Abstract
Interleukin-7 (IL-7) is critical for T-cell development and peripheral T-cell homeostasis. The survival of pro-T cells and mature T cells requires IL-7. The survival function of IL-7 is accomplished partly through induction of the antiapoptotic protein Bcl-2 and inhibition of proapoptotic proteins Bax and Bad. We show here that the proapoptotic protein Bim, a BH3-only protein belonging to the Bcl-2 family, also plays a role in peripheral T-cell survival. Deletion of Bim partially protected an IL-7-dependent T-cell line and peripheral T cells, especially cells with an effector memory phenotype, from IL-7 deprivation. However, T-cell development in the thymus was not restored in IL-7−/− Rag2−/− mice reconstituted with Bim−/− bone marrow. IL-7 withdrawal altered neither the intracellular location of Bim, which was constitutively mitochondrial, nor its association with Bcl-2; however, a reduction in its association with the prosurvival protein Mcl-1 was observed. IL-7 withdrawal did not increase Bim mRNA or protein expression but did induce changes in the isoelectric point of BimEL and its reactivity with an antiphosphoserine antibody. Our findings suggest that the maintenance of peripheral T cells by IL-7 occurs partly through inhibition of Bim activity at the posttranslational level.
Interleukin-7 (IL-7), a cytokine produced by stromal cells in lymphoid tissues, was initially discovered on the basis of its nonredundant role in the growth and differentiation of B- and T-cell progenitors in the mouse and was subsequently shown to be important for T-cell development in humans (33, 37, 40, 50). More recently, IL-7 has also been found to be essential for the homeostasis of nearly all subsets of T cells (3, 45, 48, 49). The thymic effect of IL-7 is mainly antiapoptotic, whereas the homeostatic effects on peripheral T cells include both survival and proliferation. IL-7 stimulates cell division through the stabilization of Cdc25a, an activator of cyclin-dependent kinase (21), and the destabilization of p27kip1, an inhibitor of cyclin-dependent kinase (28).
The antiapoptotic effect of IL-7 is attributed mainly to the Bcl-2 family of proteins, inasmuch as IL-7 induces the expression of antiapoptotic proteins Bcl-2 (26, 46) and Mcl-1 (36) and the inhibition of proapoptotic proteins Bax (22, 23) and Bad (29). Transgenic expression of Bcl-2 restored normal numbers of thymocytes and peripheral T cells in IL-7 receptor α chain (IL-7Rα)-deficient mice (1, 31). IL-7 inhibits Bax by preventing its translocation from the cytoplasm to the mitochondria (22). Bax is implicated as a death-promoting factor in the IL-7 survival function based on our previous observation that Bax deficiency rescues T-cell development in IL-7Rα-deficient mice early in life (23). Although Bad-deficient mice show no dramatic effects on T-cell development (44), knock-in mice with an active form of Bad show a reduction in the level of pro-T cells in the thymus (10). Our previous study showed that IL-7 inactivated Bad by inducing Bad phosphorylation (29), indicating that Bad is relevant to the IL-7 survival effect in T cells.
Bim, a proapoptotic member of the Bcl-2 family, was identified as a BH3-only protein that induces apoptosis and can be antagonized by antiapoptotic proteins of the Bcl-2 family. Bim has three isoforms, BimEL, BimL, and BimS, which are apparently produced by alternative splicing, and all can induce apoptosis (34). Bim is essential for apoptosis induced by growth factor deprivation in a broad range of cell types, including lymphocytes and neurons (14, 41). Mice lacking Bim display abnormal accumulations of single positive thymocytes in the thymus and of T and B cells in the spleen (6). Studies of Bim-deficient mice indicate important roles for Bim in the deletion of autoreactive T and B cells (7, 13) during their development and in the termination of immune responses (17, 38). Bim was previously implicated in IL-7R signaling in that genetic deletion of Bim was reported to partially restore thymic and peripheral T-cell numbers in the absence of IL-7R (39), and loss of Bim compensated for a lack of IL-7 in precursor B-cell survival (35).
The activity of Bim can be modulated at the transcriptional and posttranslational levels. The transcriptional control of Bim involves inhibition of forkhead transcription factors (FoxOs) in murine T cells (47). Posttranslational regulation of Bim activity has been reported via sequestration to the cytoplasmic dynein motor complex by interaction with the dynein light chain (42). It also has been reported that Bim protein levels are controlled by ubiquitin-dependent degradation (27, 30). In this study, we evaluated the role of Bim in the development and homeostasis of T cells controlled by IL-7, and we further examined the molecular mechanism of IL-7 regulation of Bim.
MATERIALS AND METHODS
Mice.
Bim−/− and Rag2−/− mice (in a C57BL/6 background) were purchased from The Jackson Laboratory. IL-7−/− and IL-7−/− Rag2−/− mice were obtained from R. Murray (DNAX). All mice were maintained by homozygous breeding at the National Cancer Institute (NCI), Frederick Cancer Research and Development Center, animal facility. The NCI—Frederick Animal Care and Use Committee approved all experiments on mice. In our experiments, we refer to C57BL/6 as the wild-type (WT) control for Bim−/− and IL-7−/− mice. All mice used were 8 to 12 weeks old.
Cell lines.
The IL-7-dependent thymocyte cell line D1 was generated from p53−/− mice (25). D1 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (HyClone), 2 mM l-glutamine, 1% penicillin-streptomycin, 50 μM β-mercaptoethanol (Invitrogen), and 50 μg/ml murine recombinant IL-7 (PeproTech). The retrovirus packaging cell line phoenix-Eco was maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and 1% penicillin-streptomycin.
DNA constructs.
The retroviral vector pMIG, containing green fluorescent protein (GFP) as a marker, was described previously (29). The complete coding sequences of murine BimEL, BimL, and BimS were amplified by reverse transcription-PCR (RT-PCR) from D1 total RNA. The Bim cDNAs were cut by BamHI and XhoI and were cloned into the BglII/XhoI sites of the retroviral expression vector pMIG. Potential phosphorylation sites of BimEL were changed by PCR-based site-directed mutagenesis using a QuikChange kit (Stratagene). pMIG-hU6-shBim18 was constructed by inserting the sequences of mouse BIM (GenBank accession no. AF032459; nucleotides 486 to 506) into the pMIG-hU6 vector (28).
Retroviral infection.
Individual retroviral constructs were transfected into the phoenix-Eco packaging cell line using Fugene-6 (Roche Applied Science). The retrovirus-containing supernatants were harvested after 48 h and loaded onto a RetroNectin (TaKaRa)-coated plate, and then D1 cells were added and infected overnight. Cells were analyzed for apoptosis by annexin V/7-amino-actinomycin D (7-AAD) staining, and GFP-positive cells were identified using flow cytometry for analysis or sorting (20).
Western blotting and immunoprecipitation.
