Abstract
Panton-Valentine leukocidin (PVL) is associated with staphylococcal skin and pulmonary infections. Community dissemination of PVL-producing Staphylococcus aureus strains constitutes a public health concern. Family transmission and spread of community-acquired leukocidin-positive methicillin-susceptible S. aureus ST152 isolates associated with severe clinical symptoms are herein described. Remarkably, ST152 isolates usually are methicillin resistant.
CASE REPORT
From July 2003 to December 2004, nine patients, members of a family and their relatives, had 20 episodes of skin infections, including abscesses in different body areas, bursitis, cellulitis, folliculitis, and furuncles. There was not any predisposed factor for soft tissue infection in the studied sample.
Twenty-three cultures were performed at the Microbiology Department of the hospital, and three different Staphylococcus aureus strains were recovered from different clinical samples from patients (Table 1). Treatment measurements for methicillin-resistant S. aureus (MRSA) infections in hospitals were immediately implemented (13). Two of these three strains were isolated on repeated occasions, while the third one was isolated only once. The three strains had the same antibiotic resistance profile; they were resistant to penicillin (penicillin-resistant S. aureus [PRSA]) and susceptible to clindamycin, ciprofloxacin, erythromycin, gentamicin, mupirocin, oxacillin, trimethoprim-sulfamethoxazole, rifampin, teicoplanin, and vancomycin. Molecular characterization of these PRSA strains signaled a remarkable association of community-acquired (CA) leukocidin-positive methicillin-susceptible S. aureus (MSSA) ST152 isolates with familial furunculosis.
TABLE 1.
Familial distribution of the 22 S. aureus isolatesa
| Isolate | PFGE type | PVL | Patient (age in yr) | Sample | Body location |
|---|---|---|---|---|---|
| 1 | A | + | M (39) | Abscess | Underarm |
| 2 | A | + | M (39) | Abscess | Underarm |
| 3 | A | + | M (39) | Pharyngeal | Pharynges |
| No sample | F (35) | ||||
| 4 | A | + | 1 (17) | Abscess | RL |
| 5 | A | + | 1 (17) | Abscess | RL |
| 6 | A | + | 1 (17) | Abscess | LH |
| 7 | B | − | 1 (17) | Abscess | RL and LL |
| 8 | A | + | 2 (15) | Abscess | Toe |
| 9 | A | + | 3 (12) | Abscess | Forehead |
| 10 | A | + | 4 (9) | Nasal | Nose |
| 11 | B | − | 4 (9) | Nasal | Nose |
| 12 | C | − | 4 (9) | Pharyngeal | Pharynges |
| 13 | A | + | 5 (8) | Pharyngeal | Pharynges |
| 14 | A | + | 5 (8) | Nasal | Nose |
| 15 | B | − | 5 (8) | Pharyngeal | Pharynges |
| PR S. aureusb | 6 (5) | Exudate | Underarm | ||
| 16 | A | + | Gf (17) | Exudate | Umbilicus (piercing) |
| 17 | A | − | Gf (17) | Pharyngeal | Pharynges |
| 18 | A | − | Gf (17) | Pharyngeal | Pharynges |
| 19 | B | + | Gf (17) | Abscess | RL |
| 20 | B | − | Gf, M | Nasal | Nose |
| 21 | B | − | Gf, M | Pharyngeal | Pharynges |
| No sample | Gf, F | ||||
| 22 | A | + | N (68) | Abscess | RH finger |
M, mother; F, father; Gf, girlfriend; N, neighbor; LH, left hand; LL, left leg; RH, right hand; RL, right leg; PR, penicillin resistant; +, positive; −, negative.
Lost.
The nine patients were treated with systemic and topical antibiotherapy. Systemic antibiosis was used but the antibiotic and the treatment schedule changed depending on the patients' lesions. In cases of abscesses, 7 days of treatment with oral amoxicillin-clavulanic or cloxacillin was undergone. In cases of folliculitis, cellulitis, or bursitis, 3 days of intravenous (i.v.) amoxicillin-clavulanic, cloxacillin, or erythromycin treatment was administered. Some of them also needed surgical incisions and drainage. After the patients had been provided with information and teaching, all members of the family and relatives who were infected by ≥1 PRSA strain were prescribed with nasal mupirocin treatment twice a day and chlorhexidine showers once a day for 5 days. Moreover, the simultaneous use of alcohol-based hand rubs for the same people, plus all their relatives, was implemented. These measures were repeated each time that a member of this studied population became infected by PRSA. After application of these measures, there was satisfactory outcome of the episodes. Nevertheless, 1 year after the burst was thought to be solved, a new case of skin infection was detected in one son, who presented furuncle in the nape, infected by a Panton-Valentine leukocidin (PVL)-positive PRSA strain. He received antimicrobial therapy described above in case of abscesses and additionally with nasal mupirocin and chlorhexidine showers for 5 days. All other family members were then treated for 2 weeks with oral cloxacillin and for 1 month with nasal mupirocin, yielding satisfactory outcome.
