Abstract
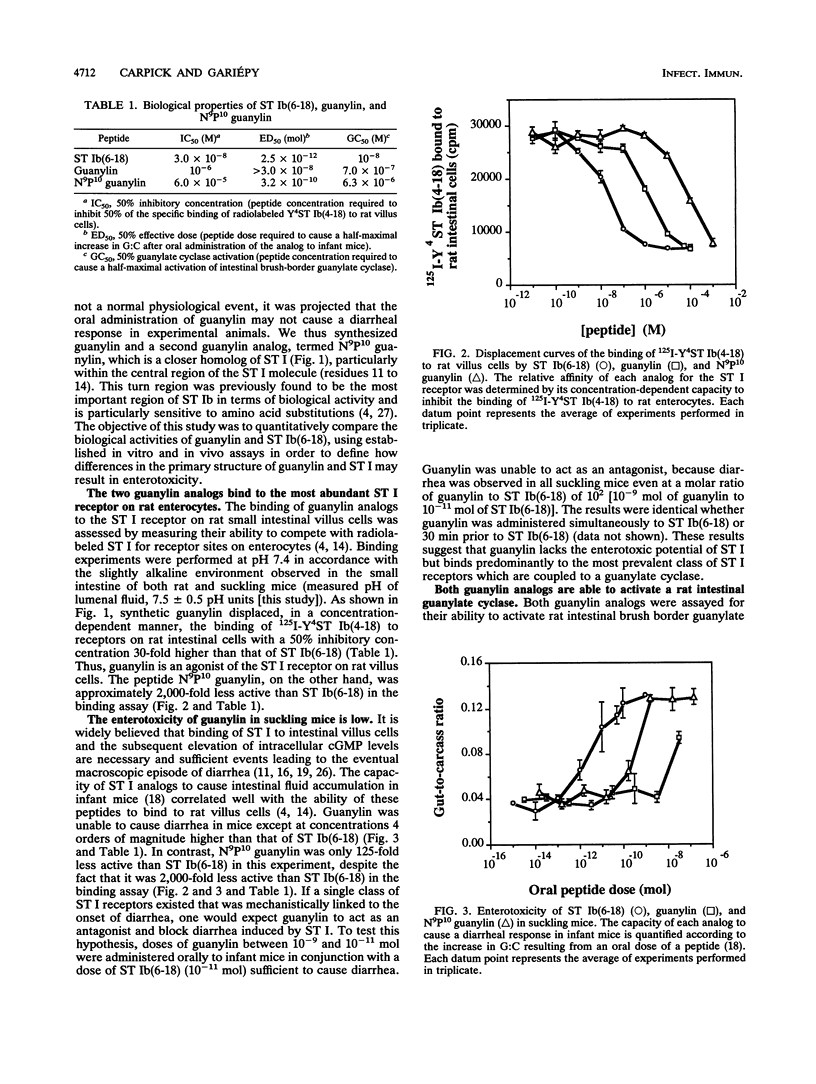
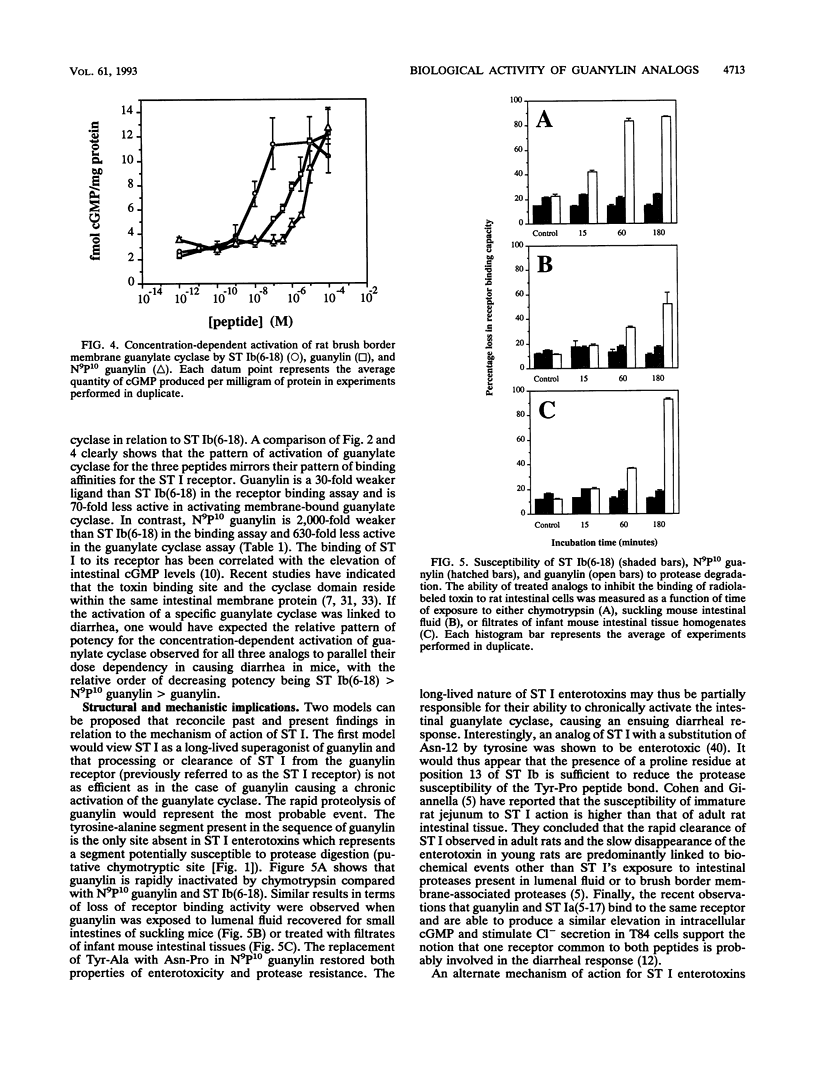
The mechanism by which bacterial heat-stable enterotoxins (ST I STA) cause diarrhea in humans and animals has been linked to the activation of an intestinal membrane-bound guanylate cyclase. Guanylin, a recently discovered rat intestinal peptide, is homologous in structure to ST I and can activate guanylate cyclase present on the human colonic carcinoma cell line T84. To directly test the mechanistic association of guanylate cyclase activation with diarrhea, we synthesized guanylin and a guanylin analog termed N9P10 guanylin and compared their biological activities with those of a synthetic ST I analog, termed ST Ib(6-18). We report that guanylin is able to inhibit the binding of a radiolabeled ST I analog to rat intestinal cells but causes diarrhea in infant mice only at doses at least 4 orders of magnitude higher than that of ST Ib(6-18). In contrast, N9P10 guanylin was enterotoxic in mice at much lower doses than guanylin but proved to be a weaker inhibitor of radiolabeled ST I than guanylin in the receptor binding assay. The pattern of guanylate cyclase activation observed for ST Ib(6-18) and the two guanylin analogs parallels the results observed in the receptor binding assay rather than those observed in the diarrheal assay. Treatment of guanylin with chymotrypsin or lumenal fluid derived from newborn mouse intestines resulted in a rapid loss of binding activity. Together, these results suggest that ST I enterotoxins may represent a class of long-lived superagonists of guanylin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimoto S., Takao T., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. Amino-acid sequence of a heat-stable enterotoxin produced by human enterotoxigenic Escherichia coli. Eur J Biochem. 1982 Dec 15;129(2):257–263. doi: 10.1111/j.1432-1033.1982.tb07047.x. [DOI] [PubMed] [Google Scholar]
- Banik N. D., Ganguly U. Diacylglycerol breakdown in plasma membrane of rat intestinal epithelial cells. Effect of E. coli heat-stable toxin. FEBS Lett. 1989 Jul 3;250(2):201–204. doi: 10.1016/0014-5793(89)80720-3. [DOI] [PubMed] [Google Scholar]
- Banik N., Ganguly U. Stimulation of phosphoinositides breakdown by the heat stable E. coli enterotoxin in rat intestinal epithelial cells. FEBS Lett. 1988 Aug 29;236(2):489–492. doi: 10.1016/0014-5793(88)80083-8. [DOI] [PubMed] [Google Scholar]