Cell lysates from 5 × 106 D1 cells were prepared with Triton X-100 lysis buffer (29) supplemented with a protease inhibitor mixture (Roche Applied Science). Equal amounts of proteins were loaded onto SDS-PAGE gels, transferred to nitrocellulose membranes, and analyzed by immunoblotting. For immunoprecipitation, equal amounts of protein were precleared with protein A/G beads (Roche Applied Science) and incubated with the appropriate antibodies at 4°C overnight. Then the antibody complexes were captured with protein A/G beads at 4°C for 2 h. After three washes with the same lysis buffer, the beads were boiled in SDS sample buffer and separated by SDS-PAGE. Antibodies specific for Bim were purchased from Calbiochem. A hamster anti-human Bcl-2 monoclonal antibody was obtained from BD Biosciences. Antiactin was purchased from Sigma, anti-Mcl-1 from Rockland, and anti-phosphoserine 4A4 from Millipore.
Mitochondrial protein preparation.
Cells were lysed in isotonic buffer (200 mM mannitol, 70 mM sucrose, 1 mM EGTA, 10 mM HEPES [pH 6.9]) by Dounce homogenization. Unbroken cells, nuclei, and heavy membranes were spun down at about 1,000 × g and discarded. The mitochondrial fraction was produced by pelleting at 12,000 × g for 20 min and washing the pellets once in isotonic buffer, followed by a final resuspension in Triton X-100 lysis buffer (62.5 mM Tris-HCl at pH 6.8, 1% Triton X-100, 10% glycerol, 50 mM dithiothreitol [DTT]) with 2% SDS. The supernatant recovered from the high-speed centrifugation represented the cytosolic fraction (22).
Two-dimensional (2D) gel electrophoresis. (i) First-dimension electrophoresis.
Protein samples (20 μl) in 180 μl IPG (immobilized pH gradient) gel rehydration buffer containing 8 M urea, 2% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 2% (vol/vol) IPG (pH 3 to 10) buffer, 40 mM DTT, and 0.01% (wt/vol) bromophenol blue were loaded onto 11-cm-long Immobiline DryStrip pH 3-10 L gels, allowing proteins to enter the gel during the overnight rehydration and equilibration process. After equilibration, the proteins were separated by isoelectric focusing using an Ettan IPGphor II electrophoresis unit (GE Healthcare). The strips were run for a total of 25 kVh. After the isoelectric focusing, strips were equilibrated for 15 min with SDS equilibration buffer containing 50 mM Tris-HCl (pH 8.8), 6 M urea, 30% glycerol, 2% SDS, and a trace of bromophenol blue. The equilibrated gel strips were used in second-dimension electrophoresis.
(ii) Second-dimension electrophoresis.
Second-dimension electrophoresis was performed using a Multiphor II (GE Healthcare) flatbed system with an ExcelGel SDS precast gel (polyacrylamide gradient, 12 to 14%). As molecular weight standard, Sea Blue was used (Invitrogen). The proteins were electroblotted, at a constant current of 250 mA by a semidry horizontal transfer technique using a Multiphor II NovaBlot unit, onto polyvinylidene difluoride Hybond-P membranes (GE Healthcare) in 25 mM Tris base, 192 mM glycine, and 20% methanol.
Cell death assay.
Cell death was quantitated by staining with annexin V-allophycocyanin and 7-AAD (BD Biosciences) according to the manufacturer's protocol, followed by flow cytometric analysis by using a FACSort flow cytometer (Becton Dickinson).
Isolation of T cells.
Lymph nodes (LNs) from Bim−/− or WT mice were harvested, and single-cell suspensions were prepared. Total T cells, CD4-positive cells, or CD8-positive cells were isolated using a Dynal Mouse T-cell, CD4, or CD8 negative isolation kit (Invitrogen), respectively, according to the manufacturer's protocol. The purity of T cells by this technique was >90%. The purified T cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 1% penicillin-streptomycin, and 50 μM β-mercaptoethanol, with or without IL-7.
Adoptive transfer of T cells.
T-cell transfer experiments were performed as previously described (28, 48). In brief, single-cell suspensions were prepared from LNs of Bim−/− or WT donor mice. LN cells were labeled with 5 μM 5,6-carboxyfluorescein diacetate succinimidyl diester (CFSE; Invitrogen) at 37°C for 10 min, followed by two washes with phosphate-buffered saline (PBS). A total of 1 × 107 CFSE-labeled cells were suspended in PBS and adoptively transferred into IL-7−/− or WT recipient mice by intravenous injection. The recipient mice received whole-body irradiation (300 rads) at least 3 h before the injection. Six days later, the host mice were killed, and spleen cells were stained with phycoerythrin (PE)-conjugated anti-CD4 (BD Biosciences) and PE-Cy5-conjugated anti-CD8 (BD Biosciences), anti-CD25 (eBioscience), anti-CD44 (eBioscience), anti-CD62L eBioscience), and anti-CD69(sBioscience). The intensity of CFSE on donor cells was analyzed by gating on either CD4+ or CD8+ cells on a FACScan flow cytometer (Becton Dickinson).
Bone marrow reconstitution.
Bone marrow (BM) cells were harvested by flushing cells from the femurs and tibias of Bim−/− and WT donor mice. BM cells were treated with a T-cell depletion kit and an autoMACS separator (Miltenyi Biotec), and 106 cells were injected into the tail veins of sublethally irradiated (300 rads) recipient IL-7−/− Rag2−/− and Rag2−/− mice. Thymuses were harvested from the recipient mice after 8 weeks. Cells were stained with antibodies against CD4 and CD8, T-cell receptor β (TCRβ), and TCRγδ and were analyzed by flow cytometry.
RESULTS
Bim promotes the death of D1 cells.
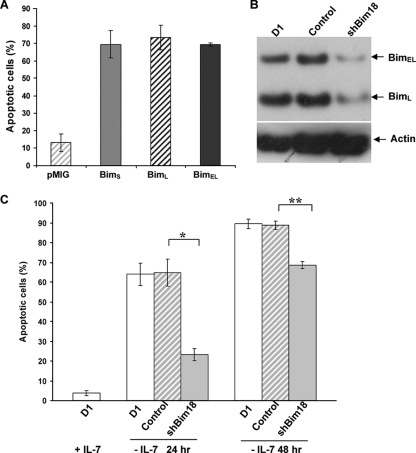
To determine whether Bim played a role in the IL-7 survival function, we first overexpressed three murine Bim isoforms, BimS, BimL, and BimEL (34), in D1 cells by retroviral infection and measured cell death by annexin V/7-AAD staining and flow cytometry analysis. We observed that all three isoforms of Bim killed D1 cells in the presence of IL-7 (Fig. 1A). We further introduced a small interfering RNA (siRNA) construct, pMIG-hU6-shBim18 (referred to below as shBim18), to knock down the expression of endogenous Bim. The expression of Bim in D1 cells was strongly inhibited by shBim18, with 64% and 62% decreases in the levels of BimEL and BimL proteins, respectively (Fig. 1B), whereas BimS protein was undetectable in D1 cells to begin with. Cell survival was strongly enhanced by shBim18 after 24 h of IL-7 withdrawal: ≈23% of cells were apoptotic, compared to 65% of control cells. This protection by shBim18 was reduced at 48 h of IL-7 deprivation (Fig. 1C), consistent with the activation of other proapoptotic proteins, as we have shown previously (23, 29). These data indicate that Bim can be an important contributing proapoptotic factor following IL-7 withdrawal.
