Abstract
The frequency of and risk factors for methicillin-resistant Staphylococcus aureus (MRSA) transmission from a MRSA index person to household contacts were assessed in this prospective study. Between January 2005 and December 2007, 62 newly diagnosed MRSA index persons (46 patients and 16 health care workers) and their 160 household contacts were included in the study analysis. Transmission of MRSA from an index person to household contacts occurred in nearly half of the cases (47%; n = 29). These 29 index persons together had 84 household contacts, of which two-thirds (67%; n = 56) became MRSA positive. Prolonged exposure time to MRSA at home was a significant risk factor for MRSA transmission to household contacts. In addition, MRSA colonization at least in the throat, younger age, and eczema in index persons were significantly associated with MRSA transmission; the presence of wounds was negatively associated with MRSA transmission. Furthermore, an increased number of household contacts and being the partner of a MRSA index person were household-related risk factors for MRSA acquisition from the index person. No predominant pulsed-field gel electrophoresis (PFGE) type was observed to be transmitted more frequently than other PFGE types. To date, screening household contacts and providing MRSA eradication therapy to those found positive simultaneously with the index person is not included in the “search-and-destroy” policy. We suggest including both in MRSA prevention guidelines, as this may reduce further spread of MRSA.
Methicillin-resistant Staphylococcus aureus (MRSA) is currently the most prevalent antibiotic-resistant pathogen in hospitals in many parts of the world, and there are a growing number of reports describing its increasing prevalence in various community populations (10-12). MRSA is an important cause of infections, and MRSA infections are increasing in both health care centers and the community. Compared to methicillin-sensitive Staphylococcus aureus (MSSA), infections with MRSA are more difficult to treat and tend to have a poorer outcome (2, 8).
Carriage of MRSA is a prerequisite for most MRSA infections and plays an important role in the dissemination of this organism within health care facilities and into the community (3, 6, 7, 9). In the Netherlands, due to the “search-and-destroy” infection control policy and a strict antibiotic policy, the number of patients colonized with MRSA is still very limited (13, 31, 34). The “Destroy” part of this policy is important, as it eliminates two out of the three known reservoirs, carriage in patients and carriage in health care workers (HCWs), whereas the third reservoir is the environment. But even in low-prevalence countries like the Netherlands, the emergence of community-acquired MRSA has caused a change in MRSA epidemiology and an increasing number of MRSA cases (13).
In the past, it has been shown that carriers of Staphylococcus aureus and MRSA can be a source of transmission of these pathogens to their household contacts (5, 17, 18, 21, 26). The exact risk factors for transmission of MRSA to household contacts have not been studied properly, but close contact, the environment, or being an HCW are thought to be plausible risk factors for transmission (28, 29, 32).
The contribution of transmission in households to the MRSA burden has not yet been studied, and because of lack of data and well-calculated scenarios, no evidence-based policy for this reservoir has been developed. For this reason, being a household contact of a MRSA carrier has not yet been established as a risk group for MRSA under the Dutch “search-and-destroy” policy.
The aims of this study are to gain insight in the frequency of and risk factors for transmission of MRSA to household contacts and therefore into the community.
(The work was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy—Infectious Diseases Society of America [ICAAC/IDSA], 24 to 28 October 2008, Washington, DC [24a].)
MATERIALS AND METHODS
Data collection.
All newly diagnosed MRSA-positive persons between January 2005 and December 2007 being admitted to or treated in the outpatient clinic at or being an HCW at the Erasmus University Medical Center Rotterdam, the Netherlands, or in a general hospital in Rotterdam, the Netherlands (Maasstad Hospital), and their household contacts were invited to participate in this prospective observational study. Index persons without household contacts were not included in this study. Informed consent was obtained from all persons and their household contacts.
Household contacts were screened for MRSA to determine whether transmission from index persons to household contacts had taken place.
Definitions.
Index persons were patients or HCWs with newly diagnosed MRSA. Household contacts were defined as persons living in the same house as the initial MRSA index person or having frequent contact in the same house (more than 2 h per day) with the index person. MRSA transmission was defined as a positive MRSA swab from the anterior nares, throat, perineum, wounds, or skin lesions, when present, in one of the household contacts during the period of exposure to the index person. The MRSA strain from the household contact had to have the same pulsed-field gel electrophoresis (PFGE) pattern as that of the index person.
