Abstract
The mechanisms by which epithelial cells regulate clathrin-mediated endocytosis (CME) of transferrin are poorly defined and generally viewed as a constitutive process that occurs continuously without regulatory constraints. In this study, we demonstrate for the first time that endocytosis of the transferrin receptor is a regulated process that requires activated Src kinase and, subsequently, phosphorylation of two important components of the endocytic machinery, namely, the large GTPase dynamin 2 (Dyn2) and its associated actin-binding protein, cortactin (Cort). To our knowledge these findings are among the first to implicate an Src-mediated endocytic cascade in what was previously presumed to be a nonregulated internalization process.
Iron is an essential element for all mammalian organisms that plays essential roles in hemoglobin and myoglobin production (23). Altered iron transport can lead to disease states such as hemochromatosis (23), anemia (5, 23), and neuronal disorders (23). The transferrin receptor (TfR) is an important component of iron regulation in cells. There are two distinct TfRs in humans sharing 45% identity that are homodimeric and bind iron-associated transferrin (Tf) at markedly different affinities (26). While significant attention has been paid toward understanding the basic endocytic machinery that supports the efficient internalization and recycling of the TfR1 and its associated iron-bound ligand, it has been assumed that this transport process is constitutive in nature. This is in direct contrast to the highly regulated internalization pathway used by members of the receptor tyrosine kinase family (RTKs) and the family of G-coupled protein receptors (GPCRs) that utilize phosphorylation and/or ubiquination as signaling modules to regulate internalization.
To test if TfR1 internalization might be regulated in a similar fashion, we focused on two essential components of the endocytic machinery: the large GTPase Dyn2 that mediates endocytic vesicle scission (35) and Cort that binds to Dyn2 via an SH3-PRD interaction and has been postulated to regulate actin dynamics to facilitate vesicle invagination and release (36, 40). Both Dyn2 and Cort have shown to be phosphorylated in vivo and in vitro by a variety of kinases (51, 58). Dyn1 interacts with (17) and is phosphorylated by Src in neuronal cells and in other excitable cells in response to activation of GPCRs and epidermal growth factor (EGF) (1, 2). While the Src phosphorylation motifs of dynamin are conserved in the epithelial expressed form of Dyn2, it is unclear if Dyn2 is phosphorylated in response to ligands that induce clathrin-based endocytosis.
Cort possesses a series of C-terminal tyrosines that are heavily Src-phosphorylated and implicated in regulating actin remodeling during cell motility (20). In this study, we demonstrate that addition of Tf to cultured epithelial cells results in an internalization of the TfR1 mediated by a Src kinase-dependent phosphoactivation of the Dyn2-Cort-based endocytic machinery. In support of these findings, dominant negative forms of c-Src kinase, when expressed in a hepatocyte-derived cell line (Clone 9), attenuate Tf internalization. Remarkably, cells exposed to Tf showed a 3- to 4-fold increase in Dyn2 and Cort phosphorylation compared to that shown by untreated cells, an increase exceeding that observed in cells treated with EGF. These findings provide new insights into the regulation of what was thought to be a constitutive endocytic process.
MATERIALS AND METHODS
Reagents and antibodies.
The anti-Dyn2 and the antipandynamin (MC63) antibodies were generated in rabbits and affinity-purified as described previously (21, 22). An anticlathrin heavy-chain monoclonal antibody (X-22) was obtained from ATCC (Rockville, MD). The anti-Cort AB3 and C-Tyr antibodies were generated by our lab and described previously (8). The Cort monoclonal antibody (4F11) was purchased from Upstate Biotechnology (Lake Placid, NY). The anti-Src (sc-18) antibody was purchased from Santa Cruz Biotechnology (San Diego, CA); the c-Src monoclonal antibody (327) was a gift from Joan S. Brugge (Harvard Medical School, Boston, MA). The phospho-Src family antibodies pY416 and pY418 were purchased from Cell Signaling Technology (Danvers, MA) and Biosource (Camarillo, CA), respectively. The phosphotyrosine pY20 was purchased from BD Transduction Laboratories (San Jose, CA), and anti-phosphotyrosine clone 4G10 was purchased from Millipore (Temecula, CA). The anti-TfR1-N antibody was raised against the peptide sequence QVDGDNSHVEMKLAADEEENADSNMKASVRKPKRFNG corresponding to amino acids 20 to 56 in full-length rat TfR1. The anti-TfR1-C antibody was raised against the peptide TSRLTTDFHNAEKTNRFV corresponding to amino acids 646 to 663 in full-length rat TfR1. The monoclonal antibody against TfR1 was purchased from Zymed Laboratories (San Francisco, CA). Goat anti-rabbit or goat anti-mouse secondary antibodies conjugated to Alexa-488 or -594 were from Invitrogen (Carlsbad, CA). Alexa-488- or -594-conjugated Tf for ligand uptake assays was from Invitrogen (Carlsbad, CA) and apo-transferrin (ATF) was purchased from ProSpec (Rehovot, Israel). All other chemicals and reagents unless otherwise stated were from Sigma (St. Louis, MO).
Cell culture and transfection.
Clone 9 cells, an epithelial cell line isolated from normal rat liver (ATCC CRL-1439; Rockville, MD), were maintained in Ham's F-12K medium. FR cells, a fibroblast cell line isolated from normal rat skin cells (ATCC CRL-1213), were maintained in Dulbecco's modification of Eagle's medium (DMEM). Mouse embryo fibroblast (MEF) cells were a gift from Andras Kapus (St. Michael's Hospital, Toronto, Canada) and were maintained in DMEM. SYF cells, an embryonic fibroblast cell line isolated from Src, Yes, and Fyn knockout mice (ATCC CRL-2459), were maintained in DMEM. The v-Src-ts MDCK cell line was a gift from Yasuyuki Fujita but was produced by Robert Friis's lab (Switzerland) and was maintained in DMEM. All media were supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin, in 5% CO2/95% air at 37°C. v-Src-ts MDCK cells are normally cultured at 40.5°C (restrictive temperature), and switched to 35°C (permissive temperature) to activate v-Src. Transfection was performed using either Lipofectamine Plus reagent kit or Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA), and Dyn2 siRNA treatment was performed as described previously (6). For TfR1, small interfering RNA (siRNA) transfection was performed with Lipofectamine RNAiMAX according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
Plasmid construction.
Green fluorescent protein (GFP)/red fluorescent protein (RFP)-tagged and untagged Dyn2 and Cort wild-type (WT) constructs were generated as previously described (7, 8). The Dyn2(aa) Y231F, Y597F, or Y231+597F point mutants, as well as a Cort B mutant (M3YF) bearing point mutations on three tyrosine residues (Y384, 429, 445F), were generated using PCR-based mutagenesis methods. The untagged c-Src WT was designed using oligonucleotide primers specific for c-Src according to the rat c-Src sequences from GenBank (accession numbers AF130457 and AF157016): c-Src 5′, ATGGGCAGCAACAAGAGCAAGCCCAAGG; c-Src 3′, CTATAGGTTCTCCCCGGGCTGGTACT. Full-length cDNA was amplified by long-distance reverse transcription-PCR (RT-PCR; Applied Biosystems, Branchburg, NJ), and the PCR fragments were ligated into the eukaryotic expression vector pCR3.1 (Invitrogen, Carlsbad, CA). Kinase-dead c-Src K297M, inactive c-Src Y419F, and active c-Src Y530F mutants were generated using PCR-based mutagenesis methods. All DNA constructs were verified by restriction enzyme digestion and sequence analysis. All plasmids were purified either by equilibrium centrifugation in a CsCl-ethidium bromide gradient (7) or with the Qiagen plasmid maxikit (Qiagen, Valencia, CA).
siRNA oligonucleotides.
siRNA oligonucleotides targeting four different regions of Dyn2 were purchased from Dharmacon Research (Lafayette, CO) and pooled before being transfected in order to enhance the reduction of Dyn2 protein levels. The target sequences and transfection methods have been described previously (6). siRNA oligonucleotides targeting four different regions of TfR (J-055550) were purchased from Dharmacon Research (Lafayette, CO) and pooled before transfection. The TfR target sequences utilized were (i) CACUAAGGGUGUACGUAAU, (ii) AUGAGGAACCAGACCGUUA, (iii) GGAUAUGGGUCUAAGUCUA, and (iv) CGGCAAGUAGAUGGAGAUA.