FIG. 1.
Bim induces the death of IL-7-dependent D1 cells. (A) D1 cells were infected with retroviruses expressing either GFP alone (pMIG) or isoforms of Bim and were cultured at 37°C for 16 h in the presence of 50 ng/ml of IL-7. Apoptotic cells were determined by annexin V/7-AAD staining. The proportion of apoptotic cells was calculated by annexin V positivity and was expressed as a percentage of vector (pMIG)-infected cells (GFP-positive cells). Values are means ± standard deviations from three experiments. (B) D1 cells were infected with retroviruses carrying either a scrambled control siRNA or a siRNA specific for Bim (shBim18). GFP-positive cells were sorted after 24 h of infection and were cultured with 50 ng/ml of IL-7. Total-cell extracts were subjected to immunoblotting with an antibody against Bim. (C) D1 cells or D1 cells in which Bim had been knocked down by siRNA were deprived of IL-7 for 24 h or 48 h, and cell death was quantitated by annexin V/7-AAD staining. Values are means ± standard deviations for three experiments (*, P ≤ 0.001; **, P = 0.002).
Bim-deficient T cells are more resistant to IL-7 deprivation in vitro.
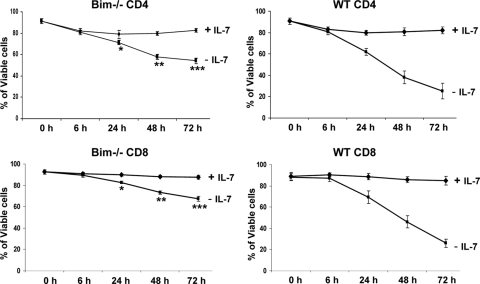
Since IL-7 is a key cytokine required for persistence of peripheral T cells, including naïve CD4 and CD8 cells (19, 45, 48), we tested whether deletion of Bim could improve primary T-cell survival in the absence of IL-7 in vitro. Bim−/− or WT CD4 and CD8 cells from LNs (axillary, inguinal, and brachial) were isolated by negative selection with magnetic beads and placed in culture without or with IL-7 (2 ng/ml). Cell death was assessed by annexin V/7-AAD staining at various time points. Both CD4 and CD8 T cells from either Bim−/− or WT mice showed the same viability when cultured with IL-7. However, CD4 and CD8 T cells from WT mice were more susceptible to IL-7 deprivation than Bim-deficient T cells after culture for 48 h or 72 h (Fig. 2). These data confirm that Bim contributes to the death of T cells in the absence of IL-7 in vitro.
FIG. 2.
Bim−/− T cells are more resistant to IL-7 deprivation than WT T cells in vitro. CD4- or CD8-positive T cells were isolated from the LNs of individual Bim−/− or WT mice by negative selection with magnetic beads. The purified cells were cultured either with IL-7 (2 ng/ml) or without IL-7 for the indicated time points. Cell viability was measured by annexin V/7-AAD staining. Results are means ± standard deviations for six individual mice in each group. P values for comparison of Bim−/− versus WT CD4 cells are as follows: *, 0.001; **, ≤0.0001; ***, ≤0.0007. P values for comparison of Bim−/− versus WT CD8 cells are as follows: *, 0.002, **, ≤0.0001; ***, ≤0.0001).
Deletion of Bim partially restores T-cell survival in IL-7-deficient hosts.
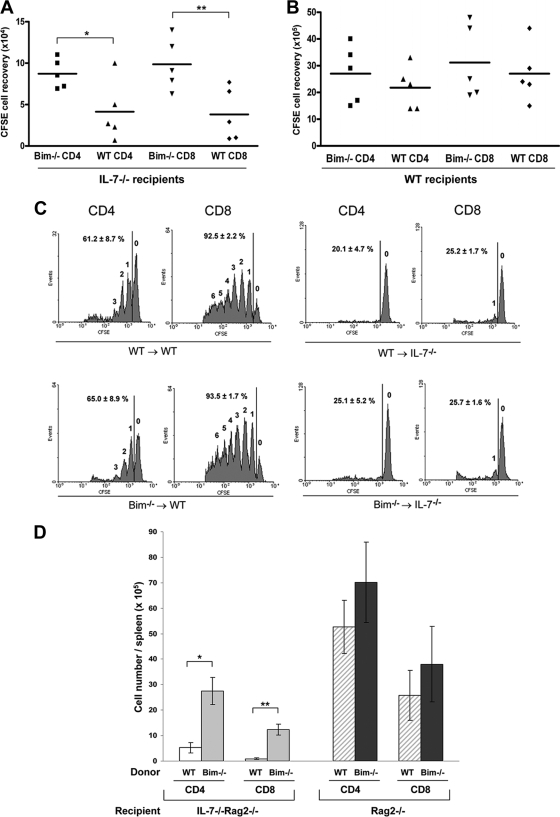
Having shown that in the D1 thymic cell line and cultured primary T cells Bim plays an important role in cell death induced by IL-7 withdrawal, we then tested the role of Bim in peripheral T cells in vivo. After transfer of T cells into a host depleted of lymphocytes, the survival and expansion of most subsets requires IL-7 (45, 48). LN cells from Bim−/− or WT mice were labeled with CFSE and transferred into sublethally irradiated IL-7−/− or WT mice. Both Bim−/− and WT donor T cells were predominantly of a naïve phenotype based on staining with anti-CD25, anti-CD44, anti-CD62L, and anti-CD69 (data not shown). Six days after transfer, donor T cells were analyzed by staining with anti-CD4 and anti-CD8, followed by flow cytometry. As shown in Fig. 3A, the recovery of Bim−/− donor T cells from IL-7-deficient hosts was modestly higher than that of WT donor T cells. In IL-7−/− hosts, the recoveries of CFSE+ donor cells per mouse were as follows: (8.7 ± 1.8) × 104 Bim−/− versus (4.1 ± 3.6) × 104 WT CD4 cells; (9.8 ± 3.2) × 104 Bim−/− versus (3.8 ± 3.2) × 104 WT CD8 cells. Thus, compared to the recovery of WT donor cells from IL-7−/− hosts, ≈2-fold increases in the recovery of Bim−/− CD4 cells and ≈3-fold increases in that of Bim−/− CD8 cells were observed. However, all groups recovered from IL-7-deficient mice contained much fewer cells than those recovered from WT hosts (≥30-fold) (Fig. 3A and B), possibly due to apoptotic factors other than Bim and also to the absence of the proliferative effects of IL-7 on T cells (28, 45). Homeostatic proliferation profiles of donor T cells were analyzed for CFSE intensity by gating on CD4 or CD8. Bim−/− and WT donor T cells proliferated in the WT host at similar rates, and few cells underwent homeostatic expansion in IL-7-deficent hosts (Fig. 3C). Thus, over a 6-day period of IL-7 deprivation using CFSE-marked T cells, Bim accounted for part of the death mechanism. To determine whether a long-term effect of Bim deletion on IL-7 deprivation could be detected, 2 × 105 T cells from Bim−/− or WT mice were transferred to irradiated IL-7−/− Rag2−/− mice, and 8 weeks later, CD4 or CD8 cells were analyzed. As shown in Fig. 3D, a ≈5-fold increase in the level of Bim−/− CD4 cells and a ≈15-fold increase in that of Bim−/− CD8 T cells were noted in the spleen compared to the levels of WT cells. Thus, a relatively modest short-term benefit of Bim depletion was magnified over time as Bim−/− T cells expanded independently of IL-7. Since most of these recovered T cells had the phenotype of effector memory cells (data not shown), it is possible that Bim is the primary killer in IL-7-deprived effector memory cells that may respond to the commensal microflora in Rag−/− mice. This slow expansion could also account for the much higher levels of reconstitution of the T-cell pool reported for IL-7R−/− Bim−/− mice (39).
