 3T3 cells that had been treated with PDGF for various times. The phosphorylation of endogenous B-Raf on S/TP sites was also assessed using antibodies that specifically recognize pS/TP motifs. By immunoblot analysis, endogenous Pin1 was found to associate with endogenous B-Raf at 30 min and 1 h following PDGF treatment (Fig. 1C), and by immunofluorescent staining, these two proteins were found to colocalize in cells treated with PDGF for 30 min, with the specificity of this colocalization confirmed by staining of Pin1−/− MEFs (Fig. 1E). Complex formation between B-Raf and Pin1 correlated with the phosphorylation of B-Raf on S/TP sites (Fig. 1C), and this interaction could be blocked when the MEK inhibitor U0126 was used to prevent ERK activation and the S/TP phosphorylation of B-Raf (Fig. 1D).
To further establish that Pin1 contributes to the dephosphorylation of B-Raf, we next examined the S/TP phosphorylation state of endogenous B-Raf in Pin1−/− MEFs and in cells where WT Pin1 had been reexpressed. As was observed in NIH 3T3 cells, phosphorylation of B-Raf on S/TP sites was strongly induced when WT Pin1 MEFs were treated with growth factor for 30 min and could barely be detected at the 0- and 4-h time points (Fig. 1F). However, in MEFs lacking Pin1, significant phosphorylation of B-Raf on S/TP sites was observed prior to growth factor addition and 4 h after growth factor treatment (Fig. 1F). In addition, basal B-Raf activity and that induced by growth factor treatment were reduced in Pin1−/− MEFs, as was the growth factor-induced phosphorylation of MEK on activating sites (Fig. 1G). Together, these findings indicate that Pin1 is needed for the efficient dephosphorylation of B-Raf and are consistent with the model that S/TP phosphorylation inhibits Raf signaling. Moreover, they reveal that B-Raf, like C-Raf, is subject to a regulatory cycle of feedback phosphorylation/dephosphorylation and that the molecules mediating these events (ERK, PP2A, and Pin1) are the same.
Analysis of the in vivo phosphorylation state of normal and oncogenic B-Raf proteins.
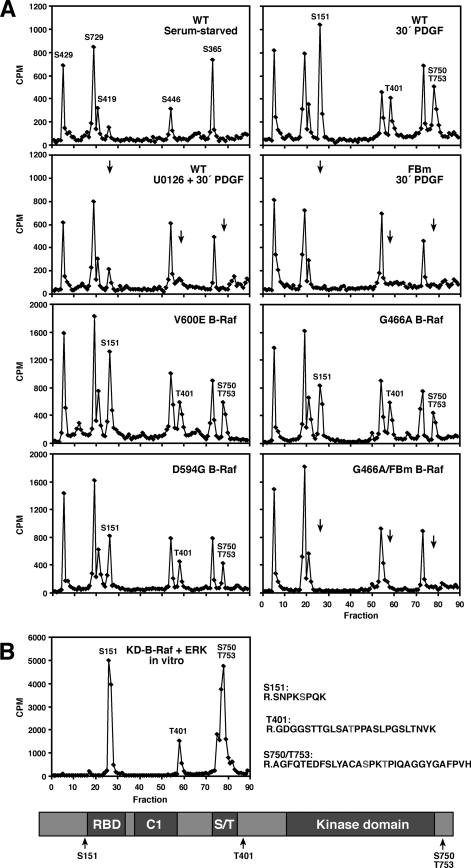
Although S750 and T753 have been identified previously as B-Raf sites that are phosphorylated by activated ERK (3), whether other residues of B-Raf are targets for feedback phosphorylation is currently unknown. Therefore, to fully characterize the feedback phosphorylation state of B-Raf, metabolic [32P]-labeling experiments were performed (Fig. 2A). Analysis of B-Raf tryptic phosphopeptides by HPLC, phosphoamino acid analysis, Edman degradation, and mass spectrometry revealed that B-Raf isolated from quiescent NIH 3T3 cells is phosphorylated on five sites: S729 and S365, two sites that serve as 14-3-3 binding sites (2); S446, a known activating site in the catalytic domain N-region (17); S429, a previously identified site of protein kinase A (PKA) phosphorylation (14); and S419. Following 30 min of PDGF treatment, little change in the phosphorylation state of these residues was observed; however, several new phosphopeptides were detected. Eluting in HPLC fractions 78 to 79 was a peptide phosphorylated on S750 and T753, the previously identified ERK sites, and eluting in fractions 26 and 58 to 59 were peptides phosphorylated at S151 and T401, respectively. All four of these identified sites are followed by a proline residue, and their phosphorylation could be blocked by pretreating cells with the MEK inhibitor U0126 (Fig. 2A), suggesting that these residues are feedback targets of the proline-directed kinase, ERK. Consistent with this model, we found that when purified activated ERK was incubated with kinase-dead B-Raf(K375M) in vitro, ERK strongly phosphorylated B-Raf on the S151, S750, and T753 sites, with phosphorylation of T401 also observed (Fig. 2B). The identity of the residues phosphorylated was confirmed by the analysis of a B-Raf mutant in which all the feedback S/TP sites were mutated to alanine (FBm-B-Raf) (Fig. 2A).
FIG. 2.
Identification of B-Raf feedback phosphorylation sites. (A) Serum-starved NIH 3T3 cells stably expressing Flag-tagged WT, FBm, V600E, G466A, G466A-FBm, or D594G B-Raf protein were metabolically labeled with [32P]orthophosphate. Cells either were left untreated or were stimulated with 100 ng/ml PDGF in the presence or absence of 10 μM U0126 prior to lysis and immunoprecipitation of the B-Raf proteins. Labeled B-Raf proteins were isolated following SDS-PAGE and were digested with trypsin. The tryptic phosphopeptides were separated by HPLC, and the radioactivity collected in the HPLC fractions is depicted. (B) Purified kinase-dead B-Raf (KD-B-Raf) was phosphorylated by activated ERK2 in vitro in the presence of [γ-32P]ATP. Labeled KD-B-Raf was isolated and analyzed as in panel A. Peptides containing the feedback sites are indicated, as are the locations of the feedback sites.