Tf uptake assay and Src kinase inhibitor studies.
Endocytosis of fluorescently conjugated Tf was assayed as previously described (20). For studies that tested Src inhibitors' effects on Tf internalization, MEF and Clone 9 cells were serum starved for 20 min and incubated in PP2 or SU6656 drug-containing media (Calbiochem, La Jolla, CA) at various concentrations (0, 5, 10, 20, and 40 μM) for 2 h prior to addition of 10 μg/ml of Alexa-594-conjugated Tf in drug-containing media for 20 min. Following a mild acid wash to removed surface-bound Tf, the cells were fixed and quantitated by fluorescence microscopy as described previously (20).
IP and blotting.
MEF cells were serum starved for 16 h using either serum-free or low-serum media (0.2% fetal bovine serum [FBS]) and then stimulated with Tf (10 μg/ml) at different time points (0 to 20 min), lysed, and sonicated. Cell debris was removed by centrifugation. The remaining cell lysate was aliquoted into equal amounts of protein for each immunoprecipitation (IP) to be performed, precleared with protein A Sepharose beads, and incubated with 4 μg of the appropriate antibodies (anti-Src [sc-18], anti-Cort [C-Tyr or AB3], anti-Dyn2 or Pan-Dyn [MC63], and anti-TfR1). Samples were incubated at 4°C for 2 h before adding protein A Sepharose beads for an additional hour. After extensive washing, samples were eluted in Laemmli sample buffer and analyzed by Western blot (WB) analysis as described previously (7), using antibodies against Dyn2, MC63, Cort (4F11, C-Tyr, or AB3), TfR1 (mono- or poly-TfR1), pY20, 4G10, p-Src Y416/Y418, and anti-c-Src (327).
Biotin binding assay and quantitation.
For biotinylation of surface TfR, MEF or Clone 9 cells were serum starved for 16 h and then stimulated with 10 μg/ml Tf for 0, 2, and 10 min (5 μg/ml bovine serum albumin [BSA] for control) at 37°C. To stop endocytosis, cells were washed three times with cold phosphate-buffered saline (PBS) and then incubated with 0.5 mg/ml N-hydroxysuccinimide (NHS)-biotin in cold PBS for 10 min on ice. To remove unbound biotin, cells were rinsed three times with 50 mM NH4Cl in cold PBS. TfR was precipitated from lysates by using anti-TfR1 antibody and was subjected to WB analysis using antibiotin and anti-TfR1 antibodies. Quantitation was done with a GS-700 imaging densitometer and Molecular Analyst software (Bio-Rad, Hercules, CA).
Purification of recombinant proteins.
His-tagged Dyn2 and Cort WT and mutants were expressed in and purified from Escherichia coli M15 (pREP4) bacteria according to the manufacturer's instructions (Qiagen, Valencia, CA).
In vitro Src phosphorylation assay.
Purified recombinant Dyn2 or Cort WT and mutants were mixed with 7.22 μl Src Mg-ATP cocktail (49.5 μl of Src kinase reaction buffer [Upstate Biotechnology, Lake Placid, NY] with 19.8 μl of 2 mM ATP and 2.18 μl of [γ-32P]ATP at room temperature) and then added to 4.4 μl active Src N1 (Sigma, St. Louis, MO). Reaction mixtures were incubated at 30°C for 30 min. Phosphorylated dynamin and Cort were resolved by SDS-PAGE and visualized by autoradiography.
Microscopy and morphological quantitation of endocytosis and TIRF.
All cell images were acquired with an Axiovert 35 or 200M microscope (Carl Zeiss, Thornwood, NY) equipped with a 63×, 1.4 NA, oil immersion lens and a cooled charge-coupled-device (CCD) camera (Orca II or Orca II ERG; Hamamatsu, Bridgewater, NJ) by the use of IPLab software (Scanalytics, Fairfax, VA) at full resolution using the same acquisition settings (exposure time = 500 milliseconds; 16-bit grayscale). Fluorescence intensity was quantitated as described previously (6). The results presented represent the average fluorescence intensity units ± the standard error (SE) measured for 50 cells for each of up to four independent experiments. For total internal reflection fluorescence (TIRF)-based live cell imaging, transfected cells grown in glass-bottom, 35-mm cell culture dishes were imaged using a Zeiss 200M microscope equipped with a heating stage, atmosphere control, a TIRF module (excitation laser lines at 488 and 514 nm), a 100× 1.45-NA lens, a Zeiss Axiovision camera, and Axiovision software (Carl Zeiss, Thornwood, NY). Images were captured every 6 s for 10 min prior to 10 μg/ml Tf addition and for 20 min postaddition.
RESULTS
Activation of a Dyn2-Cort endocytic complex by addition of Tf ligand.
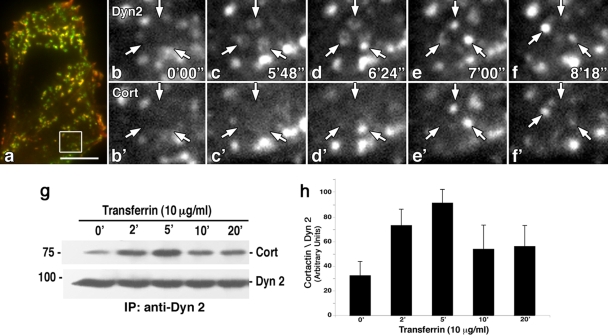
To test for a stimulatory effect of Tf on the endocytic process in epithelial cells, we performed both morphological and biochemical assays to follow corecruitment of Dyn2 and Cort to clathrin-coated pits. Cultured rat fibroblasts (FR) coexpressing Dyn2-GFP and Cort-RFP were serum starved for 16 h prior to addition of 10 μg/ml of Tf and observed by TIRF microscopy over a 15-min time period. TIRF microscopy is widely used to observe plasma membrane (PM) dynamics within 100 to 200 nm of the substrate (43). Interestingly, Dyn2-GFP formed numerous rings along the cell base that appeared to vesiculate and detach just minutes following Tf addition (Fig. 1a to f). Cort-RFP in turn associated with these putative endocytic buds just prior to this detachment process (Fig. 1a and b′ to f′). To support these morphological observations, cultured fibroblasts were treated with Tf and then lysed for Dyn2 IP followed by WB analysis for Cort (Fig. 1g and h). Importantly, we observed a transient 2- to 3-fold increase in the interaction between Dyn2 and Cort 2 to 5 min following Tf addition, a time frame that coincided with the images obtained by TIRF microscopy. This suggests that addition of Tf ligand leads to an assembly of the Dyn2-Cort endocytic complex.