FIG. 3.
Loss of Bim improves T-cell survival but not proliferation in IL-7-deficient hosts. (A) T cells were isolated from LNs of Bim−/− or WT mice, labeled with CFSE, and then transferred intravenously into irradiated IL-7−/− mice. Six days later, spleen cells were stained for CD4 and CD8. Donor T cells were analyzed for CFSE-positive cells by gating on CD4 and CD8. Numbers of CFSE-positive cells in different groups were determined for each spleen. Data were analyzed using CellQuest software (BD Biosciences). Means ± standard deviations for 2 or 3 mice from two independent experiments are shown (*, P = 0.008; **, P ≤ 0.001). (B) CFSE-positive cells were recovered from WT recipient spleens after 6 days of adoptive transfer of T cells. (C) Six days after transfer, T cells were recovered from the spleens of IL-7−/− or WT recipient mice. Donor T-cell homeostatic proliferation profiles were analyzed for CFSE intensity by gating on CD4 or CD8 using flow cytometry. Numbers above the peaks indicate the number of cell divisions. Vertical lines represent the gate used for calculating the percentage of cells that had divided. (D) T cells from Bim−/− or WT mice were transferred to irradiated Rag2−/− or IL-7−/− Rag2−/− recipients. Eight weeks later, spleens were harvested, and CD4 or CD8 T-cell recovery was determined by staining with anti-CD4 and anti-CD8.
Bim deficiency does not rescue thymic development in the absence of IL-7.
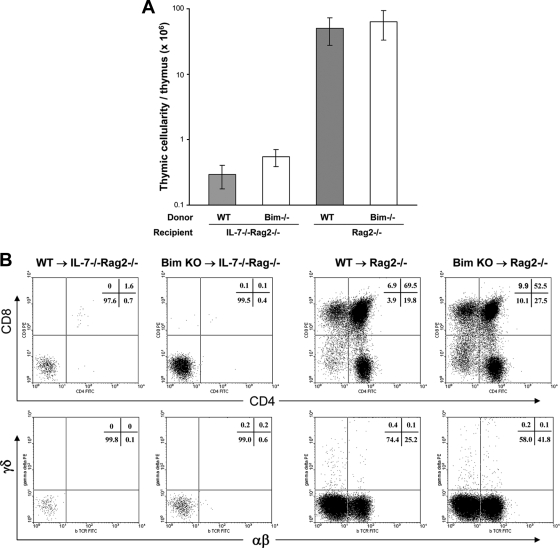
In addition to peripheral T-cell homeostasis, IL-7 is also critical for early T-cell development in the thymus, mainly to protect pro-T cells from apoptotic cell death (26). To determine whether a lack of Bim can substitute for IL-7 in T-cell development, we performed BM chimerism experiments. BM cells were prepared from 10- to 12-week-old Bim−/− or WT mice and were transplanted into irradiated IL-7−/− Rag2−/− or Rag2−/− recipients. Thymic development in recipient mice expressing or lacking IL-7 was evaluated after 8 weeks. Very low thymic cellularity was found in IL-7−/− Rag2−/− mice reconstituted with BM either from Bim−/− or from WT mice, with average levels of only 5.5 × 105 cells/thymus and 2.9 × 105 cells/thymus, respectively. In Rag2−/− hosts, which express IL-7, the thymic cellularity averaged 6.27 × 107 cells/thymus from Bim−/− BM-reconstituted mice and 4.98 × 107 cells/thymus from WT BM-reconstituted mice (Fig. 4A), levels similar to those for normal, unirradiated WT mice. T-cell development in the thymuses of different groups of recipients was further analyzed by staining with anti-CD4 and anti-CD8, or with anti-TCRαβ and TCRγδ. In the absence of IL-7, neither Bim−/− nor WT BM could restore T-cell development in the thymus. Normal T-cell development was seen in all Rag2−/− hosts, with significant production of thymocytes: single-positive CD4 and CD8 cells, double-positive (DP) cells, and double-negative (DN) cells, as well as both αβ and γδ T cells (Fig. 4B). Together, these data indicate that although Bim probably contributes to the death of adult pro-T cells in the absence of IL-7, additional death pathways are involved.
FIG. 4.
Lack of Bim does not relieve the block in T-cell development caused by the absence of IL-7. Bone marrow from Bim−/− or WT mice was used to reconstitute irradiated Rag2−/− or IL-7−/− Rag2−/− recipients. Eight weeks later, thymuses were harvested from recipients. (A) Thymic cellularity for individual recipients. Means ± standard deviations for 2 to 3 mice from two independent experiments are shown (*, P = 0.009; *, P = 0.001). (B) Thymocytes were stained for CD4 and CD8 (top) or TCRαβ and TCRδγ (bottom). Cell percentages for each quadrant are shown in the upper right corner. Values represent data for five mice from two individual experiments.
IL-7 does not regulate Bim protein synthesis or mitochondrial translocation.