The basic reproductive ratio (R0) is the total number of secondary MRSA cases generated from the total number of MRSA index persons introduced into a susceptible population of household contacts. Therefore, R0 = total number of MRSA household contacts/total number of MRSA index persons.
The total exposure time of MRSA positivity between the index person and household contacts was defined as the time between the first positive MRSA culture of the index person and the swabs taken from the household contacts. Exposure time at home was defined as the time between hospital discharge and swabs obtained from household contacts.
Data to determine risk factors for transmission from index to household contacts were collected by means of a standard questionnaire. Questions addressed whether the index person was an HCW or a patient, whether they had current skin problems or nonintact skin (due to wounds, skin lesions, or indwelling devices), and whether they had indwelling devices (e.g., drains or catheters) in situ. Household-related data concerned household composition, relationship of the household contacts to the index patient, age, sex, and number of hours of contact with the index person.
Microbiology methods.
Culture samples for MRSA were taken from the throat, the anterior nares, and the perineum. Samples from existing skin lesions, wounds, or invasive devices (drains, catheters, and external osteosynthesis material) present at the time of MRSA detection were cultured also, simultaneously. MRSA-screening swabs were first inoculated onto blood agar plates (Becton Dickinson) and thereafter put into phenol red mannitol enrichment broth containing 5 mg/liter ceftizoxime and 75 mg/liter aztreonam (33). After 24 h of incubation at 35°C, the blood agar plates were checked for the growth of any bacterial species. If fewer than ≥15 CFU were present, the sampling was deemed to have been insufficient, and a new swab was requested. After 48 h of incubation, 1 loop (1 ml) of the broth was subcultured onto a blood agar plate. Microbiological methods to identify and type MRSA are described elsewhere (20).
PFGE.
All MRSA isolates were molecularly typed by PFGE. To verify the relatedness of MRSA isolates from index patients and household contacts, the isolates from index and household contacts were compared. Intrafamilial transmission, from an index person to a household contact, was considered to be established when PFGE types were identical (30).
Statistical analysis.
All analyses were done using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL). Proportions were compared by using the chi-square test (Fisher's exact test in the case of small numbers), and continuous data with Mann-Whitney's test. Results are reported as odds ratios (OR) with 95% confidence intervals (95% CI). The statistical tests were 2-tailed; a P value of less than 0.05 was considered statistically significant.
RESULTS
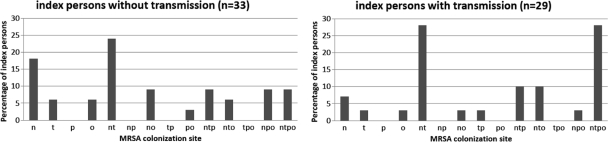
Between January 2005 and December 2007, 62 MRSA-positive persons (46 patients and 16 HCWs) and their 160 household contacts agreed to participate and were included in the analysis. The median age of the MRSA-positive persons was 33 years (range, 0 to 87 years), and 58% (n = 36) were male. The median age of the household contacts was 28 years (range, 0 to 77 years), and 49% (n = 77) were male. The index persons had a median of two MRSA-colonized sites (range, 1 to 5 sites). Fifty-four of the index persons (87%) were MRSA colonized at least in the nose, 42 index persons (68%) were MRSA colonized at least in the throat, 23 index persons (37%) were MRSA colonized at least at the perineal area, and 28 index persons (45%) were MRSA colonized at least at other sites (urine, n = 4; exit sites, n = 6; wounds, n = 17; and other, n = 1). The index persons had a median number of three household members (range, 1 to 10). Twenty-one index persons (34%) lived together with their partner, 16 (26%) with their partner and children, 10 (16%) with their parents, 11 (18%) with their parents and siblings, and 5 (8%) with other persons. The diversity of MRSA colonization sites of the index persons is depicted in Fig. 1.
FIG. 1.
Rates of MRSA colonization of sites in 62 MRSA-positive index persons. n, nose only; t, throat only; p, perineum only; o, other only; nt, nose + throat; np, nose + perineum; no, nose + other; tp, throat + perineum; po, perineum + other; ntp, nose + throat + perineum; nto, nose + throat + other; tpo, throat + perineum + other; npo, nose + perineum + other; ntpo, nose + throat + perineum + other.