FIG. 1.
Dyn2 and Cort are recruited sequentially to clathrin-coated pits in response to Tf addition. (a to f′) TIRF microscopy of live rat fibroblasts coexpressing Dyn2-GFP and Cort-RFP. (a) Low-magnification color image showing overlap of Dyn2-GFP and Cort-RFP in yellow. (b to f′) Higher-magnification images of select regions from the cells in panel a show numerous rings of Dyn2-GFP (arrows) that appear along the cell base. With no ligand added (time zero) (b and b′), no rings are present, but upon Tf addition, these rings appear, condense, and vesiculate prior to liberation from the PM (b to f). Cort-RFP is recruited to the Dyn2 ring structures just prior to the release of the Dyn2-GFP positive vesicle (arrows) (b′ to f′). Bar = 10 μm. (g) IP of Dyn2 from Tf-stimulated cultured cells shows an induced interaction with Cort. (h) Densitometric quantitation of three independent experiments as shown in panel g comparing the ratio of Cort to Dyn2 showing a 3-fold increase in association of Dyn2 and Cort by just 5 min following Tf addition. Results represent the average ± SE of three independent experiments.
As the addition of Tf ligand to cells appeared to stimulate Dyn2-Cort association at the PM, it became important to confirm that ligand addition resulted in the endocytic clearance of the TfR1 from the PM in a time frame consistent with the observations described in Fig. 1. To this end, MEF cells were treated with 10 μg/ml Tf at different time points, then fixed and prepared for immunofluorescence (IF) microscopy using an antibody to the extracellular domain of the TfR1. In serum-starved cells, the TfR1 was localized to numerous punctate spots along the cell base (Fig. 2a), while cells 5 to 20 min following Tf addition showed a marked (30 to 40%) reduction of receptor intensity at the cell surface (Fig. 2b to d). Biochemical experiments from these cells treated with Tf for different time periods followed by biotinylation of total surface receptors and IP of the TfR1 were supportive of the IF experiments and showed a progressive 20 to 30% clearance of the receptor from the cell surface by 5 to 10 min post-ligand addition (Fig. 2e and f). These findings demonstrate a ligand-induced internalization of the TfR1 that is synchronous with assembly of the endocytic machinery.
FIG. 2.
Addition of Tf induces rapid TfR1 internalization from the cell surface. (a to c) IF staining of MEF cells incubated in low-serum media and stained with an antibody to the extracellular domain of the TfR1. Cells fixed and stained after 0, 5, and 20 min following addition of 10 μg/ml Tf showed a markedly reduced surface staining (b and c). (d) Quantitative microscopy analysis of total cell fluorescence showing a 30 to 40% reduction in surface TfR1 following ligand addition at 2, 5, 10, 15, and 20 min post-ligand addition. Results represent the average ± SE of >60 cells measured in each of three independent experiments. (e) WB analysis of surface biotinylation assay results showing a reduction of the TfR1 levels at the PM following addition of Tf ligand. (f) Densitometric quantitation of the results for 3 independent experiments represented in panel e comparing the ratio of biotinylated TfR1 to total TfR1. A similar clearance of the TfR1 from the surface at 10 min post-ligand addition is observed in both IF and biochemical assays. α, anti; bar = 10 μm.
In an attempt to directly view the effects of Tf addition on the endocytic machinery, we followed the dynamics of GFP-tagged Dyn2 in Clone 9 cells by TIRF microscopy in live cells treated with 10 μg/ml ligand. In resting serum-starved cells, Dyn2 exhibited a punctate distribution along the cell base (Fig. 3a) that mimicked the pattern observed in cells stained with our Dyn2 antibody (not shown). Higher-magnification images revealed that these spots were a collection of largely static, tightly packed puncta. Addition of Tf to the cultured cells appeared to increase the formation of Dyn2-positive vesicles by 3 to 5 min that was maximized by 10 to 20 min (Fig. 3b to d). A detailed numerical counting of 5 different cells viewed 10 min before and 10 min following Tf addition showed a greater than 40% increase in Dyn2 vesicle formation subsequent to ligand addition (data not shown), supporting the concept that Tf induces endocytic clearance of the TfR1. As Dyn2 and Cort function are required for the internalization of Tf and its receptor in many cell types (61), we also utilized a biochemical approach by immunoprecipitating the TfR1 from MEFs at different times following ligand addition and subjected these to WB analysis for Dyn2 and Cort. As shown in Fig. 3e to g, there was a substantial (2- to 3-fold) increase in Dyn2 and Cort with the TfR1 receptor. While this interaction is presumed to be indirect through the association of components within the forming endocytic pit/vesicle, it does further support the novel concept of a rapid Tf ligand-induced recruitment of the endocytic machinery to the site of receptor internalization.
FIG. 3.
Tf addition stimulates an increase in vesicle budding from the PM. (a) A low-magnification TIRF microscopy image of living Clone 9 cells expressing Dyn2-GFP. A series of higher-magnification images of the boxed region in panel a are shown in panels b to d. Cells in the absence of Tf showed little, if any, Dyn2-GFP-based vesicle budding over the 10- to 20-min observation time (b). In contrast, addition of Tf (5 μg/ml) induced the formation of Dyn2 vesicles from the PM that continued over a 20-min time period (c and d). Statistical counts of this observed Dyn2-GFP vesicle formation within 10-μm by 10-μm boxes of 5 different cells viewed 10 min before and 10 min following Tf addition showed a >40% increase in Dyn2 vesicle formation subsequent to ligand addition. Bar = 10 μm. (e) IP of the TfR1 from MEFs showing an increase in associated Dyn2 and Cort minutes following Tf addition. (f and g) Densitometric quantitation of 3 independent experiments similar to that shown in panel e comparing the ratio of Dyn2 and Cort to TfR1. Results represent the average ± SE of 3 independent experiments.
Ligand binding by the TfR1 leads to stimulation of Src kinase cascades that activate endocytosis.