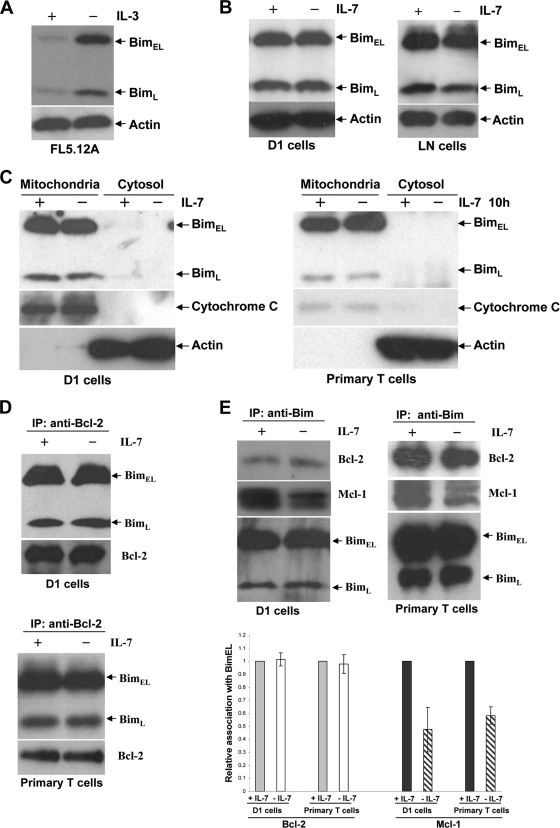
We then investigated the molecular mechanism of IL-7 regulation of Bim. It has been shown that Bim was regulated by IL-2 or IL-3 at both the transcriptional and translational levels (16, 47). We verified that IL-3 downregulated Bim protein in an IL-3 dependent cell line, FL5.12A (Fig. 5A). Therefore, we examined whether IL-7 influenced the expression of Bim in D1 cells or in LN T cells. Immunoblot analysis revealed that after 12 h of IL-7 deprivation, the total level of Bim protein remained the same as that in the presence of IL-7, in both D1 cells and LN T cells (Fig. 5B). It had been reported that the proapoptotic activity of Bim was inhibited by binding to the cytoplasmic dynein motor complex and the resulting sequestration in the cytoplasm (42). We next assessed the intracellular localization of Bim after IL-7 withdrawal relative to that in cells maintained in IL-7. Under both conditions, both the BimEL and BimL forms localized to the mitochondrial fraction and were undetectable in the cytosolic fraction (Fig. 5C). Immunoprecipitation was used to study the antiapoptotic proteins bound to Bim. Immunoprecipitation of Bcl-2 showed coimmunoprecipitation of Bim (Fig. 5D), and immunoprecipitation of Bim showed coimmunoprecipitation of both Bcl-2 and Mcl-1 (Fig. 5E). The association of Bim with Bcl-2 was not affected by IL-7. Mcl-1 is a preferred binding partner for Bim, and surprisingly, 50% or 40% less Mcl-1 protein was coimmunoprecipitated with Bim in D1 cells or primary T cells when they were cultured without IL-7 (Fig. 5E). This effect appears to result from a decline in Mcl-1 levels in cells deprived of IL-7 (not shown). Collectively, these data suggest that regulation of Bim by IL-7 occurs neither at the translational level nor by mitochondrial translocation, whereas posttranslational modification of Bim by IL-7 remains a possibility.
FIG. 5.
IL-7 does not alter Bim synthesis or mitochondrial translocation. Cells were placed in culture for 12 h with or without IL-7. (A) IL-3 was withdrawn from FL5.12A cells for 12 h. Total-cell extracts were subjected to immunoblotting with an antibody against Bim. (B) Total-cell extracts from D1 or LN cells were resolved by SDS-PAGE and subjected to immunoblotting with anti-Bim. (C) Primary T cells were isolated from spleens and LNs of 5 WT mice by negative selection with magnetic beads and were placed in culture for 10 h with or without IL-7. D1 or primary T cells were fractionated into cytoplasmic and mitochondrial fractions, and Bim protein levels were visualized by probing blots with an antibody specific for Bim. Cytochrome c oxidase and actin are shown as markers for mitochondrial and cytosolic proteins, respectively. (D) Cell extracts were prepared from D1 or primary T cells, immunoprecipitated (IP) with anti-Bcl-2, and blotted with anti-Bim or anti-Bcl-2. (E) Total proteins extracted from D1 or primary T cells were subjected to immunoprecipitation with anti-Bim, followed by blotting with anti-Bcl-2, anti-Mcl-1, and anti-Bim. The relative association of Bcl-2 or Mcl-1 with BimEL was quantified using an ImageJ program by measuring densitometry after normalization against BimEL protein.
IL-7 regulates Bim posttranslational modification.
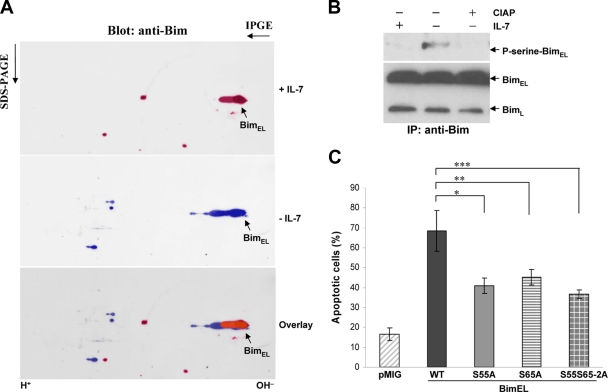
We next sought evidence of Bim protein modification using 2D gel electrophoresis. IL-7 deprivation of D1 cells for 12 h induced a prominent molecular mobility shift of BimEL (Fig. 6A) from a high isoelectric point (pI) to a low pI, suggesting that protein modification of BimEL occurred after removal of IL-7. Primary T cells showed similar Bim modification (not shown). Other studies had shown that phosphorylation of BimEL at serine 55 and serine 65 was induced by nerve growth factor deprivation and that the phosphorylation potentiated the proapoptotic activity of BimEL (9, 41). We therefore performed immunoprecipitation with anti-Bim from D1 cell lysates and treated the immunoprecipitated Bim with calf intestine alkaline phosphatase (CIAP). As shown in Fig. 6B, the serine phosphorylation of BimEL induced by IL-7 deprivation was eliminated by CIAP treatment. We further generated BimEL mutants in which serine 55 or 65 or both were replaced with alanine (the S55A, S65A, or S55S65-2A mutant). All three mutants showed less proapoptotic activity than WT BimEL in D1 cells (Fig. 6C). Thus, IL-7 may regulate Bim, at least in part, through phosphorylation.
FIG. 6.
IL-7 regulates Bim at the posttranslational level. (A) D1 cells were cultured in the present or absence of IL-7. Total-cell extracts were subjected to 2D gel electrophoresis and immunoblotting with anti-Bim. (B) Cell extracts were prepared from D1 cells cultured with or without IL-7, immunoprecipitated with anti-Bim, and treated with CIAP, followed by blotting with anti-phosphoserine 4A4 and anti-Bim. (C) D1 cells were infected with retroviruses expressing either GFP alone (pMIG), WT BimEL, or mutants of BimEL. The infected cells were cultured at 37°C for 16 h in the present of 50 ng/ml of IL-7. Apoptotic cells were determined by annexin V/7-AAD staining. The proportion of apoptotic cells was calculated as the percentage of vector (pMIG)-infected cells (GFP-positive cells) that were positive for annexin V. Values are means ± standard deviations for three experiments (*, P = 0.01; **, P = 0.042; ***, P ≤ 0.0098).