Given that oncogenic B-Raf proteins mediate constitutive ERK signaling, we next investigated whether oncogenic forms of B-Raf might also be subject to feedback phosphorylation. For these experiments, we examined the phosphorylation state of the high-activity V600E B-Raf protein as well as those of mutants with intermediate (G466A B-Raf) or impaired (D594G B-Raf) activity. As shown in Fig. 2A, all three B-Raf mutants were phosphorylated at the feedback sites; however, the level of phosphorylation was highest for the V600E and G466A proteins. Together these findings reveal that both normal and oncogenic B-Raf proteins are targets of feedback phosphorylation, and they identify S151, T401, S750, and T753 as the residues phosphorylated.
Effect of feedback phosphorylation on B-Raf protein interactions.
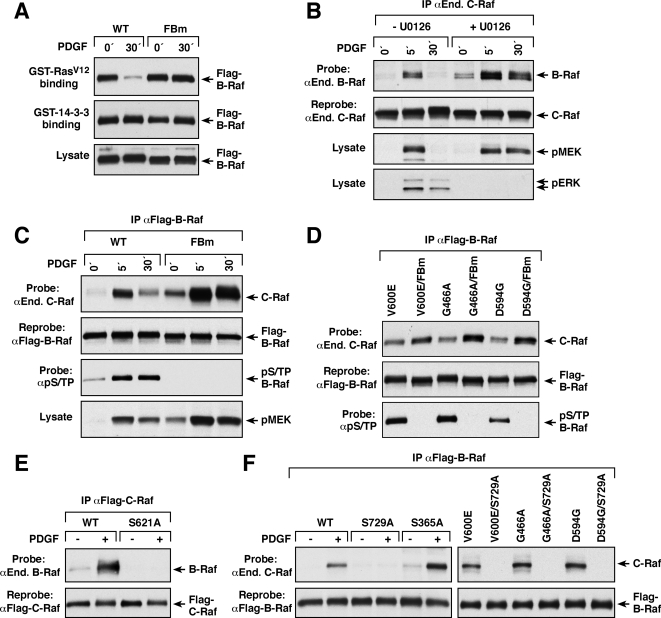
We next assessed the effect of feedback phosphorylation on B-Raf protein interactions. Because sites of feedback phosphorylation are found in close proximity to the Ras binding domain (RBD) of B-Raf (residues 155 to 227) as well as the pS365 and pS729 binding sites for 14-3-3, we first compared the abilities of WT B-Raf and FBm-B-Raf to associate with Ras and 14-3-3 under conditions where feedback phosphorylation is induced. As shown in Fig. 3A, when a GST-14-3-3 fusion protein was incubated with lysates prepared from serum-starved cells or with cells treated with PDGF for 30 min, equivalent levels of WT and FBm B-Raf proteins were found to associate with 14-3-3, and the interaction was not altered by growth factor treatment. In contrast, although equivalent amounts of WT and FBm B-Raf proteins from serum-starved cells interacted with a GST-RasV12 fusion protein, 30 min of PDGF treatment greatly reduced the amount of WT B-Raf associating with GST-RasV12 but had no effect on the interaction between FBm-B-Raf and GST-RasV12 (Fig. 3A). These findings are similar to what has been observed for C-Raf (8) and suggest that feedback phosphorylation is a conserved mechanism used to disrupt the Ras/Raf interaction.
FIG. 3.
Effect of feedback phosphorylation on B-Raf-C-Raf heterodimerization. (A) Lysates from serum-starved or PDGF-treated NIH 3T3 cells stably expressing Flag-tagged WT or FBm B-Raf were incubated with agarose beads containing either GST-14-3-3 or GST-RasV12. Binding of Flag-B-Raf to the GST proteins was determined by immunoblot analysis. Lysates were examined for Flag-B-Raf levels. (B) Serum-starved NIH 3T3 cells were treated with 100 ng/ml PDGF in the presence or absence of 10 μM U0126 prior to lysis. Endogenous (End.) C-Raf was immunoprecipitated (IP) and examined for binding of endogenous B-Raf. The blot was reprobed to confirm equal C-Raf levels. Lysates were examined for pMEK and pERK levels by immunoblot analysis. (C) Serum-starved NIH 3T3 cells stably expressing Flag-tagged WT or FBm B-Raf were treated with PDGF as indicated prior to lysis. Flag-B-Raf proteins were isolated and probed either for endogenous C-Raf binding or for pS/TP levels. Blots were reprobed to confirm equivalent Flag-B-Raf levels. Lysates were also examined for pMEK levels. (D) The indicated Flag-tagged B-Raf proteins were isolated from NIH 3T3 cells and examined for endogenous C-Raf binding and for pS/TP levels. Blots were reprobed to confirm equivalent Flag-B-Raf levels. (E) Serum-starved NIH 3T3 cells expressing Flag-tagged WT or S621A C-Raf were either left untreated or treated for 5 min with PDGF prior to lysis. Flag-C-Raf proteins were isolated and probed for endogenous B-Raf binding. The blot was reprobed to confirm equivalent Flag-C-Raf levels. (F) The indicated Flag-tagged B-Raf proteins isolated from NIH 3T3 cells treated or not treated with PDGF were examined for endogenous C-Raf binding. Blots were reprobed to confirm equivalent Flag-B-Raf levels.
Under normal growth signaling conditions, B-Raf has also been found to interact with C-Raf in a manner that is dependent on Ras binding and that can be prolonged by mutating the feedback T753 site to alanine (11, 27). Consistent with these data, we found that B-Raf interacted with C-Raf in an inducible and transient manner following growth factor treatment (Fig. 3B and C). In addition, when B-Raf feedback phosphorylation was prevented, either by U0126 treatment or by mutation of all the feedback sites, an increase in the basal level of heterodimerization with C-Raf was observed, and heterodimerization in response to growth factor treatment was increased and prolonged (Fig. 3B and C). These findings support a model whereby feedback phosphorylation disrupts Raf heterodimerization.
Unlike WT B-Raf, oncogenic B-Raf proteins have been shown to heterodimerize constitutively with C-Raf in a Ras-independent manner (11). When we next examined the effect of feedback phosphorylation on the ability of oncogenic B-Raf to form heterodimers with C-Raf, we found that the levels of endogenous C-Raf associating with B-Raf proteins of high (V600E), intermediate (G466A), and impaired (D594G) kinase activities all increased when the feedback sites were mutated, indicating that feedback phosphorylation also inhibits the heterodimerization of oncogenic B-Raf proteins (Fig. 3D).