In 33 of the 62 (53%) index cases, no transmission to their household contacts was observed. These 33 index persons together had 76 household contacts. Transmission of MRSA from an index person to household contacts occurred in 29 out of the 62 (47%) index persons. These 29 index persons together had 84 household contacts. The attack rate of MRSA transmission in the 84 household contacts was 67%, as 56 household contacts became MRSA positive. The overall or general basic reproductive ratio, R0, was 56 MRSA-positive household contacts divided by 62 MRSA index persons; therefore, the R0 = 0.90.
Risk factors for MRSA transmission to household contacts are shown in Table 1. Index persons with MRSA transmission to their household contacts were significantly younger than the index persons without transmission (25 years versus 45 years; P = 0.05). Index persons who transmitted MRSA to their household contacts had a median of three MRSA-colonized sites (range, 1 to 5), and index persons without transmission had a median of two MRSA-colonized sites (range, 1 to 5) (P = 0.15). Twenty-four of the index persons (83%) who transmitted MRSA were at least MRSA colonized in the throat, compared to 18 index persons (56%) who did not transmit MRSA (P = 0.03). Furthermore, MRSA throat colonization in combination with one or more other MRSA-colonized sites significantly increased the risk of MRSA transmission (P = 0.02; OR, 4.00; and 95% CI, 1.23 to 13.05). Index persons with eczema had significantly more risk of MRSA transmission than index persons without eczema (P = 0.05). Interestingly, the presence of wounds was negatively associated with MRSA transmission. Of the index persons without transmission (n = 33), the median total MRSA exposure time was 14.5 days (range, 2 to 330). In the group with transmission to the household contacts, the median total MRSA exposure time was 29 days (range, 1 to 1,134). The duration of MRSA exposure time at home was significantly associated with MRSA transmission to household contacts (41 days of exposure versus 15 days for those without transmission; P = 0.04).
TABLE 1.
Index person-related potential risk factors for MRSA transmission
| Potential risk factor | Transmission of MRSA (n = 29)a | No transmission of MRSA (n = 33)a | P value | OR (95% CI) |
|---|---|---|---|---|
| Characteristics of index persons | ||||
| Health care worker | 5 (17) | 11 (33) | 0.15 | 0.42 (0.13-1.39) |
| Male | 17 (59) | 19 (58) | 0.93 | 1.04 (0.38-2.87) |
| Median age in yrs (range) | 25 (0-87) | 45 (0-80) | 0.05b | |
| Age group in yrs | ||||
| 0-10 | 8 (28) | 7 (21) | 0.93 | |
| 11-20 | 1 (3) | 0 (0) | 1.00 | |
| 21-60 | 17 (59) | 22 (67) | 0.52 | 0.68 (0.21-2.23) |
| >61 | 3 (10) | 4 (12) | 0.65 | 0.66 (0.11-4.00) |
| Characteristics of MRSA colonization | ||||
| Median no. (range) of sites colonized | 3 (1-5) | 2 (1-5) | 0.15b | |
| Colonization at least in: | ||||
| Nose | 26 (90) | 28 (85) | 0.71 | 1.55 (0.34-7.13) |
| Throat (n = 61) | 24 (83) | 18 (56) | 0.03 | 3.73 (1.14-12.27) |
| Perineum (n = 61) | 13 (45) | 10 (21) | 0.28 | 1.79 (0.63-5.09) |
| Elsewhere | 14 (48) | 13 (39) | 0.48 | 1.44 (0.52-3.94) |
| Colonization only: | ||||
| In throat | 1 (3) | 2 (6) | 0.63 | 0.55 (0.05-6.44) |
| Elsewhere than throat | 5 (17) | 15 (46) | 0.02 | 0.25 (0.08-0.82) |
| Colonization in throat and elsewhere | 24 (83) | 18 (55) | 0.02 | 4.00 (1.23-13.05) |
| Skin-related risk factors | ||||
| Nonintact skin | 12 (41) | 19 (58) | 0.20 | 0.52 (0.19-1.43) |
| Skin problems | 8 (28) | 6 (18) | 0.38 | 1.71 (0.52-5.71) |
| Eczema (n = 58) | 4 (14) | 0 (0) | 0.05c | |
| Wounds | 6 (21) | 16 (42) | 0.02 | 0.28 (0.09-0.86) |
| Indwelling devices | 4 (15) | 10 (30) | 0.12 | 0.37 (0.10-1.34) |
| Exposure | ||||
| No. of household members [median (range)] | 3 (1-7) | 1 (1-10) | 0.007b | |
| Exposure time in days [median (range)] (n = 56) | 29 (1-1,134) | 14.5 (2-330) | 0.07b | |
| Exposure time at home in days [median (range)] (n = 51) | 41 (1-1,134) | 15 (1-330) | 0.04b |
Values are the number (%) of index persons with the risk factor, unless otherwise indicated.