DISCUSSION
IL-7 is uniquely required for thymocyte development and peripheral T-cell survival and proliferation. The survival effect of IL-7 has been shown by us and others to primarily involve a balance of Bcl-2 family members by induction of Bcl-2 (1, 26) and Mcl-1 (36) and by repression of posttranslational activation of the death proteins Bax (22, 23) and Bad (29). In this study, we investigated the role of Bim, another death protein from the Bcl-2 family, in the IL-7 survival function. We observe that Bim plays a nonredundant role in the survival of peripheral T cells; although the short-term effect was modest, over the long term Bim-deficient T cells with an effector memory phenotype accumulated in the periphery of IL-7-deficient mice. Bim was constitutively located in mitochondria, and thus, there was no evidence that IL-7 regulated the intracellular location of Bim as it does for the other death proteins Bax and Bad. Within mitochondria, Bim was associated with Bcl-2 in the presence or absence of IL-7. In contrast, the amount of Bim complexed with Mcl-1 declined following IL-7 withdrawal in parallel with a decline in Mcl-1. The isoelectric point and serine phosphorylation of Bim were altered following IL-7 withdrawal; this may be partly due to phosphorylation at S55 and S65. This suggests that posttranslational modification of Bim could alter its efficacy, since mutation of these sites reduced its killing capacity. Alternatively, IL-7 may primarily regulate the levels of Mcl-1, thereby countering the effects of fixed levels of Bim.
Bim has previously been implicated in cell death due to IL-2 withdrawal in vitro, for example, in a study by Bosque et al. (5), and due to IL-3 withdrawal in vitro (18). Since neither IL-2 nor IL-3 is actually required for lymphocytes in vivo, whereas IL-7 is essential, we believe that our observations have physiological relevance, which we demonstrate by performing in vivo experiments. Our interpretation of the relationship of IL-7 and Bim differs somewhat from that of a study using a different approach based on the analysis of Bim−/− IL-7R−/− mice (39). Although both studies support such a relationship, the magnitude of the rescue effect of Bim deletion was greater in the Bim−/− IL-7R−/− mouse study than in our chimeric approach. In our study, transfer of Bim−/− hematopoietic stem cells into IL-7−/− Rag2−/− mice showed no detectable thymic reconstitution, whereas the Bim−/− IL-7R−/− mouse showed a modest thymic reconstitution of about 1/10 of the WT level.
One explanation for the difference between our results using chimeric mice versus Bim−/− IL-7R−/− mice is that apoptosis of thymic stem cells in newborns could be more Bim dependent than that in the adult. IL-7R−/− mice show severely reduced thymic cellularity beginning at the DN2 and DN3 stages (50). Bim−/− IL-7R−/− mice showed increased numbers of DN2 and DN3 cells in newborns, but no significant increase in adults, compared with those in IL-7R−/− mice (39). Thus, the Bim−/− IL-7R−/− mouse may rescue thymopoiesis early in life, whereas the hematopoietic cells used in our chimeric experiments were from adult donors, and these failed to reconstitute the IL-7−/− Rag2−/− thymus. This is reminiscent of the finding that apoptosis of newborn thymocytes is also more Bax dependent than that of adult thymocytes (23). According to this model, the deletion of either Bim or Bax can compensate for IL-7-deficiency early in life, but later on, other death mechanisms may additionally come into play. For example, other BH3-only proteins, such as Bad and Noxa, may become redundant with Bim in the apoptosis of adult thymocytes, and similarly, Bak may become redundant with Bax as a downstream death protein.
What does seem clear is that, since a Bcl-2 transgene protects both newborn and adult thymocytes from IL-7 deprivation (31), a balance of Bcl-2 family members is implicated in the death pathway following IL-7 withdrawal. Thus, there is no need to invoke another death pathway that would not be protected by Bcl-2. From our studies and others, there appears to be a complex balance of Bcl-2 family members regulated by IL-7; these minimally include Bim, Bad, and Bax versus Bcl-2 and Mcl-1, and the regulation involves transcriptional and posttranslational mechanisms.
Bcl-2 has been shown to be important for the survival of pro-T cells (26, 32). A recent study indicated that antagonism between Bcl-2 and Bim was critical for the control of naïve T-cell survival. Thus, T cells from Bim+/− Bcl-2−/− mice died rapidly in vitro, whereas this death was prevented by deletion of the remaining Bim allele (51). Our data demonstrated that either CD4 or CD8 T cells from Bim−/− mice were resistant to IL-7 deprivation-driven apoptosis in vitro (Fig. 2), and deletion of Bim resulted in better survival of CD4 or CD8 T cells in the absence of IL-7 in vivo (Fig. 3). In a recent clinical trial, IL-7 treatment of patients was observed to induce Bcl-2 expression in almost all subsets of peripheral blood T cells (46). Taken together with our findings, this suggests that an important survival mechanism in peripheral T cells is IL-7-induction of Bcl-2, which in turn counteracts Bim.
Mcl-1, another antiapoptotic protein of the Bcl-2 family, is also a potential mediator of the survival effect of IL-7 on peripheral T cells. Mcl-1 is widely expressed in most subsets of mature T cells (12). Mcl-1 transcription was reported to be induced by IL-7 in pro-T cells and peripheral T cells (36). We showed here that Bim was associated with Mcl-1 in mitochondria (Fig. 5E), and our preliminary studies indicated that IL-7 withdrawal has a potent destabilizing effect on Mcl-1 protein (not shown).
Noxa is another BH3-only protein that has been shown to selectively target Mcl-1. The Noxa/Mcl-1 balance appears to be critical for triggering apoptosis in activated T cells, thereby restraining T-cell expansion (2). It remains possible that Noxa is also redundant with Bim in the IL-7 pathway, which could explain why Bim deficiency only partially protects peripheral T cells from IL-7 deficiency (Fig. 3A and B). An additional explanation of the partial effect of Bim deletion is that IL-7 does more than protect from apoptosis; it also has proliferative effects on T cells (Fig. 3C) (28), which would contribute to the high recovery of T cells from normal hosts.
Thymic development in IL-7-deficient mice was not rescued by Bim-deficient bone marrow (Fig. 4). We favor the interpretation that Bim plays a redundant role with other BH3-only proteins in this process. Bad is a candidate, since knock-in mice with an active form of Bad had significantly decreased numbers of pro-T cells in the thymus (10), which is the critical stage supported by IL-7. Our previous study indicted that IL-7 inhibited Bad activity by inducing its phosphorylation and sequestration in the cytoplasm (29).
The proapoptotic activity of Bim can be regulated in a remarkable number of ways depending on the cell type and the agent of apoptosis induction. There are reports of transcriptional induction of Bim after cytokine withdrawal from a hematopoietic cell line, mainly through activation of FoxO3 (11, 47). The proteasome-ubiquitin pathway has also been shown to induce Bim protein degradation (27, 30). However, in our study, we observed neither Bim protein (Fig. 5B) nor Bim mRNA (not shown) induction by IL-7 deprivation in T cells. In certain cell types, in the healthy state, Bim was found in the cytoplasm associated with the dynein motor complex, and upon apoptotic stimulation, Bim was released from its sequestration in the dynein motor complex and translocated to mitochondria (42). However, it was previously reported that Bim was primarily associated with mitochondria in resting, activated, and apoptotic T cells (52). We also found that the subcellular distributions of Bim in the presence and absence of IL-7 were indistinguishable, that Bim was localized in mitochondria (Fig. 5C), and that it bound to Bcl-2 and Mcl-1 (Fig. 5D and E). Thus, IL-7 inhibition of Bim did not occur by cytosolic sequestration.