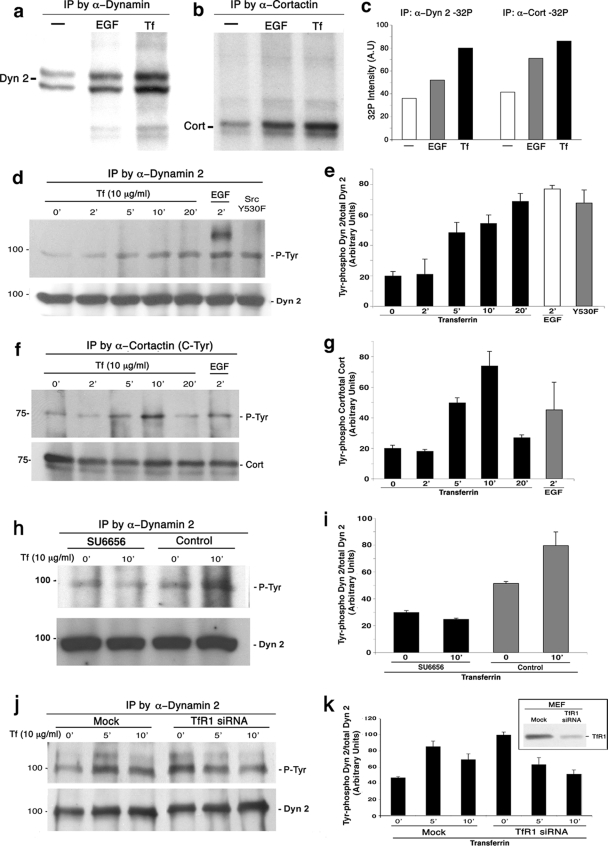
The observations described above support the premise that ligand binding to the TfR1 stimulates a deliberate recruitment of the Dyn2-Cort-based endocytic machinery to the sequestered receptor-ligand complex leading to its internalization. As is the case for many other receptor classes, ligand binding leads to an activation of a variety of different cellular kinase cascades, including Src kinase and autophosphorylation of the RTKs, such as EGFR, PDGFR (15, 41), and BARK (β-adrenergic receptor kinase), for some of the seven transmembrane GPCRs (3), to name just a few. However, few kinase cascades have been implicated in the internalization of the TfR1 in either fibroblasts or epithelial cells. To this end, we first conducted a general test for Tf-induced protein phosphorylation in cultured cells by using inorganic phosphate (32P) labeling of total cell protein followed by two-dimensional gel electrophoresis. In cultured Clone 9 cells treated with either carrier or 10 μg/ml of Tf for 10 min, we observed numerous phospho-protein spots that appeared in the ligand-treated cells (data not shown). Subsequently, specific proteins of interest were precipitated from these labeled cells to identify phospho-targets. Clone 9 cells were exposed to 0.2% BSA in phosphate-deficient medium (PDM) for 16 h, followed by 2 h of incubation in PDM including 0.1 mCi/ml of 32P. Two independent experiments were performed, in which cells were exposed to 5 μg/ml of Tf for 5 min, 30 ng/ml of EGF, or 10% FBS or remained in PDM as a control. Following these incubations, either the TfR1, Dyn2, or Cort was immunoprecipitated from cell lysates and separated by SDS-PAGE for subsequent autoradiography. No phosphorylation of the TfR1 was observed in control or ligand-treated cells (not shown), but interestingly, while both Dyn2 and Cort were modestly phosphorylated under serum-starved conditions, the inclusion of Tf in the cell culture media markedly increased 32P labeling of both Cort and Dyn2 >2-fold compared to that in serum-starved cells (Fig. 4a to c). This protein phosphorylation appeared to equal or exceed that observed in cells stimulated with 10% FBS or 30 ng/ml of EGF and could be markedly attenuated by incubating cells for 1 h in 10 μM of the Src family inhibitor SU6656 prior to ligand addition (Fig. 4h and i). It should be noted, however, that the molar ratio of the TfR to the EGFR in these cells is likely to be high; thus, this difference would be minimized if the receptor concentrations were normalized to each other. Nevertheless, the levels of Src, Dyn2, and Cort phosphorylation by stimulation of the TfR are noteworthy.
FIG. 4.
Dyn2 and Cort are tyrosine phosphorylated after Tf stimulation. Addition of Tf to cultured cells induces substantial phosphorylation of both Dyn2 and Cort. (a and b) Autoradiographs of Dyn2 or Cort precipitated from Clone 9 cells following incubation for 16 h in 0.2% BSA in PDM, including 32P for 2 h without stimulation or 5 min in 30 ng/ml of EGF, or 5 μg/ml Tf. (c) Quantitative scanning of the precipitated proteins revealed a 2- to 3-fold increase in phosphorylation following stimulation with Tf compared to resting cells. (d and f) MEF cells were stimulated with either Tf or EGF or expressed active c-Src Y530F protein as a positive control, at the indicated time points. Dyn2 (d) or Cort (f) was precipitated from cell lysates and analyzed by WB analysis for phosphotyrosine (pY20 antibody) and the precipitated protein. (e and g) Densitometric quantitation of the phosphotyrosine bands from 3 independent experiments, similar to those shown in panels d and f and normalized to the total levels of the respective protein. Tyrosine phosphorylation of both Dyn2 and Cort increases significantly (4- to 5-fold) following addition of Tf, compared to resting cells. This increase in tyrosine phosphorylation is at levels similar to, or even exceeding, that induced by stimulation with EGF. (h and i) The Src kinase inhibitor SU6656 prevents Tf-stimulated phosphorylation of Dyn2. MEF cells were serum starved and then treated with 20 μM SU6656 drug for 2 h prior to, and included with, stimulation with 10 μg/ml Tf for 20 min. Following Tf stimulation, cells were lysed, and Dyn2 was precipitated and analyzed by WB analysis with Y416 antibody. Pretreatment with the SU6656 drug completely prevented the Src-mediated phosphoactivation of Dyn2. Results represent the average ± SE of 3 independent experiments. α, anti.
As both Dyn2 and Cort appeared to be significantly phosphorylated in response to Tf ligand, we next tested whether this represented a tyrosine modification. MEF cells and rat primary hepatocytes (not shown) were treated with Tf, and the pTyr status of Cort and Dyn2 precipitated from these lysates was determined. As shown in Fig. 4d to g, both Dyn2 and Cort exhibited a substantial increase (3- to 4-fold) in tyrosine phosphorylation 10 to 20 min post-ligand addition, levels that mimicked those observed in cells treated with EGF or expressing a constitutively active Src protein (c-Src Y530F mutant). Importantly, this tyrosine phosphorylation of Dyn2 and Cort is concomitant with a ligand-stimulated activation of Src kinase, as shown later. To confirm that activation of the TfR alone was responsible for tyrosine phosphorylation of the endocytic machinery and did not result from a contaminant within the Tf ligand preparation, stimulating another unidentified signaling cascade, we performed experiments with the iron-free apo-Tf as the ligand. While the iron-bound ligand stimulated Dyn2 phosphorylation as before in 5 to 10 min, the apo-Tf ligand induced no such increase. In fact, Dyn2 phosphorylation was actually decreased by 70 to 80% over the same time period with the iron-free reagent (repeated three times) (data not shown).
There are two distinct TfR genes that are expressed in a tissue-specific manner. The TfR1 is ubiquitously expressed, while the TfR2 is found largely in hepatocytes and some hemopoietic cells (26, 55) and is believed to be trafficked differently than TfR1. To ensure that activation of the tyrosine phosphorylation cascade was a result of stimulating the higher-affinity TfR1 only, we first tested whether the liver-derived Clone 9 cells used in this study expressed both or only one TfR form. WB analysis using isoform specific antibodies to each receptor revealed a high expression of the TfR2 in rat liver homogenate and isolated hepatocytes but no expression in the Clone 9 cells (data not shown). Finally, to test that the observed Tf ligand-induced phosphorylation of Dyn2 is dependent upon activation of its receptor, cells were treated with siRNA to reduce TfR1 levels, and Dyn2 phospho-levels were measured. MEF cells treated with TfR siRNA for 72 h exhibited a 90% reduction in receptor levels as assessed by WB analysis (Fig. 4k). Mock-treated cells exhibited a 2- to 3-fold increase in Dyn2 phosphorylation following ligand addition. In contrast, siRNA-treated cells with reduced TfR expression exhibited higher Dyn2 phosphorylation under resting conditions, which actually decreased upon Tf stimulation (Fig. 4j and k).