Mann-Whitney test (two-sided).
Fisher's exact test.
Index persons with transmission had a significantly higher median number of household contacts than index persons without transmission (3.0 versus 1.0, P = 0.007). Thirty-five percent of the index persons (n = 10) with MRSA transmission lived together with their partner and children, 17% (n = 5) with their partner, 28% (n = 8) with their parents and siblings, 17% (n = 5) with their parents, and 3% (n = 1) with other persons. This is in contrast to the index persons without transmission, where 18% of the index persons (n = 6) without MRSA transmission lived together with their partner and children, 49% (n = 5) with their partner, 9% (n = 3) with their parents and siblings, 15% (n = 5) with their parents, and 9% (n = 3) with other persons. In addition, 24 index persons (83%) with MRSA transmission had more than one household contact, in contrast to 16 index persons (49%) without transmission (P = 0.005; OR, 5.100; and 95% CI, 1.57 to 16.61). The risk of MRSA transmission to household contacts was highest among partners of the index person (P = 0.02; OR, 5.20; and 95% CI, 1.10 to 24.52). The hours of contact per day between index persons and household members were not associated with an increased risk of MRSA transmission. Household-related determinants of transmission are shown in Table 2.
TABLE 2.
Risk factors for MRSA transmission to household members
| Potential risk factor | Transmission of MRSA (n = 56)a | No transmission of MRSA (n = 28)a | P value | OR (95% CI) |
|---|---|---|---|---|
| Female | 28 (50) | 12 (43) | 0.54 | 1.33 (0.54-3.22) |
| Median age in yrs (range) | 23 (0-77) | 18 (4-50) | 0.77b | |
| Relationship to index person | ||||
| Partner | 16 (29) | 2 (7) | 0.02 | 5.20 (1.10-24.52) |
| Child | 11 (20) | 8 (29) | 0.36 | 0.61 (0.21-1.75) |
| Parent | 16 (29) | 9 (32) | 0.74 | 0.84 (0.32-2.26) |
| Sibling | 8 (14) | 4 (14) | 1.00c | |
| Other | 5 (9) | 5 (18) | 0.29 | 0.45 (0.12-1.71) |
| Contact with index person in h/dayd | ||||
| <1 | 2 (5) | 2 (11) | 0.39 | 1.00 |
| 1-4 | 3 (8) | 4 (22) | 0.82 | 0.75 (0.06-8.83) |
| 5-10 | 11 (30) | 8 (28) | 0.49 | 1.10 (0.24-20.40) |
| >10 | 21 (57) | 7 (39) | 0.31 | 3.00 (0.35-25.46) |
Values are the number (%) of household contacts with the risk factor, unless otherwise indicated.
Mann-Whitney test (two-sided).
Fisher's exact test.
The number of hours of contact per day with the index person was only obtained for 55/84 household contacts.
PFGE strains of index persons and their MRSA-positive household contacts were compared to determine if MRSA transmission took place. Thirty different PFGE patterns were identified in the 62 MRSA-positive index persons. The 29 index persons that had apparently transmitted their strain to their household contacts had 20 genotypically different MRSA strains. All isolates from MRSA-positive household members had the same PFGE pattern as the isolate from their index person. There was no dominant PFGE type that was transmitted more frequently than other PFGE types.