Phosphorylation of Bim has been observed previously, and there is controversy regarding its functional effects. Serines 55 and 65 in BimEL have been shown to be the targets of phosphorylation by extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), or p38 mitogen-activated protein (MAP) kinase. ERK-mediated phosphorylation in fibroblasts and epithelial cells has been correlated with a decrease in the apoptotic activity of BimEL, perhaps by targeting of BimEL for degradation (15, 27). In an IL-3-dependent B-cell line, phosphorylation of Bim by ERK also was reported to inhibit its apoptotic function, but not through its degradation (16). The opposite conclusion was reached regarding BimEL phosphorylation by JNK or p38 MAP kinase, which was reported to enhance its proapoptotic activity in a Bax-dependent manner (4, 9, 41). Our evidence for Bim phosphorylation was based on a shift in charge, reactivity with an anti-phosphoserine antibody, and the effects of deleting candidate serines. We also attempted to analyze Bim directly by mass spectroscopy (not shown). We were able to detect Bim peptides from Flag-tagged Bim immunoprecipitates and found tentative phosphorylation at serines 55 and 65 of BimEL in the absence of IL-7, although these phosphorylation sites could not be verified in the spectrum because of the low quantity and quality of Bim protein isolated from D1 cells. The following evidence favors the model in which JNK/p38 increases Bim-mediated apoptosis during IL-7 withdrawal from T cells: (i) IL-7 withdrawal induced activation of JNK and p38 MAP kinases (24, 43); (ii) phosphorylated BimEL was detected in thymocytes, and the phosphorylation enhanced its binding to antiapoptotic Bcl-xL (8); (iii) IL-7 deprivation induced a shift in the molecular mobility of BimEL from a high pI to a low pI (Fig. 6A), as well as serine phosphorylation of BimEL (Fig. 6B); and (iv) mutation of the possible serine phosphorylation sites decreased the proapoptotic activity of BimEL (Fig. 6C). Therefore, IL-7 withdrawal may promote apoptosis by inducing phosphorylation of Bim.
Overall, these findings suggest that, in the presence of IL-7, Bim is constrained by Mcl-1 and Bcl-2. Following IL-7 withdrawal, levels of Mcl-1 and Bcl-2 decline, releasing free Bim. Phosphorylation of Bim also may occur and promote the function of Bim, which is to activate the proapoptotic proteins Bax and Bak (41, 53). This induces mitochondrial leakage of cytochrome c, triggering the apoptotic cascade.
Acknowledgments
We thank Rodney Wiles and Steve Stull for animal technical assistance, Kathleen Noer and Roberta Matthai for flow cytometry assistance, Zhen Xiao and Timothy D. Veenstra for mass spectroscopy, Daniel McVicar for valuable suggestions, and Joost J. Opphenheim and Thomas Sayers for comments on the manuscript.
This work was supported by the Intramural Research Program of the NIH, NCI, and with federal funds from the NCI, NIH, under contract N01-CO-12400.
Footnotes
Published ahead of print on 23 November 2009.
REFERENCES
- 1.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I. L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89:1033-1041. [DOI] [PubMed] [Google Scholar]
- 2.Alves, N. L., I. A. Derks, E. Berk, R. Spijker, R. A. van Lier, and E. Eldering. 2006. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity 24:703-716. [DOI] [PubMed] [Google Scholar]
- 3.Baccala, R., D. Witherden, R. Gonzalez-Quintial, W. Dummer, C. D. Surh, W. L. Havran, and A. N. Theofilopoulos. 2005. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 174:4606-4612. [DOI] [PubMed] [Google Scholar]
- 4.Becker, E. B., J. Howell, Y. Kodama, P. A. Barker, and A. Bonni. 2004. Characterization of the c-Jun N-terminal kinase-BimEL signaling pathway in neuronal apoptosis. J. Neurosci. 24:8762-8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosque, A., I. Marzo, J. Naval, and A. Anel. 2007. Apoptosis by IL-2 deprivation in human CD8+ T cell blasts predominates over death receptor ligation, requires Bim expression and is associated with Mcl-1 loss. Mol. Immunol. 44:1446-1453. [DOI] [PubMed] [Google Scholar]
- 6.Bouillet, P., D. Metcalf, D. C. Huang, D. M. Tarlinton, T. W. Kay, F. Kontgen, J. M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735-1738. [DOI] [PubMed] [Google Scholar]
- 7.Bouillet, P., J. F. Purton, D. I. Godfrey, L. C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J. M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415:922-926. [DOI] [PubMed] [Google Scholar]
- 8.Bunin, A., F. W. Khwaja, and G. J. Kersh. 2005. Regulation of Bim by TCR signals in CD4/CD8 double-positive thymocytes. J. Immunol. 175:1532-1539. [DOI] [PubMed] [Google Scholar]
- 9.Cai, B., S. H. Chang, E. B. Becker, A. Bonni, and Z. Xia. 2006. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J. Biol. Chem. 281:25215-25222. [DOI] [PubMed] [Google Scholar]
- 10.Datta, S. R., A. M. Ranger, M. Z. Lin, J. F. Sturgill, Y. C. Ma, C. W. Cowan, P. Dikkes, S. J. Korsmeyer, and M. E. Greenberg. 2002. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 3:631-643. [DOI] [PubMed] [Google Scholar]
- 11.Dijkers, P. F., K. U. Birkenkamp, E. W. Lam, N. S. Thomas, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2002. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 156:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzhagalov, I., A. Dunkle, and Y. W. He. 2008. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J. Immunol. 181:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enders, A., P. Bouillet, H. Puthalakath, Y. Xu, D. M. Tarlinton, and A. Strasser. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erlacher, M., V. Labi, C. Manzl, G. Bock, A. Tzankov, G. Hacker, E. Michalak, A. Strasser, and A. Villunger. 2006. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 203:2939-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewings, K. E., K. Hadfield-Moorhouse, C. M. Wiggins, J. A. Wickenden, K. Balmanno, R. Gilley, K. Degenhardt, E. White, and S. J. Cook. 2007. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 26:2856-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada, H., B. Quearry, A. Ruiz-Vela, and S. J. Korsmeyer. 2004. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc. Natl. Acad. Sci. U. S. A. 101:15313-15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes, P. D., G. T. Belz, K. A. Fortner, R. C. Budd, A. Strasser, and P. Bouillet. 2008. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 28:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba, T. 2004. Cytokine-mediated cell survival. Int. J. Hematol. 80:210-214. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, Q., W. Q. Li, F. B. Aiello, R. Mazzucchelli, B. Asefa, A. R. Khaled, and S. K. Durum. 2005. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16:513-533. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, Q., W. Q. Li, R. R. Hofmeister, H. A. Young, D. R. Hodge, J. R. Keller, A. R. Khaled, and S. K. Durum. 2004. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol. Cell. Biol. 24:6501-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaled, A. R., D. V. Bulavin, C. Kittipatarin, W. Q. Li, M. Alvarez, K. Kim, H. A. Young, A. J. Fornace, and S. K. Durum. 2005. Cytokine-driven cell cycling is mediated through Cdc25A. J. Cell Biol. 169:755-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled, A. R., K. Kim, R. Hofmeister, K. Muegge, and S. K. Durum. 1999. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc. Natl. Acad. Sci. U. S. A. 96:14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaled, A. R., W. Q. Li, J. Huang, T. J. Fry, A. S. Khaled, C. L. Mackall, K. Muegge, H. A. Young, and S. K. Durum. 2002. Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity 17:561-573. [DOI] [PubMed] [Google Scholar]