- Carpick B. W., Gariépy J. Structural characterization of functionally important regions of the Escherichia coli heat-stable enterotoxin STIb. Biochemistry. 1991 May 14;30(19):4803–4809. doi: 10.1021/bi00233a023. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Giannella R. A. Jejunal toxin inactivation regulates susceptibility of the immature rat to STa. Gastroenterology. 1992 Jun;102(6):1988–1996. doi: 10.1016/0016-5085(92)90324-r. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus L. A., Jaso-Friedmann L., Robertson D. C. Characterization of the mechanism of action of Escherichia coli heat-stable enterotoxin. Infect Immun. 1984 May;44(2):493–501. doi: 10.1128/iai.44.2.493-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte L. R., Eber S. L., Turner J. T., Freeman R. H., Fok K. F., Currie M. G. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest. 1993 Jun;91(6):2423–2428. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz J. C., Jaso-Friedman L., Robertson D. C. Binding of Escherichia coli heat-stable enterotoxin to rat intestinal cells and brush border membranes. Infect Immun. 1984 Feb;43(2):622–630. doi: 10.1128/iai.43.2.622-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Judd A. K., Schoolnik G. K. Importance of disulfide bridges in the structure and activity of Escherichia coli enterotoxin ST1b. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8907–8911. doi: 10.1073/pnas.84.24.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Schoolnik G. K. Design of a photoreactive analogue of the Escherichia coli heat-stable enterotoxin STIb: use in identifying its receptor on rat brush border membranes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):483–487. doi: 10.1073/pnas.83.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Drake K. W. Effect of purified Escherichia coli heat-stable enterotoxin on intestinal cyclic nucleotide metabolism and fluid secretion. Infect Immun. 1979 Apr;24(1):19–23. doi: 10.1128/iai.24.1.19-23.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Luttrell M., Thompson M. Binding of Escherichia coli heat-stable enterotoxin to receptors on rat intestinal cells. Am J Physiol. 1983 Oct;245(4):G492–G498. doi: 10.1152/ajpgi.1983.245.4.G492. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Limitations of the infant mouse test for Escherichia coli heat stable enterotoxin. Can J Comp Med. 1979 Oct;43(4):371–379. [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Hugues M., Crane M., Hakki S., O'Hanley P., Waldman S. A. Identification and characterization of a new family of high-affinity receptors for Escherichia coli heat-stable enterotoxin in rat intestinal membranes. Biochemistry. 1991 Nov 5;30(44):10738–10745. doi: 10.1021/bi00108a019. [DOI] [PubMed] [Google Scholar]