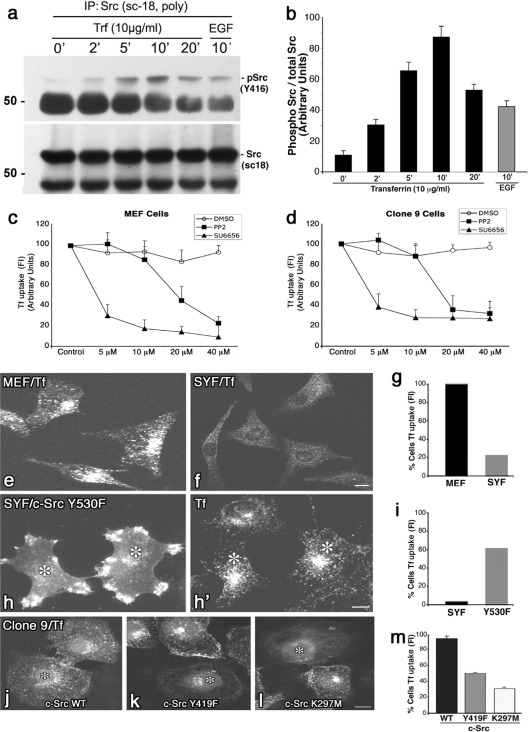
Because treatment of cells with physiological concentrations of Tf ligand appears to activate Src kinase (Fig. 5a and b), resulting in the internalization of the TfR1, it was important to test if inhibiting Src activity might attenuate endocytic uptake of this receptor. Several different approaches were utilized. First, we tested whether drugs known to inhibit Src family kinases, such as PP2 or SU6656, might attenuate Tf internalization. While previous studies have reported mixed results showing partial inhibition (61) or no inhibition (52) of internalization using PP2, we are not aware that any experiments have been performed with the more specific SU6656 inhibitor. To this end, we tested the effects of different concentrations of these inhibitors on Tf internalization in two different cell types (MEF and Clone 9). Serum-starved cells were incubated in vehicle alone or various increasing concentrations of PP2 or SU6656 for 2 h prior to addition of 10 μg/ml Alexa-594-Tf in drug-containing medium for 20 min. Cells were then fixed and acid washed, and the amount of internalized Tf was quantitated by fluorescence measurements. As shown in Fig. 5c and d, Tf internalization was reduced significantly (60 to 70%) at 20 to 40 μM PP2 in either cell type, while the SU6656 drug was even more efficacious and attenuated uptake by 70 to 80% at just 5 to 10 μM. This morphological observation correlates well with the finding that SU6656 reduces the Tf-stimulated phosphorylation of Dyn2 (Fig. 4h and i). Second, a cell line isolated from embryonic mice knocked out for Src, Yes, and Fyn (SYF cells) was tested for an ability to internalize Alexa-594-conjugated Tf. Normal control cells (MEF) take up this ligand readily, while the SYF triple knockout cells do not (Fig. 5e and f). Furthermore, “rescue” experiments performed on SYF cells reexpressing active c-Src Y530 protein showed that the c-Src Y530 protein-expressing cells were subsequently able to internalize Tf ligand to levels approaching that of wild-type cells (Fig. 5e, h, and h′), suggesting that c-Src activity plays an important role in this process. Third, in support of these findings, Clone 9 cells expressing an inactive form (11, 57) of c-Src (Y419F mutant) or the kinase-dead form K297M mutant (Fig. 5h and j) had significantly reduced levels of Tf internalization compared to those of cells expressing WT c-Src (Fig. 5k and l). Finally, an MDCK cell line expressing a temperature-sensitive mutant of v-Src was also utilized. At the restrictive temperature (40.5°C), the v-Src is misfolded and inactive, while shifting the cells to the permissive temperature (35°C) results in a refolding and, thus, activation of v-Src (4, 53) that has been shown to induce cell dissemination and motility (see Fig. S1a and d in the supplemental material). At the restrictive temperature, these cells internalize modest levels of Alexa-594-Tf. However, ligand internalization was increased dramatically (6-fold) upon a shift to 35°C (see Fig. S1b, e, and f in the supplemental material), further supporting an important role for Src kinase activity in regulating endocytosis of Tf and its receptor.
FIG. 5.
Active Src kinase is required for the endocytic uptake of Tf. (a and b) WB analysis and corresponding quantitation of activated Src precipitated from MEFs that were treated with Tf (10 μg/ml) over 0 to 20 min. Ligand addition induced a 7- to 10-fold activation of Src by 5 to 10 min. This activation exceeded that observed by EGF addition and occurs at a time corresponding to endocytic vesicle formation, and an increased physical interaction between the TfR1, Dyn2, and Cort. (c and d) Src inhibitory drugs reduce Tf internalization in MEF or Clone 9 cells. Serum-starved cells were incubated in vehicle alone or various increasing concentrations of PP2 or SU6656 for 2 h prior to addition of 10 μg/ml Alexa-594 Tf for 20 min. Cells were then fixed, and fluorescence was quantitated for Tf internalization. A marked reduction of Tf internalization was observed at low or moderate concentrations of either drug. Data are presented as means ± the standard deviation. (e and f) IF images of MEF control cells (e) or MEFs cultured from SYF−/− mice (f) that were incubated with Alexa-594-labeled Tf at 37°C for 20 min. While the control MEFs internalized substantial amounts of ligand, the SYF−/− cells internalized 80% less (g). Expression of an exogenous, active c-Src (Y530F) in the SYF−/− cells (h) rescued over 60% of the cells to internalize Alexa-594 Tf (h′ and i) compared to the untransfected cells. (j to m) Clone 9 cells that were incubated with Alexa-594-labeled Tf at 37°C for 20 min and then fixed and viewed by IF. Control cells (j) internalized substantially more Tf than did cells expressing inactive forms of c-Src, such as the c-Src Y419F mutant (k) or the c-Src K297M mutant (l) that were reduced by 50% and 70%, respectively (m). Asterisks represent transfected cells. Results represent the averages ± SE of >100 cells measured in each of 3 independent experiments. Bars = 10 μm.
Tyrosine phosphorylation of Dyn2 and Cort is essential for endocytic uptake of the TfR1.
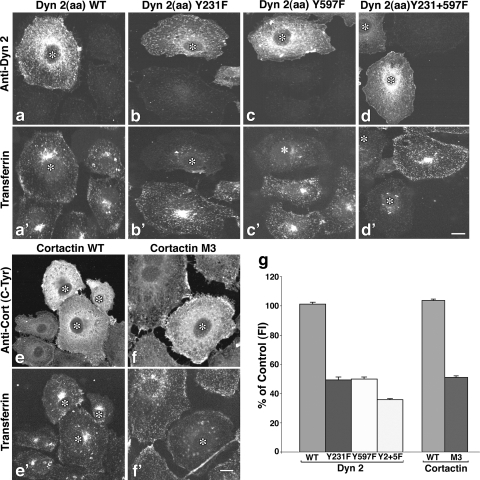
The observations described above indicate that Tf ligand binding to its receptor activates Src, leading to Dyn2 and Cort phosphorylation and their subsequent recruitment to the endocytic site. To extend these findings, we directly tested whether phosphorylation of these proteins is indeed important for supporting receptor-ligand internalization. It is well known that Dyn2 and Cort are required for clathrin-based uptake of Tf in epithelial cells (30, 37), but a role for phosphoactivation of these proteins in the endocytic process has not been tested. To examine this role, a series of Src phosphorylation-defective mutants of Dyn2 and Cort were made to the previously identified phosphorylation sites that include the Dyn1 Y231F, Y597F (2), Cort Y384F, Y429F, and Y445F (Cort M3YF) mutants (25) (see Fig. S2a and b in the supplemental material). Bacterially expressed Dyn2 and Cort were used in an in vitro Src phosphorylation assay to confirm that these mutant proteins were phosphorylation defective. As shown in Fig. S2c in the supplemental material, while both WT Dyn2 and Cort were significantly phosphorylated by adding Src kinase, little, if any, 32P was incorporated by the mutant proteins. These control experiments suggest that the mutant constructs utilized in this study are suitable to test the role of c-Src phosphorylation in living cells. As shown in Fig. 6a and e, cells expressing WT proteins internalized Tf at levels equal to those of surrounding untransfected cells (Fig. 6a′ and e′). In dramatic contrast to control cells, those expressing the c-Src phosphorylation-defective forms of Dyn2 and Cort showed a marked reduction in Tf uptake. Cells expressing the Dyn2 Y231F or Y597F mutant proteins (Fig. 6b, b′, c, c′, and g) exhibited a 50% reduction in internalized Tf, while cells expressing a Dyn2 double mutant showed a further reduction to 40% (Fig. 6d, d′, and g). Similarly, cells expressing the triple Y-F mutation (M3YF) of Cort (Fig. 6) also exhibited a 50% reduction in endocytic uptake of Tf compared to control cells (Fig. 6f, f′, and g).