- 24.Khaled, A. R., A. N. Moor, A. Li, K. Kim, D. K. Ferris, K. Muegge, R. J. Fisher, L. Fliegel, and S. K. Durum. 2001. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol. Cell. Biol. 21:7545-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, K., A. R. Khaled, D. Reynolds, H. A. Young, C. K. Lee, and S. K. Durum. 2003. Characterization of an interleukin-7-dependent thymic cell line derived from a p53−/− mouse. J. Immunol. Methods 274:177-184. [DOI] [PubMed] [Google Scholar]
- 26.Kim, K., C. K. Lee, T. J. Sayers, K. Muegge, and S. K. Durum. 1998. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J. Immunol. 160:5735-5741. [PubMed] [Google Scholar]
- 27.Ley, R., K. E. Ewings, K. Hadfield, E. Howes, K. Balmanno, and S. J. Cook. 2004. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J. Biol. Chem. 279:8837-8847. [DOI] [PubMed] [Google Scholar]
- 28.Li, W. Q., Q. Jiang, E. Aleem, P. Kaldis, A. R. Khaled, and S. K. Durum. 2006. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J. Exp. Med. 203:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, W. Q., Q. Jiang, A. R. Khaled, J. R. Keller, and S. K. Durum. 2004. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J. Biol. Chem. 279:29160-29166. [DOI] [PubMed] [Google Scholar]
- 30.Marani, M., D. Hancock, R. Lopes, T. Tenev, J. Downward, and N. R. Lemoine. 2004. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene 23:2431-2441. [DOI] [PubMed] [Google Scholar]
- 31.Maraskovsky, E., L. A. O'Reilly, M. Teepe, L. M. Corcoran, J. J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89:1011-1019. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki, Y., K. Nakayama, K. Nakayama, T. Tomita, M. Isoda, D. Y. Loh, and H. Nakauchi. 1997. Role of bcl-2 in the development of lymphoid cells from the hematopoietic stem cell. Blood 89:853-862. [PubMed] [Google Scholar]
- 33.Namen, A. E., S. Lupton, K. Hjerrild, J. Wignall, D. Y. Mochizuki, A. Schmierer, B. Mosley, C. J. March, D. Urdal, and S. Gillis. 1988. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 333:571-573. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor, L., A. Strasser, L. A. O'Reilly, G. Hausmann, J. M. Adams, S. Cory, and D. C. Huang. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17:384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver, P. M., M. Wang, Y. Zhu, J. White, J. Kappler, and P. Marrack. 2004. Loss of Bim allows precursor B cell survival but not precursor B cell differentiation in the absence of interleukin 7. J. Exp. Med. 200:1179-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opferman, J. T., A. Letai, C. Beard, M. D. Sorcinelli, C. C. Ong, and S. J. Korsmeyer. 2003. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671-676. [DOI] [PubMed] [Google Scholar]
- 37.Parrish, Y. K., I. Baez, T. A. Milford, A. Benitez, N. Galloway, J. W. Rogerio, E. Sahakian, M. Kagoda, G. Huang, Q. L. Hao, Y. Sevilla, L. W. Barsky, E. Zielinska, M. A. Price, N. R. Wall, S. Dovat, and K. J. Payne. 2009. IL-7 dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J. Immunol. 182:4255-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellegrini, M., G. Belz, P. Bouillet, and A. Strasser. 2003. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl. Acad. Sci. U. S. A. 100:14175-14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellegrini, M., P. Bouillet, M. Robati, G. T. Belz, G. M. Davey, and A. Strasser. 2004. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med. 200:1189-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puel, A., S. F. Ziegler, R. H. Buckley, and W. J. Leonard. 1998. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 20:394-397. [DOI] [PubMed] [Google Scholar]
- 41.Putcha, G. V., S. Le, S. Frank, C. G. Besirli, K. Clark, B. Chu, S. Alix, R. J. Youle, A. LaMarche, A. C. Maroney, and E. M. Johnson, Jr. 2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38:899-914. [DOI] [PubMed] [Google Scholar]
- 42.Puthalakath, H., D. C. Huang, L. A. O'Reilly, S. M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3:287-296. [DOI] [PubMed] [Google Scholar]
- 43.Rajnavolgyi, E., N. Benbernou, B. Rethi, D. Reynolds, H. A. Young, M. Magocsi, K. Muegge, and S. K. Durum. 2002. IL-7 withdrawal induces a stress pathway activating p38 and Jun N-terminal kinases. Cell. Signal. 14:761-769. [DOI] [PubMed] [Google Scholar]
- 44.Ranger, A. M., J. Zha, H. Harada, S. R. Datta, N. N. Danial, A. P. Gilmore, J. L. Kutok, M. M. Le Beau, M. E. Greenberg, and S. J. Korsmeyer. 2003. Bad-deficient mice develop diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 100:9324-9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 46.Sportès, C., F. T. Hakim, S. A. Memon, H. Zhang, K. S. Chua, M. R. Brown, T. A. Fleisher, M. C. Krumlauf, R. R. Babb, C. K. Chow, T. J. Fry, J. Engels, R. Buffet, M. Morre, R. J. Amato, D. J. Venzon, R. Korngold, A. Pecora, R. E. Gress, and C. L. Mackall. 2008. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205:1701-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl, M., P. F. Dijkers, G. J. Kops, S. M. Lens, P. J. Coffer, B. M. Burgering, and R. H. Medema. 2002. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 168:5024-5031. [DOI] [PubMed] [Google Scholar]
- 48.Tan, J. T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K. I. Weinberg, and C. D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. U. S. A. 98:8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan, J. T., B. Ernst, W. C. Kieper, E. LeRoy, J. Sprent, and C. D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Freeden-Jeffry, U., P. Vieira, L. A. Lucian, T. McNeil, S. E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojciechowski, S., P. Tripathi, T. Bourdeau, L. Acero, H. L. Grimes, J. D. Katz, F. D. Finkelman, and D. A. Hildeman. 2007. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J. Exp. Med. 204:1665-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, Y., B. J. Swanson, M. Wang, D. A. Hildeman, B. C. Schaefer, X. Liu, H. Suzuki, K. Mihara, J. Kappler, and P. Marrack. 2004. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc. Natl. Acad. Sci. U. S. A. 101:7681-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zong, W. X., T. Lindsten, A. J. Ross, G. R. MacGregor, and C. B. Thompson. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]