Abstract
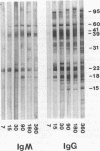
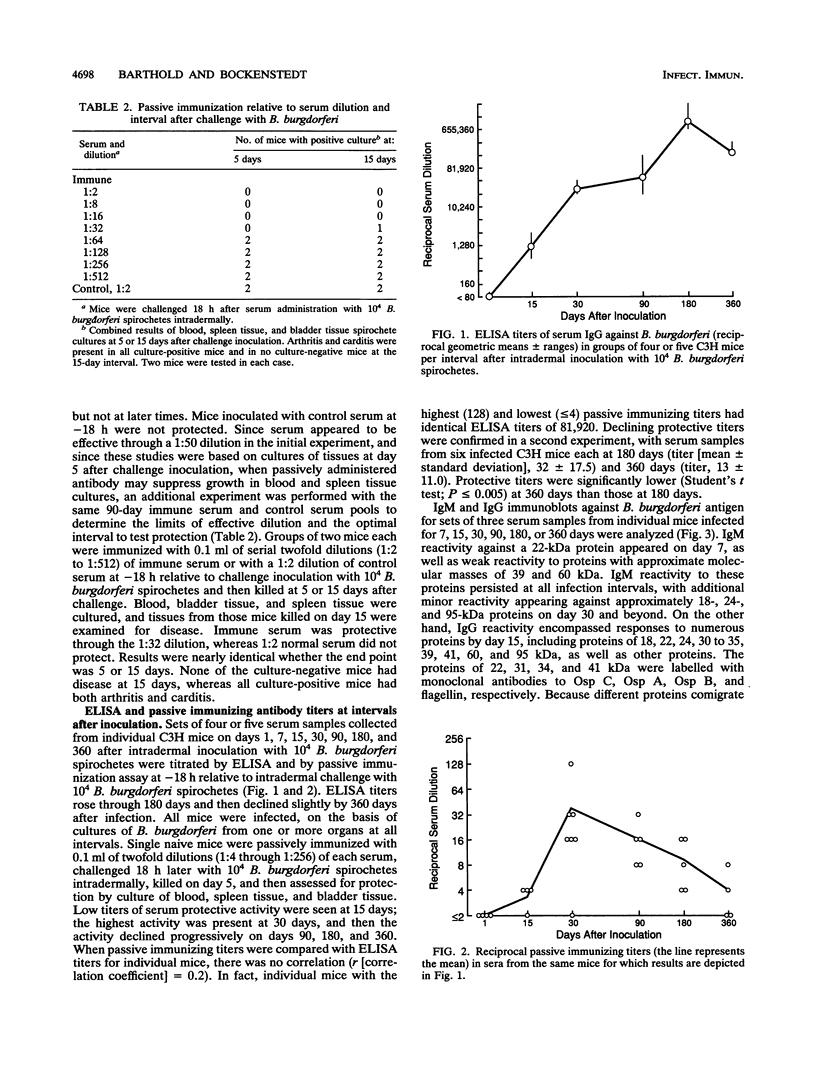
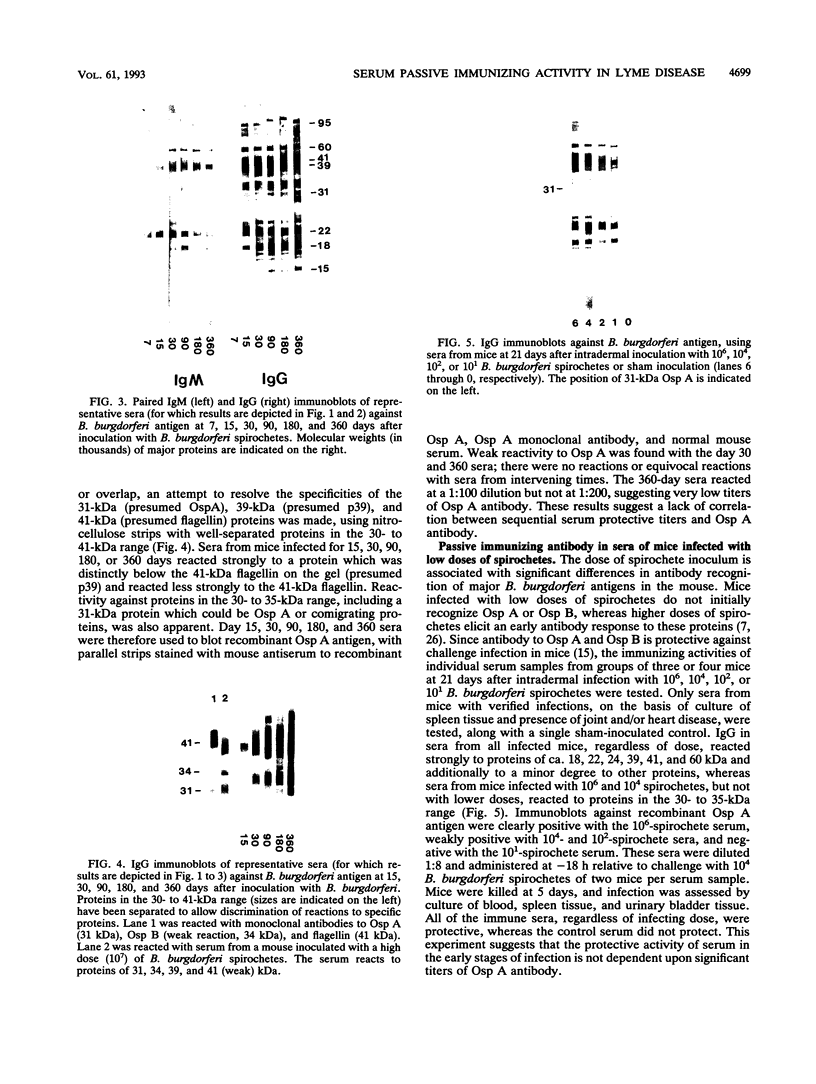
A single injection of serum from C3H mice at 90 days after intradermal inoculation with 10(4) Borrelia burgdorferi spirochetes protected naive mice when administered subcutaneously at -18 h relative to intradermal challenge inoculation with 10(4) B. burgdorferi spirochetes. When immune serum was given at intervals (-18, 0, +24, +48, and +96 h) relative to intradermal challenge with 10(4) B. burgdorferi spirochetes, it was protective if given before or at the time of challenge but not at later times. Protection with 90-day serum given at -18 h was effective at dilutions up to 1:32, but not 1:64, on the basis of culture or disease at either 5 or 15 days after challenge. Passive immunizing activity was also present in sera from mice at 21 days after intradermal challenge with 10(4), 10(2), or 10(1) spirochetes, indicating that the immunizing component was not dose dependent and probably not related to antibody to outer surface protein A. Passive immunizing titers of sera from mice at days 1, 15, 30, 90, 180, and 360 after intradermal B. burgdorferi inoculation appeared as early as day 15, were highest on day 30, and then declined progressively on days 90, 180, and 360. Immunizing titers of sera from mice at 360 days after intradermal B. burgdorferi inoculation were identical in passively immunized mice challenged with the original inoculum or with B. burgdorferi isolated at 360 days after inoculation, suggesting that there was no antigenic discrimination between the original inoculum and late isolates. These results suggest that protective antibody is produced early in the course of B. burgdorferi infection and is unrelated to antibody to outer surface protein A. In addition, the decline of protective serum titers over time despite persistent infection suggests that the antigens eliciting the protective response are either modified or suppressed, but antigenic modification could not be demonstrated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. F., Duray P. H., Magnarelli L. A. Prevalence of Borrelia burgdorferi in white-footed mice and Ixodes dammini at Fort McCoy, Wis. J Clin Microbiol. 1987 Aug;25(8):1495–1497. doi: 10.1128/jcm.25.8.1495-1497.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel M. J., Allan S., Jacobson R. H., Lauderdale T. L., Chang Y. F., Shin S. J., Thomford J. W., Todhunter R. J., Summers B. A. Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis. 1993 Mar;167(3):651–664. doi: 10.1093/infdis/167.3.651. [DOI] [PubMed] [Google Scholar]