FIG. 6.
Tyrosine phosphorylation-defective mutants of Dyn2 and Cort inhibit Tf uptake. (a to f′) Clone 9 cells expressing WT Dyn2 (a) or Cort (e) or mutants in which tyrosine residues previously shown to be sites of c-Src phosphorylation were altered (Dyn2 Y231F [b], Y597F [c], Y231+597F [d], and Cort Y384,429,445F [f] mutants) were incubated with Alexa-594-labeled Tf (a′ to f′) at 37°C for 20 min and analyzed by fluorescence microscopy. Transfected cells (*) were revealed by staining with antibodies against Dyn2 (a to d) or Cort (e and f). (g) As indicated by the images, quantitation of internalized Tf based on fluorescence intensity measurements taken from 3 independent experiments showed that inhibition of either Dyn2 or Cort tyrosine phosphorylation reduced Tf uptake by 50 to 70%. Results represent the average ± SE of >100 cells measured in each of three independent experiments. Bar = 10 μm.
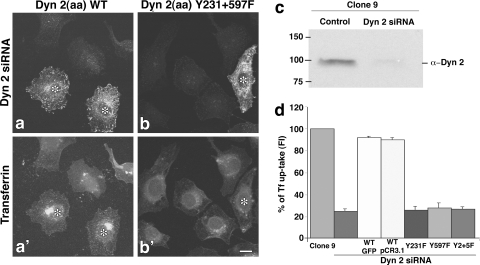
As the expression of dominant negative forms of Dyn2 significantly attenuated Tf internalization in Clone 9 cells, it was also important to extend our findings by attempting to rescue Dyn2-depleted cells by the reexpression of either the Dyn2 WT or phosphomutants. Clone 9 cells were treated with Dyn2 siRNA to reduce over 90% of endogenous protein levels (Fig. 7c) and then transfected to express either WT Dyn2 or phospho-defective mutants [Dyn2(aa) Y231F, Y597F, Y231+597F mutants] and challenged to internalize Tf. As predicted, Dyn2 siRNA-treated cells, rescued with exogenous WT Dyn2, internalized Tf 4- to 5-fold more than did the cells with reduced Dyn2 levels (Fig. 7a, a′, and d). Importantly, knockdown cells transfected with the phosphomutant Dyn2 showed no increase in Tf internalization compared to the Dyn2-depleted cells, indicating that the altered protein does not support clathrin-based uptake of this receptor (Fig. 7b, b′, and d).
FIG. 7.
Effects of Dyn2 tyrosine phosphomutants on Tf uptake in cells depleted of Dyn2. (a to b′) Clone 9 cells depleted of Dyn2 via siRNA and transfected to reexpress WT Dyn2 (a) or a Dyn2 tyrosine point mutant (Dyn2 Y231+597F [b]) were allowed to internalize Alexa-594-labeled Tf at 37°C for 20 min (a′ and b′). Dyn2 was stained (a and b) to detect the reexpressed WT or mutant (*) and analyzed by fluorescence microscopy. (c) WB analysis confirmed that Dyn2 protein levels were reduced by siRNA treatment by 90%. (d) Quantitation of Tf uptake based on fluorescence intensity measurements in control Clone 9 cells and Dyn2 siRNA-treated cells alone or following reexpression of the indicated constructs shows that Dyn2 tyrosine phosphomutants are unable to rescue Tf uptake in cells depleted of endogenous Dyn2. Data are presented as means ± SE. Bar = 10 μm.
DISCUSSION
The findings described above provide strong evidence for activation of the endocytic machinery (Fig. 1 and 3) and the corresponding internalization of the TfR1 (Fig. 2) by ligand stimulation. This is consistent with the premise established by others that ligand binding to its receptor can regulate the sequestration of this cargo, leading to the efficient assembly, formation, and liberation of clathrin-coated pits from the PM, compared to abortive disassembly of “empty” clathrin pits (14, 43). The internalization and recycling of the TfR have been closely studied by multiple groups for over 2 decades. While some have observed that internalization of the TfR is linear over time regardless of Tf addition (24), others have observed an increase in the endocytic rate of this receptor in the presence of ligand (19, 48, 49, 60). This concept of regulated internalization has been supported by studies implicating protein kinase C (PKC) (29, 47) and casein kinase activation (12), increases in cytoplasmic calcium (46), and, most recently, Src activity (61). Despite these findings, there is a common perception that internalization of nutritive receptor-ligand complexes such as Tf, asialoglycoprotein, and others does not stimulate endocytosis but instead that these complexes are internalized by a perpetually active endocytic machinery.
Thus, this current study suggests that not only is the endocytic internalization of the TfR1 allowed to proceed by ligand binding but it is actually stimulated by this action. From the observations described here, we conclude that Src kinase provides an important part of this regulatory function. First, Src is highly activated in cells treated with Tf ligand (Fig. 4). Second, inactive or kinase-dead c-Src kinase expressed in Clone 9 cells significantly attenuates Tf uptake (Fig. 5k to m). Third, a temperature-sensitive v-Src-expressing MDCK cell line internalizes only modest levels of Tf at the restrictive temperature, while this is greatly increased upon a shift to the permissive temperature that activates v-Src (see Fig. S1 in the supplemental material). Fourth, SYF cells isolated from Src knockout mice embryos internalize only modest levels of Tf (compared with MEF cells) unless “rescued” by exogenous expression of an active Src protein (Fig. 5e to i). It is important to note that while several of the experiments in this study utilize expression of c-Src kinase, it is currently unclear if this is the specific Src form responsible for the observed TfR-mediated signaling, as there are other Src family members that could participate. For this reason, we have used Src as a generic name for the broader family.
As both Dyn2 and its actin-binding partner Cort play an important role in the endocytic process (8, 13, 33, 44, 45, 50) and are phosphorylated on specific tyrosines during the internalization of EGF or GCPRs (1, 2, 28), we tested whether these proteins are Src “activated” during the ligand-stimulated internalization of the TfR1. We found that these proteins form a complex with the TfR1 in response to Tf stimulation and are markedly tyrosine phosphorylated as the receptor is endocytosed (Fig. 3). Phosphomutants of these proteins that cannot act as Src substrates significantly attenuate TfR1 internalization (Fig. 6), while cells with reduced levels of Dyn2 (by siRNA-mediated knockdown) can be rescued by the expression of Dyn2 WT (Fig. 7a and d).