Previous studies have shown that, for both normal and oncogenic B-Raf proteins to heterodimerize with C-Raf, the C-terminal 14-3-3 binding site of C-Raf (S621) must be intact (11, 27) (Fig. 3E). To determine whether binding of 14-3-3 to B-Raf is also required for heterodimerization, B-Raf proteins containing alanine substitutions in the two 14-3-3 binding sites, S365 and S729 (2), were examined for their abilities to heterodimerize with C-Raf in response to growth factor treatment. Not surprisingly, given that mutation of the S365 14-3-3 binding site enhances the membrane localization of B-Raf (2), increased heterodimerization with C-Raf was observed for S365A B-Raf compared to WT B-Raf (Fig. 3F). In contrast, S729A B-Raf failed to heterodimerize with C-Raf in response to growth factor treatment, and mutation of this site disrupted the constitutive interaction of oncogenic B-Raf proteins and C-Raf (Fig. 3F), indicating that heterodimerization with C-Raf is dependent on the C-terminal S729 14-3-3 binding site of B-Raf.
Effect of feedback phosphorylation and heterodimerization on the transformation potential of oncogenic B-Raf proteins.
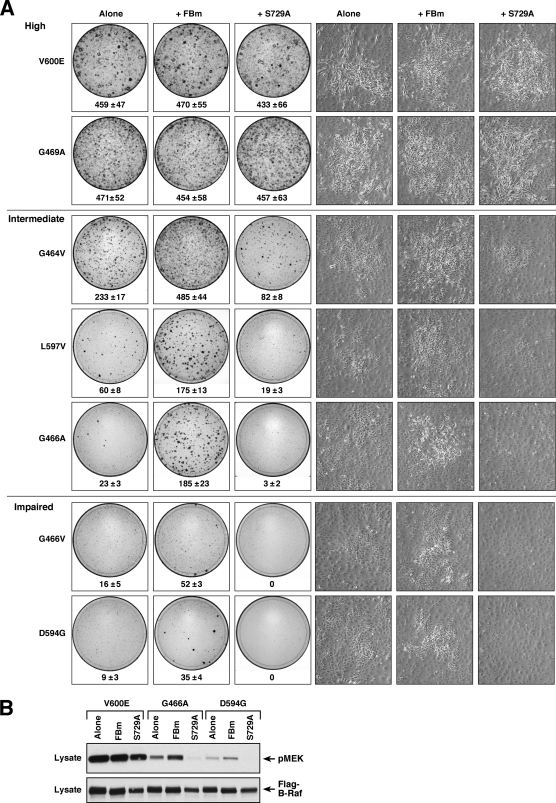
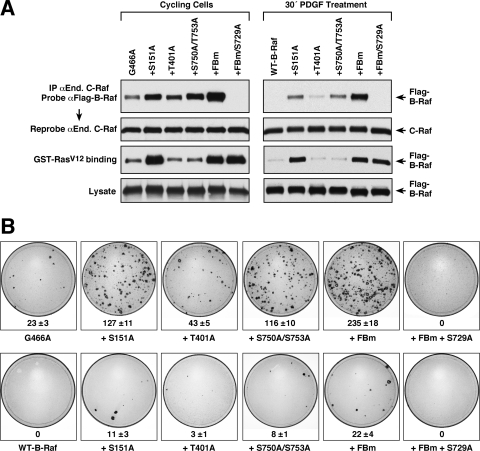
Previous studies have found that all oncogenic B-Raf proteins can activate C-Raf and that heterodimerization with C-Raf is required for kinase-impaired oncogenic B-Raf proteins to mediate ERK activation in vivo (31). Therefore, to further investigate both the impact of feedback phosphorylation and the contribution of heterodimerization to oncogenic B-Raf function, we examined the transformation potential of oncogenic B-Raf proteins containing mutations in either the feedback phosphorylation sites (which exhibit increased heterodimerization) or the S729 14-3-3 binding site (which are unable to heterodimerize). For these studies, FBm or S729A mutations were incorporated into a number of oncogenic B-Raf proteins that exhibit various levels of kinase activity. The proteins were then expressed in NIH 3T3 cells and examined for their abilities to alter cell morphology and induce focus formation. As shown in Fig. 4A, the FBm or S729A mutation had no effect on transformation induced by the V600E or G469A B-Raf protein, both of which possess high kinase activity. However, mutation of the feedback sites significantly increased the transforming activities of B-Raf proteins with intermediate or impaired kinase activity (Fig. 4A). The total number of foci observed and, often, the sizes of the foci were increased, and cells within the foci exhibited a more transformed appearance. In contrast, the S729A mutation reduced the transforming activities of the oncogenic proteins with intermediate or impaired kinase activity, causing a reduction in focus number and a flatter cell morphology (Fig. 4A). Examination of activated phospho-MEK levels revealed that the FBm and S729 mutations had no effect on MEK activation induced by the high-activity V600E B-Raf protein; however, the FBm and S729A mutations increased and decreased, respectively, the abilities of the intermediate G466A and kinase-impaired D594G B-Raf proteins to activate MEK (Fig. 4B), indicating a correlation between the transformation potential of these proteins and their ability to activate ERK cascade signaling in vivo.
FIG. 4.
Feedback phosphorylation and heterodimerization impact the transformation potential of oncogenic B-Raf proteins. (A) NIH 3T3 cells were infected with recombinant retroviruses expressing the indicated B-Raf proteins. After 2 weeks of culture, cell morphology was examined by light microscopy, and focus formation was visualized by staining with methylene blue. Focus plates from a representative experiment are shown. The mean number of foci from at least 3 independent experiments ± standard deviation is given below each plate. (B) Lysates from NIH 3T3 cells stably expressing the indicted B-Raf proteins were examined for activated pMEK levels by immunoblot analysis.