- Kubota H., Hidaka Y., Ozaki H., Ito H., Hirayama T., Takeda Y., Shimonishi Y. A long-acting heat-stable enterotoxin analog of enterotoxigenic Escherichia coli with a single D-amino acid. Biochem Biophys Res Commun. 1989 May 30;161(1):229–235. doi: 10.1016/0006-291x(89)91585-4. [DOI] [PubMed] [Google Scholar]
- Kuno T., Kamisaki Y., Waldman S. A., Gariepy J., Schoolnik G., Murad F. Characterization of the receptor for heat-stable enterotoxin from Escherichia coli in rat intestine. J Biol Chem. 1986 Jan 25;261(3):1470–1476. [PubMed] [Google Scholar]
- Levine M. M. Travellers' diarrhoea: prospects for successful immunoprophylaxis. Scand J Gastroenterol Suppl. 1983;84:121–134. [PubMed] [Google Scholar]
- Mann E. A., Cohen M. B., Giannella R. A. Comparison of receptors for Escherichia coli heat-stable enterotoxin: novel receptor present in IEC-6 cells. Am J Physiol. 1993 Jan;264(1 Pt 1):G172–G178. doi: 10.1152/ajpgi.1993.264.1.G172. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Hardy J. W., Hug M. I., Echeverria P., Falkow S. Isolation and nucleotide sequence determination of a gene encoding a heat-stable enterotoxin of Escherichia coli. Infect Immun. 1983 Mar;39(3):1167–1174. doi: 10.1128/iai.39.3.1167-1174.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome P. M., Burgess M. N., Mullan N. A. Effect of Escherichia coli heat-stable enterotoxin on cyclic GMP levels in mouse intestine. Infect Immun. 1978 Oct;22(1):290–291. doi: 10.1128/iai.22.1.290-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Okamoto K., Yukitake J., Miyama A. Reduction of enterotoxic activity of Escherichia coli heat-stable enterotoxin by substitution for an asparagine residue. Infect Immun. 1988 Aug;56(8):2144–2148. doi: 10.1128/iai.56.8.2144-2148.