DISCUSSION
Our study shows that MRSA transmission from an index person to household contacts occurs in approximately half the cases (47%). Furthermore, when an index person transmits MRSA to household contacts, two-thirds of all household contacts (67%) will become MRSA colonized. Risk factors for MRSA transmission were identified both in MRSA index persons and household contacts. MRSA carriage at least in the throat, the duration of MRSA exposure time at home, eczema, and younger age were all significant risk factors for transmission to household contacts. Furthermore, MRSA transmission was significantly associated with index persons who had more than one household contact. A household-contact-related risk factor for acquisition of MRSA was being a partner of the index person.
As our study was conducted in the Netherlands, where the prevalence of MRSA is among the lowest in the world (13), the MRSA transmission rate in our study may not be representative for countries were the MRSA prevalence is higher. In these latter countries, in cases where a similar PFGE strain is cultured from a household contact, it is more difficult to determine whether the index person was the MRSA source or whether the strain was picked up from another source in the community. In such circumstances, it remains equivocal whether intrafamilial transmission has occurred. As our study demonstrated a large diversity of different PFGE types in combination with a very low prevalence of MRSA in our community, we can better ascertain that the household contact obtained the MRSA from the index person.
Our study did not collect information on the recent antibiotic history of the MRSA-positive index cases or the MRSA-positive contacts. Therefore, we cannot be certain if this has influenced the outcome of our study.
In previous studies, it has been demonstrated that the environment of the MRSA index person may have acted as an intermediate source for transmission to household contacts (4, 27, 28). In that case, the index had first contaminated the household environment and, therefore, the environment served as a MRSA source for the household contacts. In this study, we did not attempt to establish the potential role of the environment in MRSA transmission, as we did not collect environmental specimens in the homes of the index persons. A study by Boyce et al. (4) showed that the frequency of environmental contamination by MRSA in a hospital was higher when patients had MRSA-positive wounds or urine than when MRSA was present in other body sites. Studies of MRSA contamination of the environment in a household setting have yet to be reported. Thus, when household contacts share equipment or personal items of a MRSA index person, it is plausible that they also are at risk of becoming colonized with MRSA.
The spread of MRSA among household contacts has been reported previously, but the observed MRSA transmission rates to household contacts are variable (5, 14, 16, 18, 22, 23, 36). Johansson et al. (18) observed that in 22 of 51 index persons (43%), MRSA was transmitted to one or more household contacts. However, Calfee et al. (5) observed a lower MRSA transmission rate than in our study, as 21 of the 88 index persons (24%) transmitted MRSA to their household contacts. A recent study reported 19% MRSA transmission to household contacts from index persons treated in home health care settings (23). Most studies reporting on MRSA transmission from index persons to household members differ in study methods, and therefore, comparison of study outcomes with that of our study cannot yield robust conclusions (14, 15, 22, 23, 36). These studies differ mostly in the sites cultured. Ho et al. (16) observed MRSA transmission in 12 index persons with 46 household members at risk. Six (13%) of the household members became MRSA positive. In this study, swabs were taken from the anterior vestibule of the nose, axillary skin, and cutaneous or wound lesions. Thus, no perineal or throat swabs were obtained. The lack of perineal and, especially, throat swabs has also been observed in other studies (14, 22, 36). Therefore, these studies have probably underestimated the MRSA carriage of index persons and MRSA transmission to household contacts (24, 35).
Several risk factors for MRSA transmission were observed in our study. First, MRSA throat carriage of the index person significantly increases the risk for transmission to household contacts. Studies of MRSA transmission into the environment showed that respiratory secretions can contribute to transmission into the environment (27, 29), suggesting that transmission of MRSA can occur by the dispersal of MRSA from the throat by coughing, sneezing, or kissing. For this reason, it is important to establish colonization by swabbing not only the anterior nares but also the throat. Second, we demonstrated that the risk of transmission from index persons to household contacts depends on MRSA exposure time at home. Calfee et al. demonstrated that index persons who returned home while known to be MRSA positive transmitted MRSA to their household members significantly more often than index persons who returned to a (residential) care home (5). Healthcare workers had a significant shorter exposure time at home than the MRSA-positive patients. This can be explained by the fact that HCWs are offered eradication therapy immediately after MRSA carriage is detected, as MRSA-positive HCWs are not allowed to work in the Netherlands. Furthermore, our study revealed that index persons who transmitted MRSA to one or more of their household contacts had on average more household contacts than those who did not transmit, i.e., their households were possibly more crowded. Crowding has been shown before to be a risk factor for transmission of MRSA (5, 18).