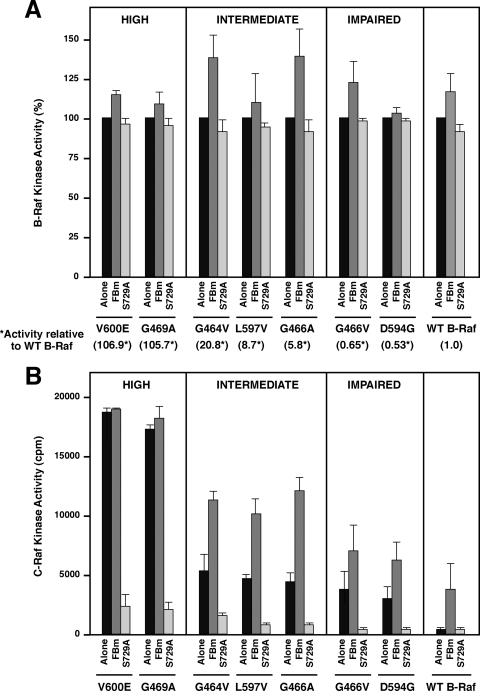
We next examined the effects of the FBm and S729A mutations on the intrinsic kinase activities of the oncogenic B-Raf proteins and on their abilities to activate endogenous C-Raf. In immune complex kinase assays, no significant change in B-Raf kinase activity was observed when the S729 site was mutated; however, the FBm proteins exhibited 1.1- to 1.4-fold increases in kinase activity (Fig. 5A). When the activation state of endogenous C-Raf was examined, we found that C-Raf activity was highest in cells expressing the high-activity B-Raf proteins and that, although no significant change in C-Raf activity was observed in cells expressing the FBm versions of these mutants, C-Raf activity was increased approximately 2-fold in cells expressing the FBm versions of the intermediate- and impaired-activity mutants (Fig. 5B). Not unexpectedly, for all of the oncogenic B-Raf proteins, the S729A mutation, which disrupts heterodimerization with C-Raf, caused a >90% decrease in C-Raf activity levels (Fig. 5B). Together, these findings indicate a correlation between the changes in the transformation potentials of the intermediate and impaired oncogenic B-Raf proteins and their abilities to heterodimerize and activate C-Raf.
FIG. 5.
Raf kinase activity in cells expressing the oncogenic B-Raf proteins. (A) NIH 3T3 cells expressing the indicated Flag-tagged oncogenic B-Raf proteins were lysed in RIPA buffer. Flag-B-Raf proteins were isolated and examined for intrinsic kinase activity using kinase-inactive MEK as an exogenous substrate. The activity of each B-Raf protein containing the oncogenic mutation alone is set at 100%. Each bar represents the mean ± standard deviation from at least 3 independent experiments where activity measurements have been normalized for B-Raf protein levels. The fold activity of each oncogenic mutant (alone) relative to that of WT B-Raf (alone) is given in parentheses at the bottom. (B) Endogenous C-Raf was isolated from RIPA lysates of cells expressing the indicated B-Raf proteins, and C-Raf kinase activity was determined using kinase-inactive MEK as an exogenous substrate. Each bar represents the mean ± standard deviation from at least 3 independent experiments where activity measurements have been normalized for C-Raf protein levels.
Analysis of specific B-Raf feedback phosphorylation sites.
To investigate the contributions of the various feedback sites to the overall effect of feedback phosphorylation on B-Raf function, we generated a panel of mutants in which specific feedback phosphorylation sites were incorporated into either WT B-Raf or the intermediate-activity G466A B-Raf protein. The mutant proteins were then examined for their abilities to heterodimerize with C-Raf and to bind activated Ras under conditions where feedback phosphorylation was induced (in cycling cells for the G466A mutants and in cells treated with PDGF for 30 min for the WT B-Raf mutants). As shown in Fig. 6A, only mutation of the S151 feedback site, which is in close proximity to the Ras binding domain (residues 155 to 227), was found to significantly increase binding to activated Ras. In contrast, mutation of S151A, T401A, and S750A T753A were all found to increase C-Raf binding (Fig. 6A), a finding consistent with peptide studies suggesting that there are multiple points of contact between heterodimerized B- and C-Raf proteins (27). Interestingly, when the S729A mutation was introduced into the FBm mutant, binding to C-Raf was abolished (Fig. 6A), indicating that the increased heterodimerization observed when the feedback sites are mutated is still dependent on 14-3-3 binding.
FIG. 6.
Analysis of individual sites of B-Raf feedback phosphorylation. (A) Lysates were prepared either from cycling cells or from PDGF-treated cells expressing the indicated Flag-tagged B-Raf proteins. The lysates were incubated with agarose beads containing GST-RasV12, and the binding of Flag-B-Raf proteins to GST-RasV12 was determined by immunoblot analysis. Endogenous C-Raf proteins were also immunoprecipitated from the lysates and examined for Flag-B-Raf binding. Blots were reprobed to confirm equivalent C-Raf levels, and lysates were examined for Flag-B-Raf levels. (B) NIH 3T3 cells were infected with recombinant retroviruses expressing the indicated B-Raf proteins, and focus formation was visualized after 2 weeks in culture. Focus plates from a representative experiment are shown. The mean number of foci ± standard deviation from at least 3 independent experiments is given below each plate.
Next, the panel of mutants was examined for the ability to induce focus formation. As shown in Fig. 6B, the S151A, T401A, and S750 T753A mutations all increased the transformation potential of G466A B-Raf and caused WT B-Raf to induce foci. The mutations appeared to be additive in their effect in that the largest numbers of foci were observed for the FBm proteins, in which all feedback sites were mutated (Fig. 6B). Strikingly, the increased transformation potential of the FBm proteins was abolished when the S729 14-3-3 binding site was also mutated (Fig. 6B), demonstrating that 14-3-3 binding is required for the increased transformation potential of the FBm mutants.
Pin1 contributes to the recycling of oncogenic B-Raf proteins.