Currently, the mechanisms that might activate the Src pathway from a ligand-bound TfR1 are undefined. This receptor does possess an YXXφ-based AP-2 binding motif, and several earlier studies (10, 18, 27, 39) have identified a role for serine/threonine phosphorylation on the μ2 subunit of AP-2 in receptor sequestration to the clathrin basket. Our current study focuses on the regulation of the vesiculation machinery and the surprising fact that it appears to be activated by the TfR1. We have not observed any phosphorylation of the TfR1 itself when precipitated from 32P-labeled cells stimulated with Tf. While the structure of the human TfR1-Tf complex has recently been solved (9), it is unclear what conformational changes, if any, might occur in the short 75-amino-acid cytoplasmic receptor tail to initiate downstream signaling cascades. Recent studies have identified physical connections between distinct receptor classes, such as the T-cell receptor (TCR), which is an RTK, and a GCPR, such as CXCR4 (32). These interactions between dissimilar receptors are believed to be mediated, in part, by an expanding family of immunoreceptor tyrosine-based activation motifs (ITAMs) that are situated in the cytoplasmic tails of many receptors expressed in both immune and epithelial cells (16, 38, 56). Upon tyrosine phosphorylation, ITAMs may bind to the cellular domain of other receptors directly or indirectly via an adaptor intermediary. It will be of significant interest to test if the TfR1 can interact and activate other cell surface receptors upon ligand binding. Finally, it should be noted that a recent study has demonstrated that a Golgi-associated pool of Src kinase is markedly activated as nascent cargo traffics from the endoplasmic reticulum (ER) to the Golgi apparatus (42). This kinase activity appears to be required for the release of secretory vesicles from the trans-Golgi network (TGN) that utilizes a vesiculation machinery similar in many respects to that of clathrin-coated pits at the cell surface (54). Thus, there is a functional precedent for Src regulation of membrane dynamics during vesicle formation that does not entail activation by RTKs.
Currently, the precise mechanistic consequences of Src-phosphorylation of Dyn2 and Cort are only partially defined. Src-mediated phosphorylation of Dyn1 in HEK293 cells appears to activate polymer assembly and GTPase activity (1, 2). Phosphorylation of the C-terminal tyrosines of Cort is believed to alter actin assembly (34) and could have profound effects on clathrin-coated membrane invagination and scission. Indeed, Zhu et al. have shown that the binding affinity of Src-phosphorylated Cort for Dyn2 is increased 5-fold (61). These findings extend our original observations that Dyn2 precipitated from platelet-derived growth factor (PDGF)-stimulated cells shows highly increased levels of associated Cort (31). Thus, it is appealing to predict that ligand-induced signaling by the TfR1 stimulates tyrosine phosphorylation of Cort, leading to recruitment of activated Dyn2 and, subsequently, vesicle formation. These observations are consistent with the findings of Wilde and colleagues (59), who observed a significant phosphorylation of clathrin heavy chain by Src kinase upon EGF stimulation leading to coat recruitment to the PM. Thus, it appears that Src function, activated by ligand-receptor interactions, plays an essential role in mobilizing the endocytic machinery to the site of internalization. Future studies of this newly identified TfR signaling pathway are likely to provide insight into iron homeostasis in the liver and regulation of the endocytic process for many other receptor forms.
Supplementary Material
Acknowledgments
This study was supported by grant RO1-DK44650 to M.A.M. and the Optical Morphology Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK84567).
Thanks go to Barbara Schroeder, Susan Chi, and Shaun Weller for carefully reading the manuscript.
Footnotes
Published ahead of print on 7 December 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahn, S., J. Kim, C. L. Lucaveche, M. C. Reedy, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 2002. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 277:26642-26651. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, S., S. Maudsley, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 1999. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 274:1185-1188. [DOI] [PubMed] [Google Scholar]
- 3.Andreeva, A. V., M. A. Kutuzov, and T. A. Voyno-Yasenetskaya. 2007. Scaffolding proteins in G-protein signaling. J. Mol. Signal. 2:13. [DOI] [PMC free article] [PubMed]
- 4.Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler, E., A. V. Hoffbrand, and J. D. Cook. 2003. Iron deficiency and overload. Hematology Am. Soc Hematol. Educ. Program 2003:40-61. [DOI] [PubMed] [Google Scholar]
- 6.Cao, H., J. Chen, M. Awoniyi, J. R. Henley, and M. A. McNiven. 2007. Dynamin 2 mediates fluid-phase micropinocytosis in epithelial cells. J. Cell Sci. 120:4167-4177. [DOI] [PubMed] [Google Scholar]
- 7.Cao, H., F. Garcia, and M. A. McNiven. 1998. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9:2595-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, H., J. D. Orth, J. Chen, S. G. Weller, J. E. Heuser, and M. A. McNiven. 2003. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 23:2162-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, Y., O. Zak, P. Aisen, S. C. Harrison, and T. Walz. 2004. Structure of the human transferrin receptor-transferrin complex. Cell 116:565-576. [DOI] [PubMed] [Google Scholar]
- 10.Conner, S. D., and S. L. Schmid. 2002. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 156:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper, J. A., and A. MacAuley. 1988. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc. Natl. Acad. Sci. U. S. A. 85:4232-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotlin, L. F., M. A. Siddiqui, F. Simpson, and J. F. Collawn. 1999. Casein kinase II activity is required for transferrin receptor endocytosis. J. Biol. Chem. 274:30550-30556. [DOI] [PubMed] [Google Scholar]
- 13.De Camilli, P., K. Takei, and P. S. McPherson. 1995. The function of dynamin in endocytosis. Curr. Opin. Neurobiol. 5:559-565. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich, M., W. Boll, A. Van Oijen, R. Hariharan, K. Chandran, M. L. Nibert, and T. Kirchhausen. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591-605. [DOI] [PubMed] [Google Scholar]
- 15.Erpel, T., and S. A. Courtneidge. 1995. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr. Opin. Cell Biol. 7:176-182. [DOI] [PubMed] [Google Scholar]
- 16.Fodor, S., Z. Jakus, and A. Mocsai. 2006. ITAM-based signaling beyond the adaptive immune response. Immunol. Lett. 104:29-37. [DOI] [PubMed] [Google Scholar]
- 17.Foster-Barber, A., and J. M. Bishop. 1998. Src interacts with dynamin and synapsin in neuronal cells. Proc. Natl. Acad. Sci. U. S. A. 95:4673-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh, P., and S. Kornfeld. 2003. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol. 160:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gironès, N., and R. J. Davis. 1989. Comparison of the kinetics of cycling of the transferrin receptor in the presence or absence of bound diferric transferrin. Biochem. J. 264:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Head, J. A., D. Jiang, M. Li, L. J. Zorn, E. M. Schaefer, J. T. Parsons, and S. A. Weed. 2003. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol. Biol. Cell 14:3216-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henley, J. R., and M. A. McNiven. 1996. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J. Cell Biol. 133:761-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hentze, M. W., M. U. Muckenthaler, and N. C. Andrews. 2004. Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285-297. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins, C. R., and I. S. Trowbridge. 1983. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J. Cell Biol. 97:508-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, C., J. Liu, C. C. Haudenschild, and X. Zhan. 1998. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273:25770-25776. [DOI] [PubMed] [Google Scholar]