- Armstrong A. L., Barthold S. W., Persing D. H., Beck D. S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992 Aug;47(2):249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- Asbrink E., Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):161–163. doi: 10.1111/j.1699-0463.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991 Feb;163(2):419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Persing D. H., Armstrong A. L., Peeples R. A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991 Aug;139(2):263–273. [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Sidman C. L., Smith A. L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992 Nov;47(5):605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., de Souza M. S., Janotka J. L., Smith A. L., Persing D. H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993 Sep;143(3):959–971. [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Barthold S., Deponte K., Marcantonio N., Kantor F. S. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect Immun. 1993 May;61(5):2104–2107. doi: 10.1128/iai.61.5.2104-2107.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister S. M., Schell R. F., Case K. L., Lovrich S. D., Day S. P. Characterization of the borreliacidal antibody response to Borrelia burgdorferi in humans: a serodiagnostic test. J Infect Dis. 1993 Jan;167(1):158–164. doi: 10.1093/infdis/167.1.158. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Lade B. N., Barbour A. G. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr Purif. 1990 Nov;1(2):159–168. doi: 10.1016/1046-5928(90)90011-m. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Johnson R. C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Proc Soc Exp Biol Med. 1986 Feb;181(2):263–269. doi: 10.3181/00379727-181-42251. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Marcantonio N., Deponte K., Kantor F. S., Flavell R. A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992 Feb;60(2):657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Jauris S., Lottspeich F., Preac-Mursic V., Wilske B., Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992 Feb;6(4):503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Gern L., Schaible U. E., Simon M. M. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis. 1993 Apr;167(4):971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Luft B. J., Munoz P., Dattwyler R. J., Gorevic P. D. Cross-antigenicity between the major surface proteins (ospA and ospB) and other proteins of Borrelia burgdorferi. J Immunol. 1990 Jan 1;144(1):284–289. [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M., Duray P. H. Experimental infection of the hamster with Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:258–263. doi: 10.1111/j.1749-6632.1988.tb31859.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986 Sep;53(3):713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody K. D., Barthold S. W., Terwilliger G. A., Beck D. S., Hansen G. M., Jacoby R. O. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg. 1990 Feb;42(2):165–174. doi: 10.4269/ajtmh.1990.42.165. [DOI] [PubMed] [Google Scholar]