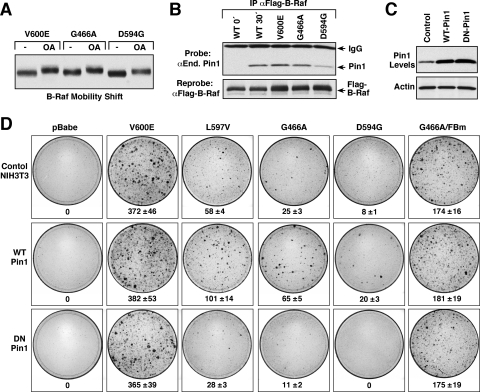
Given that oncogenic B-Raf proteins are targets of feedback phosphorylation, we next examined whether they might also be dephosphorylated and recycled in a manner involving the PP2A phosphatase and the Pin1 prolyl-isomerase. As indicated in Fig. 7A, when PP2A was inhibited with okadaic acid treatment, slower-migrating forms of the V600E, G466A, and D594G B-Raf proteins were found to accumulate. Moreover, given their constitutive phosphorylation on S/TP sites (Fig. 3D), these oncogenic B-Raf mutants were found to interact constitutively with Pin1 (Fig. 7B), indicating that oncogenic B-Raf proteins are dephosphorylated and recycled.
FIG. 7.
Pin1 contributes to the recycling of oncogenic B-Raf proteins. (A) NIH 3T3 cells expressing V600E, G466A, or D594G B-Raf protein were treated or not with 0.5 μM OA for 1 h. Lysates were prepared, and the electrophoretic mobility of the B-Raf proteins was examined by immunoblot analysis. (B) The indicated Flag-B-Raf proteins were immunoprecipitated (IP) and examined for binding of endogenous (End.) Pin1 by immunoblot analysis. The blot was reprobed to confirm equivalent Flag-B-Raf levels. (C) Lysates from control NIH 3T3 cells or cells stably expressing either WT or DN Pin1 were compared for Pin1 expression levels. (D) Control NIH 3T3 cells or cells stably expressing either WT or DN Pin1 were infected with recombinant retroviruses expressing the indicated B-Raf proteins. Focus formation was visualized after 2 weeks in culture. Focus plates from a representative experiment are shown. The mean number of foci ± standard deviation from 3 independent experiments is given below each plate.
Interestingly, expression levels of the Pin1 prolyl-isomerase have been reported to be upregulated in numerous human cancers, including cancers where oncogenic B-Raf signaling has been implicated, such as melanoma, thyroid carcinoma, and non-Hodgkin's lymphoma (NHL) (1). In regard to oncogenic B-Raf proteins, one might predict that increased levels of Pin1 could facilitate the dephosphorylation of the feedback sites, perhaps allowing for faster recycling and heterodimer reassembly. Therefore, to investigate the effect of Pin1 overexpression on oncogenic B-Raf signaling, focus-forming assays were performed in NIH 3T3 cells stably overexpressing (∼3-fold over endogenous levels) either WT Pin1 or a dominant negative (DN) Pin1 protein, which binds substrates but lacks isomerase activity (Fig. 7C and D). We found that overexpression of the Pin1 proteins on their own did not induce focus formation and that they had no significant effect on the transformation potential of the high-activity V600E B-Raf protein. In contrast, the numbers of foci induced by intermediate-activity (L597V and G466A) and impaired-activity (D594G) B-Raf mutants were increased in cells overexpressing WT Pin1, whereas focus formation was reduced in cells overexpressing DN Pin1. Consistent with the model that Pin1 influences B-Raf signaling by facilitating the dephosphorylation of the feedback sites, overexpression of the Pin1 proteins had no effect on the transformation potential of G466A FBm-B-Raf, which lacks the sites of feedback phosphorylation.
Effects of feedback phosphorylation and heterodimerization on other mutationally activated B-Raf and C-Raf proteins.
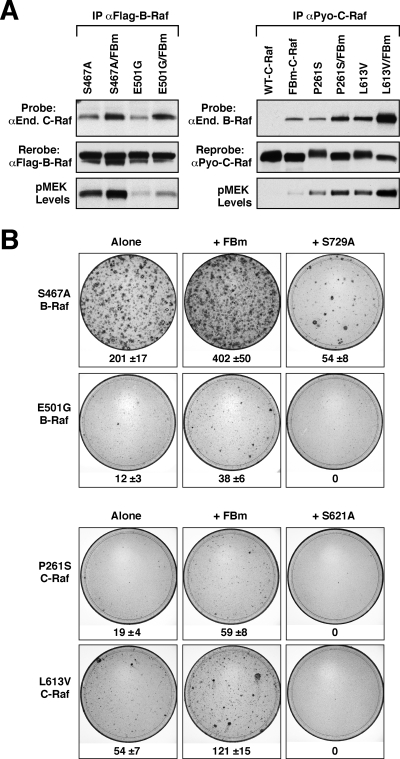
To investigate whether feedback phosphorylation and heterodimerization can impact other mutationally activated Raf proteins, we first generated B-Raf and C-Raf proteins that contain mutations identified in CFC (S467A and E501G B-Raf) and Noonan's (P261S and L613V C-Raf) syndrome patients, respectively (23-26). Versions of these proteins were then made in which either the feedback phosphorylation sites or the C-terminal 14-3-3 binding site (S729A for B-Raf and S621A for C-Raf) was mutated. As with the oncogenic B-Raf-proteins, we found that B-Raf proteins containing CFC mutations heterodimerized constitutively with C-Raf and that mutation of the feedback sites increased the level of heterodimerization and resulted in elevated MEK activation (Fig. 8A). The transforming activity of these proteins was also increased when the feedback sites were mutated, whereas mutation of the C-terminal 14-3-3 binding site reduced or abolished their ability to induce foci (Fig. 8B). It should be noted that in agreement with the study of Rodriguez-Viciana et al. (26), we found that E501G B-Raf had impaired kinase activity; however, in contrast to the finding of Rodriguez-Viciana et al. that S467A B-Raf is a high-activity mutant, we found that S467A B-Raf had intermediate kinase activity compared to those of the WT and V600E B-Raf proteins (data not shown).
FIG. 8.
Effects of feedback phosphorylation and heterodimerization on B-Raf and C-Raf proteins containing CFC and Noonan's syndrome mutations. (A) Flag-B-Raf or Pyo-C-Raf proteins were immunoprecipitated (IP) from cell lysates and examined for endogenous (End.) C-Raf or endogenous B-Raf binding, respectively. Blots were reprobed for Flag-B-Raf or Pyo-C-Raf levels, and lysates were examined for pMEK levels. (B) NIH 3T3 cells were infected with recombinant retroviruses expressing the indicated B-Raf or C-Raf proteins, and focus formation was visualized after 2 weeks of culture. Focus plates from a representative experiment are shown. The mean number of foci ± standard deviation from 3 independent experiments is given below each plate.