Third, people with eczema tend to transmit MRSA to their household contacts more frequently than people without eczema. A possible explanation is that eczema sites usually are not covered by bandages; therefore, dispersion of MRSA on skin particles is not impeded and, thus, contamination of the environment with MRSA is more likely. Similarly, we showed that covered wounds or skin lesions protect significantly against transmission of MRSA from index persons to their household contacts. This is contrary to the results of a study by Moore et al. showing that broken skin or chronic skin lesions contribute to the acquisition of MRSA in roommate contacts with MRSA colonization or infection (25). We assume that in our setting, due to strict aseptic wound care, including proper coverage of wounds and hygienic precautions, the risk of MRSA transmission may be significantly lowered.
Interestingly, the median age of index persons that did experience household transmission was significantly lower than the median age of those without transmission (25 years versus 45 years). This is confirmed by the results of the studies performed by Johansson et al. and Calfee et al. (5, 18). This age-related effect is not readily explained but may be associated with more-crowded living conditions in the household in the younger age groups than for the elderly.
The relationship of a household member to a MRSA index person also was a risk factor in our study. Partners of MRSA index persons are more likely to become MRSA colonized than other household contacts of the index person. A possible explanation may be that partners share bed linen and have bodily contact, which is a known risk factor for MRSA acquisition (15). No other household-related determinants for MRSA transmission could be revealed in our study because no further clinical data from household contacts was available.
Intrafamilial transmission of MRSA has raised important issues, such as whether household screening should be routinely performed to search for carriers and treat them. Failure to identify MRSA-positive household contacts may cause MRSA recolonization of the index patient and also contribute to the spread of MRSA into the community. Silent recolonization of index persons can reintroduce MRSA into the hospital. Even though the R0 as measured in the household contacts was <1, MRSA transmission may contribute to the spread of MRSA in specific community populations of the index person or MRSA-positive household contacts (1, 19).
To date, screening household contacts and providing eradication therapy to those found positive simultaneously with the index person is not included in the “search-and-destroy” policy in the Netherlands or elsewhere. We suggest including both in prevention guidelines for MRSA, as this may reduce further spread of MRSA strains into the community and in health care settings.
Acknowledgments
This study was supported by a Doelmatigheid grant from the Erasmus University Medical Center Rotterdam, the Netherlands.
We thank A. Ott (Laboratory for Infectious Diseases, Groningen, the Netherlands) for his contribution to the design of this study.
The authors report no conflict of interest.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2009. Methicillin-resistant Staphylococcus aureus among players on a high school football team—New York City, 2007. MMWR Morb. Mortal. Wkly. Rep. 58:52-55. [PubMed] [Google Scholar]
- 2.Blot, S. I., K. H. Vandewoude, E. A. Hoste, and F. A. Colardyn. 2002. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch. Intern. Med. 162:2229-2235. [DOI] [PubMed] [Google Scholar]
- 3.Boyce, J. M. 1989. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect. Dis. Clin. North Am. 3:901-913. [PubMed] [Google Scholar]
- 4.Boyce, J. M., G. Potter-Bynoe, C. Chenevert, and T. King. 1997. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect. Control Hosp. Epidemiol. 18:622-627. [PubMed] [Google Scholar]
- 5.Calfee, D. P., L. J. Durbin, T. P. Germanson, D. M. Toney, E. B. Smith, and B. M. Farr. 2003. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect. Control Hosp. Epidemiol. 24:422-426. [DOI] [PubMed] [Google Scholar]