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Sato T., Kubota H., Hata Y., Katsube Y., Shimonishi Y. Molecular structure of the toxin domain of heat-stable enterotoxin produced by a pathogenic strain of Escherichia coli. A putative binding site for a binding protein on rat intestinal epithelial cell membranes. J Biol Chem. 1991 Mar 25;266(9):5934–5941. [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Savarino S. J., Fasano A., Watson J., Martin B. M., Levine M. M., Guandalini S., Guerry P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Shimonishi Y., Hidaka Y., Koizumi M., Hane M., Aimoto S., Takeda T., Miwatani T., Takeda Y. Mode of disulfide bond formation of a heat-stable enterotoxin (STh) produced by a human strain of enterotoxigenic Escherichia coli. FEBS Lett. 1987 May 4;215(1):165–170. doi: 10.1016/0014-5793(87)80134-5. [DOI] [PubMed] [Google Scholar]
- Singh S., Singh G., Heim J. M., Gerzer R. Isolation and expression of a guanylate cyclase-coupled heat stable enterotoxin receptor cDNA from a human colonic cell line. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1455–1463. doi: 10.1016/0006-291x(91)91736-v. [DOI] [PubMed] [Google Scholar]
- Takao T., Shimonishi Y., Kobayashi M., Nishimura O., Arita M., Takeda T., Honda T., Miwatani T. Amino acid sequence of heat-stable enterotoxin produced by Vibrio cholerae non-01. FEBS Lett. 1985 Dec 2;193(2):250–254. doi: 10.1016/0014-5793(85)80163-0. [DOI] [PubMed] [Google Scholar]
- Thompson M. R., Giannella R. A. Different crosslinking agents identify distinctly different putative Escherichia coli heat-stable enterotoxin rat intestinal cell receptor proteins. J Recept Res. 1990;10(1-2):97–117. doi: 10.3109/10799899009064660. [DOI] [PubMed] [Google Scholar]
- Weikel C. S., Spann C. L., Chambers C. P., Crane J. K., Linden J., Hewlett E. L. Phorbol esters enhance the cyclic GMP response of T84 cells to the heat-stable enterotoxin of Escherichia coli (STa). Infect Immun. 1990 May;58(5):1402–1407. doi: 10.1128/iai.58.5.1402-1407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Ikemura H., Watanabe H., Aimoto S., Shimonishi Y., Hara S., Takeda T., Miwatani T., Takeda Y. Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 1985 Feb 11;181(1):138–142. doi: 10.1016/0014-5793(85)81129-7. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Camerato T. R., Goeddel D. V. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991 Sep 25;266(27):17912–17918. [PubMed] [Google Scholar]
- de Sauvage F. J., Horuk R., Bennett G., Quan C., Burnier J. P., Goeddel D. V. Characterization of the recombinant human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1992 Apr 5;267(10):6479–6482. [PubMed] [Google Scholar]
- de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]