Similar to what was observed for the mutationally activated B-Raf proteins, C-Raf proteins containing the Noonan's syndrome mutations were found to heterodimerize constitutively with endogenous B-Raf. Mutation of the C-Raf feedback phosphorylation sites increased the level of B-Raf binding, which correlated with elevated MEK activation (Fig. 8A). In addition, the transforming activity of the C-Raf mutant proteins increased when the feedback sites were mutated and decreased when the S621 14-3-3 binding site was mutated (Fig. 8B). Together, these findings suggest that feedback phosphorylation and Raf heterodimerization can also influence the aberrant signaling observed in developmental disorders caused by mutationally activated Raf proteins.
DISCUSSION
The B-Raf kinase initiates ERK cascade signaling as a Ras pathway effector and when activated by mutational events. In this study, we report that both normal and mutant B-Raf proteins are subject to a feedback phosphorylation/dephosphorylation cycle that can impact the signaling potential of B-Raf.
Previous studies have found that both the C-Raf and B-Raf proteins are targets of ERK-dependent feedback phosphorylation. In the case of C-Raf, six sites of feedback phosphorylation have been identified, five of which are direct targets of activated ERK (8). For B-Raf, previous work by Brummer et al. (3) identified the C-terminal S750 and T753 residues as sites phosphorylated by activated ERK. Through metabolic labeling experiments, we find here that in addition to the S750 and T753 sites, B-Raf is feedback phosphorylated on two other sites, S151 and T401. Interestingly, like the feedback sites identified for C-Raf (8), the B-Raf sites of feedback phosphorylation are distributed throughout the protein: S151 is located in the N-terminal region close to the RBD; S750 and T753 are found at the B-Raf C terminus; and T401 is located upstream of the kinase domain (Fig. 2B). These residues are phosphorylated by activated ERK in vitro, and under normal signaling conditions, their phosphorylation occurs following growth factor treatment and can be blocked by inhibition of MEK (Fig. 9A to C). As has been observed for C-Raf, we find that the hyperphosphorylated B-Raf protein is subsequently dephosphorylated in a manner requiring the activities of the PP2A phosphatase and Pin1 prolyl-isomerase, indicating that the feedback phosphorylation/dephosphorylation cycle is a conserved regulatory mechanism for the Raf proteins.
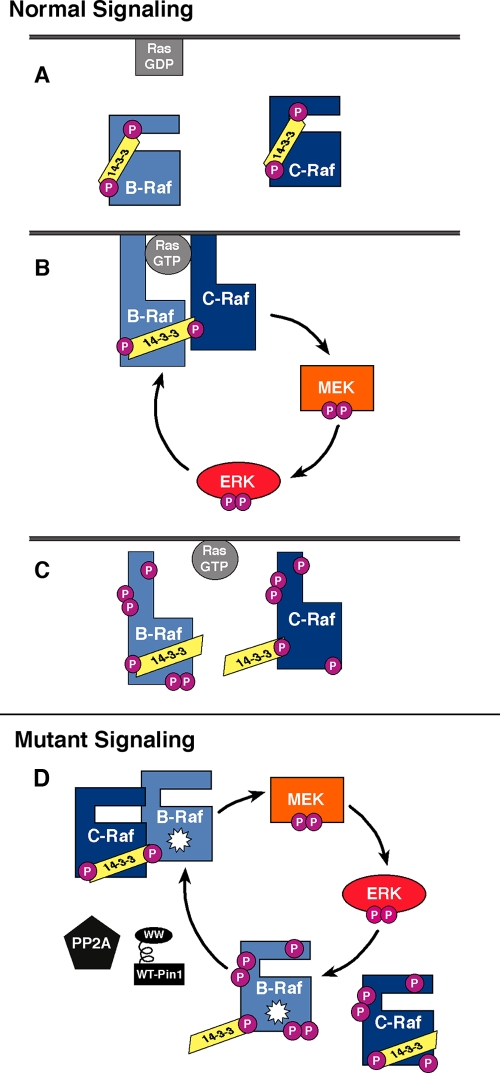
FIG. 9.
Model for the effect of feedback phosphorylation and heterodimerization on normal and mutant B-Raf signaling. (A) Prior to growth factor stimulation, RasGDP is at the membrane, and inactive B-Raf and C-Raf are in the cytosol bound by 14-3-3. (B) Growth factor stimulation promotes the conversion of Ras to its activated GTP-bound form, which recruits B-Raf and C-Raf to the plasma membrane, where they heterodimerize in a manner dependent on 14-3-3 binding to their C-terminal binding sites. The Raf proteins become activated and initiate the phosphorylation cascade that results in MEK and ERK activation. Active ERK phosphorylates numerous targets, including the Raf proteins. (C) Under normal signaling conditions, ERK-dependent feedback phosphorylation inhibits the binding of both B-Raf and C-Raf to RasGTP and disrupts B-Raf-C-Raf heterodimers, thus downmodulating Raf signaling. The hyperphosphorylated C-Raf and B-Raf proteins are subsequently dephosphorylated and returned to a signaling-competent state through the activities of the PP2A phosphatase and the Pin1 prolyl-isomerase (not shown) (D) Mutationally activated Raf proteins constitutively heterodimerize in the cytosol and promote constitutive ERK activation. ERK-dependent feedback phosphorylation of the mutant Raf proteins disrupts heterodimerization. The feedback-phosphorylated Raf proteins are dephosphorylated in a manner requiring PP2A and Pin1, which then permits heterodimers to reassemble.
Through mutational analysis, we find that feedback phosphorylation disrupts the abilities of B-Raf to bind activated Ras and to heterodimerize with C-Raf. Although phosphorylation of the S151 site appears to have the greatest effect on Ras binding, our results indicate that phosphorylation of all the feedback sites contributes to the inhibition of C-Raf binding. This finding is consistent with those of peptide binding studies conducted by Rushworth et al. (27) indicating that there are multiple points of contact between heterodimerized B-Raf and C-Raf proteins. Interestingly, these peptide binding studies also indicate that homodimerized B-Raf and C-Raf proteins have multiple contact points (27), suggesting that feedback phosphorylation of the Raf proteins may disrupt Raf homodimers as well.