- 6.Casewell, M. W., and R. L. Hill. 1986. Elimination of nasal carriage of Staphylococcus aureus with mupirocin (“pseudomonic acid”)-a controlled trial. J. Antimicrob. Chemother. 17:365-372. [DOI] [PubMed] [Google Scholar]
- 7.Casewell, M. W., and R. L. Hill. 1985. In-vitro activity of mupirocin (“pseudomonic acid”) against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 15:523-531. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 9.Darouiche, R., C. Wright, R. Hamill, M. Koza, D. Lewis, and J. Markowski. 1991. Eradication of colonization by methicillin-resistant Staphylococcus aureus by using oral minocycline-rifampin and topical mupirocin. Antimicrob. Agents Chemother. 35:1612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, E. A., A. M. Emmerson, G. M. Hogg, M. F. Patterson, and M. D. Shields. 1987. An outbreak of infection with a methicillin-resistant Staphylococcus aureus in a special care baby unit: value of topical mupirocin and of traditional methods of infection control. J. Hosp. Infect. 10:120-128. [DOI] [PubMed] [Google Scholar]
- 11.Davis, K. A., J. J. Stewart, H. K. Crouch, C. E. Florez, and D. R. Hospenthal. 2004. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39:776-782. [DOI] [PubMed] [Google Scholar]
- 12.Doebbeling, B. N., D. L. Breneman, H. C. Neu, R. Aly, B. G. Yangco, H. P. Holley, Jr., R. J. Marsh, M. A. Pfaller, J. E. McGowan, Jr., B. E. Scully, et al. 1993. Elimination of Staphylococcus aureus nasal carriage in health care workers: analysis of six clinical trials with calcium mupirocin ointment. The Mupirocin Collaborative Study Group. Clin. Infect. Dis. 17:466-474. [DOI] [PubMed] [Google Scholar]
- 13.European Antimicrobial Resistance Surveillance System. 2007. EARSS Annual report 2006. EARSS, Bilthoven, The Netherlands. http://www.earss.rivm.nl.
- 14.Eveillard, M., Y. Martin, N. Hidri, Y. Boussougant, and M. L. Joly-Guillou. 2004. Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: prevalence, duration, and transmission to households. Infect. Control Hosp. Epidemiol. 25:114-120. [DOI] [PubMed] [Google Scholar]
- 15.Hall, A. J., D. Bixler, and L. E. Haddy. 2009. Multiclonal outbreak of methicillin-resistant Staphylococcus aureus infections on a collegiate football team. Epidemiol. Infect. 137:85-93. [DOI] [PubMed] [Google Scholar]
- 16.Ho, P. L., C. Cheung, G. C. Mak, C. W. Tse, T. K. Ng, C. H. Cheung, T. L. Que, R. Lam, R. W. Lai, R. W. Yung, and K. Y. Yuen. 2007. Molecular epidemiology and household transmission of community-associated methicillin-resistant Staphylococcus aureus in Hong Kong. Diagn. Microbiol. Infect. Dis. 57:145-151. [DOI] [PubMed] [Google Scholar]
- 17.Huijsdens, X. W., M. G. van Santen-Verheuvel, E. Spalburg, M. E. Heck, G. N. Pluister, B. A. Eijkelkamp, A. J. de Neeling, and W. J. Wannet. 2006. Multiple cases of familial transmission of community-acquired methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 44:2994-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson, P. J., E. B. Gustafsson, and H. Ringberg. 2007. High prevalence of MRSA in household contacts. Scand. J. Infect. Dis. 39:764-768. [DOI] [PubMed] [Google Scholar]
- 19.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 20.Kerremans, J. J., J. Maaskant, H. A. Verbrugh, W. B. van Leeuwen, and M. C. Vos. 2008. Detection of methicillin-resistant Staphylococcus aureus in a low-prevalence setting by polymerase chain reaction with a selective enrichment broth. Diagn. Microbiol. Infect. Dis. 61:396-401. [DOI] [PubMed] [Google Scholar]
- 21.Larsen, A. R., S. Bocher, M. Stegger, R. Goering, L. V. Pallesen, and R. Skov. 2008. Epidemiology of European community-associated methicillin-resistant Staphylococcus aureus clonal complex 80 type IV strains isolated in Denmark from 1993 to 2004. J. Clin. Microbiol. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, P. L., J. C. Tsai, Y. W. Chiu, F. Y. Chang, Y. W. Chen, C. F. Hsiao, and L. K. Siu. 2008. Methicillin-resistant Staphylococcus aureus carriage, infection and transmission in dialysis patients, healthcare workers and their family members. Nephrol. Dial. Transplant. 23:1659-1665. [DOI] [PubMed] [Google Scholar]