- Preac Mursic V., Patsouris E., Wilske B., Reinhardt S., Gross B., Mehraein P. Persistence of Borrelia burgdorferi and histopathological alterations in experimentally infected animals. A comparison with histopathological findings in human Lyme disease. Infection. 1990 Nov-Dec;18(6):332–341. doi: 10.1007/BF01646399. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Weber K., Pfister H. W., Wilske B., Gross B., Baumann A., Prokop J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. 1989 Nov-Dec;17(6):355–359. doi: 10.1007/BF01645543. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Piesman J., Hunt A. R., Keen M. G., Happ C. M., Johnson B. J. The hamster immune response to tick-transmitted Borrelia burgdorferi differs from the response to needle-inoculated, cultured organisms. J Immunol. 1992 Dec 1;149(11):3648–3653. [PubMed] [Google Scholar]
- Schaible U. E., Gern L., Wallich R., Kramer M. D., Prester M., Simon M. M. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993 May;36(2):219–226. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Lovrich S. D., Callister S. M., Schell R. F. Depletion of complement and effects on passive transfer of resistance to infection with Borrelia burgdorferi. Infect Immun. 1991 Oct;59(10):3815–3818. doi: 10.1128/iai.59.10.3815-3818.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Callister S. M., Lovrich S. D., Day S. P., Coe J. E. Immunoglobulin G2 confers protection against Borrelia burgdorferi infection in LSH hamsters. Infect Immun. 1992 Jul;60(7):2677–2682. doi: 10.1128/iai.60.7.2677-2682.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Hejka A. G., England D. M. Passive immunization prevents induction of Lyme arthritis in LSH hamsters. Infect Immun. 1990 Jan;58(1):144–148. doi: 10.1128/iai.58.1.144-148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Lovrich S. D., Callister S. M., Coe J. E. Characterization of the protective antibody response to Borrelia burgdorferi in experimentally infected LSH hamsters. Infect Immun. 1991 Jun;59(6):1916–1921. doi: 10.1128/iai.59.6.1916-1921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears J. E., Fikrig E., Nakagawa T. Y., Deponte K., Marcantonio N., Kantor F. S., Flavell R. A. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991 Sep 15;147(6):1995–2000. [PubMed] [Google Scholar]
- Simpson W. J., Burgdorfer W., Schrumpf M. E., Karstens R. H., Schwan T. G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991 Feb;29(2):236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Schrumpf M. E., Schwan T. G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990 Jun;28(6):1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snydman D. R., Schenkein D. P., Berardi V. P., Lastavica C. C., Pariser K. M. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med. 1986 Jun;104(6):798–800. doi: 10.7326/0003-4819-104-6-798. [DOI] [PubMed] [Google Scholar]
- Stanek G., Klein J., Bittner R., Glogar D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med. 1990 Jan 25;322(4):249–252. doi: 10.1056/NEJM199001253220407. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Zöller L., Burkard S., Schäfer H. Validity of western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991 Jan;29(1):174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]