- 26.Kawabata, H., R. Yang, T. Hirama, P. T. Vuong, S. Kawano, A. F. Gombart, and H. P. Koeffler. 1999. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J. Biol. Chem. 274:20826-20832. [DOI] [PubMed] [Google Scholar]
- 27.Keen, J. H., M. H. Chestnut, and K. A. Beck. 1987. The clathrin coat assembly polypeptide complex. Autophosphorylation and assembly activities. J. Biol. Chem. 262:3864-3871. [PubMed] [Google Scholar]
- 28.Kim, J., S. Ahn, R. Guo, and Y. Daaka. 2003. Regulation of epidermal growth factor receptor internalization by G protein-coupled receptors. Biochemistry 42:2887-2894. [DOI] [PubMed] [Google Scholar]
- 29.Klausner, R. D., J. Harford, and J. van Renswoude. 1984. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc. Natl. Acad. Sci. U. S. A. 81:3005-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruchten, A. E., and M. A. McNiven. 2006. Dynamin as a mover and pincher during cell migration and invasion. J. Cell Sci. 119:1683-1690. [DOI] [PubMed] [Google Scholar]
- 31.Krueger, E. W., J. D. Orth, H. Cao, and M. A. McNiven. 2003. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14:1085-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar, A., T. D. Humphreys, K. N. Kremer, P. S. Bramati, L. Bradfield, C. E. Edgar, and K. E. Hedin. 2006. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity 25:213-224. [DOI] [PubMed] [Google Scholar]
- 33.Liu, J. P., and P. J. Robinson. 1995. Dynamin and endocytosis. Endocr. Rev. 16:590-607. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Quiles, N., H. Y. Ho, M. W. Kirschner, N. Ramesh, and R. S. Geha. 2004. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell. Biol. 24:5269-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNiven, M. A. 1998. Dynamin: a molecular motor with pinchase action. Cell 94:151-154. [DOI] [PubMed] [Google Scholar]
- 36.McNiven, M. A., L. Kim, E. W. Krueger, J. D. Orth, H. Cao, and T. W. Wong. 2000. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 151:187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNiven, M. A., and H. M. Thompson. 2006. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science 313:1591-1594. [DOI] [PubMed] [Google Scholar]
- 38.Mócsai, A., C. L. Abram, Z. Jakus, Y. Hu, L. L. Lanier, and C. A. Lowell. 2006. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 7:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olusanya, O., P. D. Andrews, J. R. Swedlow, and E. Smythe. 2001. Phosphorylation of threonine 156 of the mu2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 11:896-900. [DOI] [PubMed] [Google Scholar]
- 40.Orth, J. D., and M. A. McNiven. 2003. Dynamin at the actin-membrane interface. Curr. Opin. Cell Biol. 15:31-39. [DOI] [PubMed] [Google Scholar]
- 41.Parsons, J. T., and S. J. Parsons. 1997. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 9:187-192. [DOI] [PubMed] [Google Scholar]
- 42.Pulvirenti, T., M. Giannotta, M. Capestrano, M. Capitani, A. Pisanu, R. S. Polishchuk, E. San Pietro, G. V. Beznoussenko, A. A. Mironov, G. Turacchio, V. W. Hsu, M. Sallese, and A. Luini. 2008. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 10:912-922. [DOI] [PubMed] [Google Scholar]
- 43.Puthenveedu, M. A., and M. von Zastrow. 2006. Cargo regulates clathrin-coated pit dynamics. Cell 127:113-124. [DOI] [PubMed] [Google Scholar]
- 44.Qualmann, B., and M. M. Kessels. 2002. Endocytosis and the cytoskeleton. Int. Rev. Cytol. 220:93-144. [DOI] [PubMed] [Google Scholar]
- 45.Robinson, M. S. 1994. The role of clathrin, adaptors and dynamin in endocytosis. Curr. Opin. Cell Biol. 6:538-544. [DOI] [PubMed] [Google Scholar]
- 46.Sainte-Marie, J., V. Lafont, E. I. Pecheur, J. Favero, J. R. Philippot, and A. Bienvenue. 1997. Transferrin receptor functions as a signal-transduction molecule for its own recycling via increases in the internal Ca2+ concentration. Eur. J. Biochem. 250:689-697. [DOI] [PubMed] [Google Scholar]
- 47.Schonhorn, J. E., T. Akompong, and M. Wessling-Resnick. 1995. Mechanism of transferrin receptor down-regulation in K562 cells in response to protein kinase C activation. J. Biol. Chem. 270:3698-3705. [DOI] [PubMed] [Google Scholar]
- 48.Schwake, L., A. W. Henkel, H. D. Riedel, T. Schlenker, M. Both, A. Migala, B. Hadaschik, N. Henfling, and W. Stremmel. 2002. Regulation of transferrin-induced endocytosis by wild-type and C282Y-mutant HFE in transfected HeLa cells. Am. J. Physiol. Cell Physiol. 282:C973-C979. [DOI] [PubMed] [Google Scholar]
- 49.Schwake, L., A. W. Henkel, H. D. Riedel, and W. Stremmel. 2002. Patch-clamp capacitance measurements: new insights into the endocytic uptake of transferrin. Blood Cells Mol. Dis. 29:459-464. [DOI] [PubMed] [Google Scholar]
- 50.Sever, S. 2002. Dynamin and endocytosis. Curr. Opin. Cell Biol. 14:463-467. [DOI] [PubMed] [Google Scholar]
- 51.Shajahan, A. N., B. K. Timblin, R. Sandoval, C. Tiruppathi, A. B. Malik, and R. D. Minshall. 2004. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 279:20392-20400. [DOI] [PubMed] [Google Scholar]
- 52.Sorkina, T., F. Huang, L. Beguinot, and A. Sorkin. 2002. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J. Biol. Chem. 277:27433-27441. [DOI] [PubMed] [Google Scholar]
- 53.Takeda, H., and S. Tsukita. 1995. Effects of tyrosine phosphorylation on tight junctions in temperature-sensitive v-src-transfected MDCK cells. Cell Struct. Funct. 20:387-393. [DOI] [PubMed] [Google Scholar]
- 54.Thompson, H. M., and M. A. McNiven. 2001. Dynamin: switch or pinchase? Curr. Biol. 11:R850. [DOI] [PubMed]
- 55.Trinder, D., and E. Baker. 2003. Transferrin receptor 2: a new molecule in iron metabolism. Int. J. Biochem. Cell Biol. 35:292-296. [DOI] [PubMed] [Google Scholar]
- 56.Underhill, D. M., and H. S. Goodridge. 2007. The many faces of ITAMs. Trends Immunol. 28:66-73. [DOI] [PubMed] [Google Scholar]
- 57.Vojtechová, M., F. Senigl, E. Sloncova, and Z. Tuhackova. 2006. Regulation of c-Src activity by the expression of wild-type v-Src and its kinase-dead double Y416F-K295N mutant. Arch. Biochem. Biophys. 455:136-143. [DOI] [PubMed] [Google Scholar]
- 58.Weed, S. A., and J. T. Parsons. 2001. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20:6418-6434. [DOI] [PubMed] [Google Scholar]
- 59.Wilde, A., E. C. Beattie, L. Lem, D. A. Riethof, S. H. Liu, W. C. Mobley, P. Soriano, and F. M. Brodsky. 1999. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 96:677-687. [DOI] [PubMed] [Google Scholar]
- 60.Yashunsky, V., S. Shimron, V. Lirtsman, A. M. Weiss, N. Melamed-Book, M. Golosovsky, D. Davidov, and B. Aroeti. 2009. Real-time monitoring of transferrin-induced endocytic vesicle formation by mid-infrared surface plasmon resonance. Biophys. J. 97:1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu, J., D. Yu, X. C. Zeng, K. Zhou, and X. Zhan. 2007. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J. Biol. Chem. 282:16086-16094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.