- 23.Lucet, J. C., X. Paoletti, C. Demontpion, M. Degrave, D. Vanjak, C. Vincent, A. Andremont, V. Jarlier, F. Mentre, and M. H. Nicolas-Chanoine. 2009. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch. Intern. Med. 169:1372-1378. [DOI] [PubMed] [Google Scholar]
- 24.Mertz, D., R. Frei, B. Jaussi, A. Tietz, C. Stebler, U. Fluckiger, and A. F. Widmer. 2007. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 45:475-477. [DOI] [PubMed] [Google Scholar]
- 24a.Mollema, F. P. N., J. Richardus, H. A. Verbruch, N. Vaessen, and M. C. Vos. 2008. Untreated MRSA carriership: a source of transmission into the community, abstr. K-1705. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 48th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 25.Moore, C., J. Dhaliwal, A. Tong, S. Eden, C. Wigston, B. Willey, and A. McGeer. 2008. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition in roommate contacts of patients colonized or infected with MRSA in an acute-care hospital. Infect. Control Hosp. Epidemiol. 29:600-606. [DOI] [PubMed] [Google Scholar]
- 26.Nahmias, A. J., M. H. Lepper, V. Hurst, and S. Mudd. 1962. Epidemiology and treatment of chronic staphylococcal infections in the household. Am. J. Public Health Nations Health 52:1828-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohr, U., A. Kaminski, M. Wilhelm, L. Jurzik, S. Gatermann, and G. Muhr. 2009. Colonization of patients and contamination of the patients' environment by MRSA under conditions of single-room isolation. Int. J. Hyg. Environ. Health 212:209-215. [DOI] [PubMed] [Google Scholar]
- 28.Scott, E., S. Duty, and M. Callahan. 2008. A pilot study to isolate Staphylococcus aureus and methicillin-resistant S. aureus from environmental surfaces in the home. Am. J. Infect. Control. 36:458-460. [DOI] [PubMed] [Google Scholar]
- 29.Snyder, G. M., K. A. Thom, J. P. Furuno, E. N. Perencevich, M. C. Roghmann, S. M. Strauss, G. Netzer, and A. D. Harris. 2008. Detection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on the gowns and gloves of healthcare workers. Infect. Control Hosp. Epidemiol. 29:583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vos, M. C., M. D. Behrendt, D. C. Melles, F. P. N. Mollema, W. de Groot, G. Parlevliet, A. Ott, D. Horst-Kreft, A. van Belkum, and H. A. Verbrugh. 2009. 5 years of experience implementing a methicillin-resistant Staphylococcus aureus search and destroy policy at the largest university medical center in The Netherlands. Infect. Control Hosp. Epidemiol. 30:977-984. [DOI] [PubMed] [Google Scholar]
- 32.Weese, J. S., H. Dick, B. M. Willey, A. McGeer, B. N. Kreiswirth, B. Innis, and D. E. Low. 2006. Suspected transmission of methicillin-resistant Staphylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet. Microbiol. 115:148-155. [DOI] [PubMed] [Google Scholar]
- 33.Wertheim, H., H. A. Verbrugh, C. van Pelt, P. de Man, A. van Belkum, and M. C. Vos. 2001. Improved detection of methicillin-resistant Staphylococcus aureus using phenyl mannitol broth containing aztreonam and ceftizoxime. J. Clin. Microbiol. 39:2660-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wertheim, H. F., M. C. Vos, H. A. Boelens, A. Voss, C. M. Vandenbroucke-Grauls, M. H. Meester, J. A. Kluytmans, P. H. van Keulen, and H. A. Verbrugh. 2004. Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J. Hosp. Infect. 56:321-325. [DOI] [PubMed] [Google Scholar]
- 35.Widmer, A. F., D. Mertz, and R. Frei. 2008. Necessity of screening of both the nose and the throat to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit. J. Clin. Microbiol. 46:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zafar, U., L. B. Johnson, M. Hanna, K. Riederer, M. Sharma, M. G. Fakih, M. C. Thirumoorthi, R. Farjo, and R. Khatib. 2007. Prevalence of nasal colonization among patients with community-associated methicillin-resistant Staphylococcus aureus infection and their household contacts. Infect. Control Hosp. Epidemiol. 28:966-969. [DOI] [PubMed] [Google Scholar]