Previous studies have shown that the C-terminal 14-3-3 binding site of C-Raf, S621, must be intact in order for B-Raf and C-Raf to heterodimerize (11, 27). Here we find that the analogous 14-3-3 binding site on B-Raf, S729, is highly phosphorylated in cells and is also required for normal and oncogenic B-Raf proteins to heterodimerize with C-Raf. Moreover, we find that the increased heterodimerization observed when the B-Raf feedback sites are mutated is abolished when the S729A site is also mutated. Taken together, these findings suggest a model whereby the binding of a 14-3-3 dimer to the C-terminal pS621 site of C-Raf and the C-terminal pS729 site of B-Raf provides the stable docking event that then allows the two proteins to make additional contacts (Fig. 9). As a result, feedback phosphorylation on multiple sites throughout both the C-Raf and B-Raf proteins may be required to disrupt their interaction.
In the context of oncogenic B-Raf signaling, we find that feedback phosphorylation and heterodimerization with C-Raf can impact the transformation potential of certain oncogenic B-Raf proteins. More specifically, we find that mutation of the feedback sites, which increases Raf heterodimerization, or mutation of the S729A site, which prevents Raf heterodimerization, had a significant effect on the transformation potential of B-Raf proteins with intermediate or impaired kinase activity but had no effect on the high-kinase-activity V600E and G469A B-Raf proteins. Further analysis of G466A B-Raf, an intermediate-activity protein, revealed that mutation of individual feedback sites throughout the protein increased its transforming activity in a manner that correlated with its ability to bind and activate C-Raf. Moreover, the effect of preventing feedback phosphorylation was abolished when the S729A site was mutated, providing further support for the model that the increased oncogenic activity of the FBm proteins was related to their increased ability to heterodimerize with and activate C-Raf.
Perhaps not surprisingly, given that the oncogenic B-Raf proteins are subject to feedback phosphorylation, they also are dephosphorylated in a manner requiring PP2A and Pin1 (Fig. 9D). In addition, we found that the transforming potential of intermediate- and impaired-activity B-Raf proteins, whose oncogenic potential was increased by mutation of the feedback sites, could be altered by overexpression of Pin1. More specifically, the oncogenic activity of these proteins was increased in cells overexpressing WT Pin1, whereas their transforming activity was reduced in cells that overexpress a dominant negative Pin1 protein, which binds substrates but lacks isomerase activity. Pin1 is known to participate in the dephosphorylation of other proteins that might impact oncogenesis, such as Cdc25C and Myc; however, in these experiments, it appears that the effect of Pin1 overexpression is related to the ability of Pin1 to facilitate the PP2A-mediated dephosphorylation of the feedback pS/TP sites. In particular, we found that overexpression of the Pin1 proteins alone did not induce transformation and that they had no effect on the transformation potential of G466A FBm-B-Raf, which lacks the feedback sites, or V600E B-Raf, whose function is not altered by feedback phosphorylation. The involvement of the Pin1 prolyl-isomerase in the dephosphorylation of the feedback sites may be of clinical relevance, given that Pin1 levels have been reported to be upregulated in numerous human cancers, including cancers where oncogenic B-Raf signaling has been implicated, such as melanoma, thyroid carcinoma, and NHL (1). Thus, in cancers such as NHL, where intermediate- and impaired-activity oncogenic B-Raf proteins have been detected (15), increased expression of Pin1 might aid in inducing cellular transformation.
Mutationally activated Raf proteins have also been associated with certain developmental disorders. For example, B-Raf mutations have been identified in patients with CFC syndrome (23, 26), and mutations in C-Raf have been isolated from patients with Noonan's and LEOPARD syndromes (24, 25). Like the oncogenic B-Raf proteins, we find that both B-Raf and C-Raf proteins containing mutations associated with these developmental disorders constitutively heterodimerize and that heterodimerization is increased when the feedback phosphorylation sites are mutated. Moreover, as was observed for the oncogenic B-Raf proteins with intermediate and impaired activity, we find that mutation of the feedback sites increased the transforming activity of these mutationally activated Raf proteins in a manner that correlated with increased Raf heterodimerization and ERK cascade activation.
In conclusion, our findings here, together with those of previous studies, indicate that both C-Raf and B-Raf are regulated by a feedback phosphorylation/dephosphorylation cycle. Importantly, perturbations in this cycle can impact the ability of mutationally activated B-Raf and C-Raf proteins to heterodimerize, which in turn can modulate their signaling activity. As a result, targeting of the regulatory machinery controlling these events may prove useful in certain cancers and disorders with constitutive Raf signaling.
Acknowledgments
We thank members of the Laboratory of Cell and Developmental Signaling for helpful discussions and Melissa McKay for critical reading of the manuscript.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Bao, L., A. Kimzey, G. Sauter, J. M. Sowadski, K. P. Lu, and D. G. Wang. 2004. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 164:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brummer, T., P. Martin, S. Herzog, Y. Misawa, R. J. Daly, and M. Reth. 2006. Functional analysis of the regulatory requirements of B-Raf and the B-Raf(V600E) oncoprotein. Oncogene 25:6262-6276. [DOI] [PubMed] [Google Scholar]
- 3.Brummer, T., H. Naegele, M. Reth, and Y. Misawa. 2003. Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene 22:8823-8834. [DOI] [PubMed] [Google Scholar]
- 4.Chong, H., H. G. Vikis, and K. L. Guan. 2003. Mechanisms of regulating the Raf kinase family. Cell. Signal. 15:463-469. [DOI] [PubMed] [Google Scholar]
- 5.Cutler, R. E., Jr., R. M. Stephens, M. R. Saracino, and D. K. Morrison. 1998. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl. Acad. Sci. U. S. A. 95:9214-9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon, A. S., and W. Kolch. 2002. Untying the regulation of the Raf-1 kinase. Arch. Biochem. Biophys. 404:3-9. [DOI] [PubMed] [Google Scholar]
- 7.Dhomen, N., and R. Marais. 2007. New insight into BRAF mutations in cancer. Curr. Opin. Genet. Dev. 17:31-39. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty, M. K., J. Muller, D. A. Ritt, M. Zhou, X. Z. Zhou, T. D. Copeland, T. P. Conrads, T. D. Veenstra, K. P. Lu, and D. K. Morrison. 2005. Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17:215-224. [DOI] [PubMed] [Google Scholar]
- 9.Emuss, V., M. Garnett, C. Mason, and R. Marais. 2005. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 65:9719-9726. [DOI] [PubMed] [Google Scholar]
- 10.Favre, B., P. Turowski, and B. A. Hemmings. 1997. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 272:13856-13863. [DOI] [PubMed] [Google Scholar]
- 11.Garnett, M. J., S. Rana, H. Paterson, D. Barford, and R. Marais. 2005. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell 20:963-969. [DOI] [PubMed] [Google Scholar]
- 12.Hagemann, C., and U. R. Rapp. 1999. Isotype-specific functions of Raf kinases. Exp. Cell Res. 253:34-46. [DOI] [PubMed] [Google Scholar]
- 13.Hmitou, I., S. Druillennec, A. Valluet, C. Peyssonnaux, and A. Eychene. 2007. Differential regulation of B-raf isoforms by phosphorylation and autoinhibitory mechanisms. Mol. Cell. Biol. 27:31-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konig, S., B. Guibert, C. Morice, P. Vernier, and J. V. Barnier. 2001. Phosphorylation by PKA of a site unique to B-Raf kinase. C. R. Acad. Sci. III 324:673-681. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J. W., N. J. Yoo, Y. H. Soung, H. S. Kim, W. S. Park, S. Y. Kim, J. H. Lee, J. Y. Park, Y. G. Cho, C. J. Kim, Y. H. Ko, S. H. Kim, S. W. Nam, J. Y. Lee, and S. H. Lee. 2003. BRAF mutations in non-Hodgkin's lymphoma. Br. J. Cancer 89:1958-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, K. P., and X. Z. Zhou. 2007. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 8:904-916. [DOI] [PubMed] [Google Scholar]
- 17.Marais, R., Y. Light, H. F. Paterson, C. S. Mason, and C. J. Marshall. 1997. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J. Biol. Chem. 272:4378-4383. [DOI] [PubMed] [Google Scholar]
- 18.Mason, C. S., C. J. Springer, R. G. Cooper, G. Superti-Furga, C. J. Marshall, and R. Marais. 1999. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18:2137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay, M. M., and D. K. Morrison. 2007. Integrating signals from RTKs to ERK/MAPK. Oncogene 26:3113-3121. [DOI] [PubMed] [Google Scholar]
- 20.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, D. K., G. Heidecker, U. R. Rapp, and T. D. Copeland. 1993. Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem. 268:17309-17316. [PubMed] [Google Scholar]
- 22.Muslin, A., J. Tanner, P. Allen, and A. Shaw. 1996. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84:889-897. [DOI] [PubMed] [Google Scholar]
- 23.Niihori, T., Y. Aoki, Y. Narumi, G. Neri, H. Cave, A. Verloes, N. Okamoto, R. C. Hennekam, G. Gillessen-Kaesbach, D. Wieczorek, M. I. Kavamura, K. Kurosawa, H. Ohashi, L. Wilson, D. Heron, D. Bonneau, G. Corona, T. Kaname, K. Naritomi, C. Baumann, N. Matsumoto, K. Kato, S. Kure, and Y. Matsubara. 2006. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat. Genet. 38:294-296. [DOI] [PubMed] [Google Scholar]
- 24.Pandit, B., A. Sarkozy, L. A. Pennacchio, C. Carta, K. Oishi, S. Martinelli, E. A. Pogna, W. Schackwitz, A. Ustaszewska, A. Landstrom, J. M. Bos, S. R. Ommen, G. Esposito, F. Lepri, C. Faul, P. Mundel, J. P. Lopez Siguero, R. Tenconi, A. Selicorni, C. Rossi, L. Mazzanti, I. Torrente, B. Marino, M. C. Digilio, G. Zampino, M. J. Ackerman, B. Dallapiccola, M. Tartaglia, and B. D. Gelb. 2007. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat. Genet. 39:1007-1012. [DOI] [PubMed] [Google Scholar]
- 25.Razzaque, M. A., T. Nishizawa, Y. Komoike, H. Yagi, M. Furutani, R. Amo, M. Kamisago, K. Momma, H. Katayama, M. Nakagawa, Y. Fujiwara, M. Matsushima, K. Mizuno, M. Tokuyama, H. Hirota, J. Muneuchi, T. Higashinakagawa, and R. Matsuoka. 2007. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat. Genet. 39:1013-1017. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Viciana, P., O. Tetsu, W. E. Tidyman, A. L. Estep, B. A. Conger, M. S. Cruz, F. McCormick, and K. A. Rauen. 2006. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science 311:1287-1290. [DOI] [PubMed] [Google Scholar]
- 27.Rushworth, L. K., A. D. Hindley, E. O'Neill, and W. Kolch. 2006. Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 26:2262-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubbert, S., K. Shannon, and G. Bollag. 2007. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7:295-308. [DOI] [PubMed] [Google Scholar]
- 29.Therrien, M., N. R. Michaud, G. M. Rubin, and D. K. Morrison. 1996. KSR modulates signal propagation within the MAPK cascade. Genes Dev. 10:2684-2695. [DOI] [PubMed] [Google Scholar]
- 30.Tran, N. H., X. Wu, and J. A. Frost. 2005. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J. Biol. Chem. 280:16244-16253. [DOI] [PubMed] [Google Scholar]
- 31.Wan, P. T., M. J. Garnett, S. M. Roe, S. Lee, D. Niculescu-Duvaz, V. M. Good, C. M. Jones, C. J. Marshall, C. J. Springer, D. Barford, and R. Marais. 2004. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855-867. [DOI] [PubMed] [Google Scholar]
- 32.Wellbrock, C., M. Karasarides, and R. Marais. 2004. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5:875-885. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe, M. B., M. Schutkowski, M. Shen, X. Z. Zhou, P. T. Stukenberg, J. U. Rahfeld, J. Xu, J. Kuang, M. W. Kirschner, G. Fischer, L. C. Cantley, and K. P. Lu. 1997. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278:1957-1960. [DOI] [PubMed] [Google Scholar]