Molecular analyses.
Methicillin susceptibility was ensured by PCR testing of the methicillin-resistant gene mecA (17). PRSA isolates were typed by pulsed-field gel electrophoresis (PFGE) after SmaI digestion and by multiple-locus variable-number tandem repeat analysis (MLVA) (18, 20). PFGE permitted the establishment of the clonal relationship among isolates. MLVA confirmed the results obtained by PFGE. Later, multilocus sequence typing (MLST) was applied for the unambiguous assessment of the evolutionary relatedness between isolates (18). Moreover, all isolates were analyzed by PCR to detect genetic sequences encoding enterotoxins; exfoliative toxins; alpha-, beta-, gamma-, and delta-hemolysins; LukE-LukD leukotoxins; PVL; and different adhesins as collagen, elastin, and laminin, as well as fibrinogen and fibronectin binding factors (2, 10, 14, 15). The correct assignment of the amplicons was verified by sequencing.
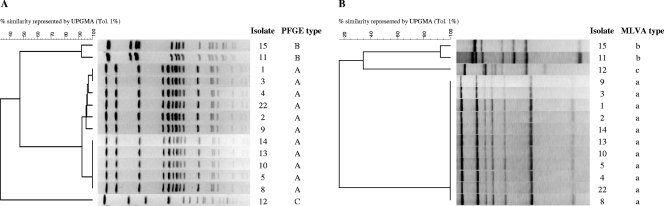
Nine people were screened and were all colonized with S. aureus. None harbored MRSA. The nine colonized people carried PRSA. The results of PFGE and MLVA for eight patients are shown in Fig. 1. PFGE patterns showed that 15 isolates belonged to PFGE type A, six to PFGE type B, and one to the sporadic type C. The same grouping distribution was obtained by MLVA. Genes encoding PVL were found in 13 isolates from clone A and in only one isolate from clone B. In total, eight people (88.9%) from the studied group harbored PVL-positive S. aureus isolates. Interestingly, only the two type A isolates that were negative for PVL were LukED positive. Moreover, most isolates (≥80%) belonging to clone A were positive for alpha-hemolysin (14 of 15 isolates [93%]), beta-hemolysin (13 of 15 [87%]), delta-hemolysin (14 οf 15 [93%]), and collagen (12 οf 15 [80%]) and laminin (15 οf 15 [100%]) binding proteins.
FIG. 1.
PFGE patterns of SmaI-digested DNA (A) and MLVA profiles of 14 S. aureus isolates recovered from clinical samples from family members or relatives (B). Each lane corresponds to a single S. aureus isolate from a different PFGE type (A, B, C) or MLVA type (a, b, c) recovered from different patients, as follows: isolates 1, 2, and 3, isolates from the mother; isolates 4 and 5, from the 17-year-old son; isolate 8, from the 15-year-old son; isolate 9, from the 12-year-old son; isolates 10, 11, and 12, from the 9-year-old son; isolates 13, 14, and 15, from the 8-year-old son; isolate 22, from the neighbor taking care of the children.
Discussion.
During the last year, emergence and spread of virulent CA S. aureus clones have been a rising problem, constituting a now serious global health concern (9, 11). CA staphylococcal infections have been repeatedly associated with skin and soft tissue infections. Staphylococcal furunculosis can be seriously complicated if the infecting strain harbors genes for production of the PVL (8). CA-MRSA strains carrying PVL have been described as a worldwide emergent problem, and also, PVL-positive MSSA strains have been already associated with staphylococcal skin and pulmonary infections (7, 10, 22). Worryingly, cases of human-to-human transmission of PVL-positive MSSA occurring out of a family, which implies no transmission by family contact, have also been already described (3, 4). The presence of the lukPV genes seems to be related to an increased likelihood of complications in children with S. aureus musculoskeletal infections and to the development of multiple furuncles with more-intense erythema in healthy young adults (12). In our study, eight people were found to be infected by one PVL gene-positive MSSA clone. Members of this group had had relapsing episodes of skin infections, including furuncles, abscesses, and cellulitis, over 18 months.
Clustering of isolates showed total reproducibility when two types of analyses, i.e., PFGE and MLVA, were applied. In summary, out of all isolates (n = 22), 15 isolates (68.2%) showed PFGE type A and MLVA type I, six isolates (27.3%) PFGE type B and MLVA type II, and one (4.5%) PFGE type C and MLVA type III. Interestingly, Fig. 1B shows that MLVA demonstrates the genetic difference existing between the sporadic PFGE clone C and the health concerning PFGE clone A. Most of the 15 type A isolates were recovered from infections (nine isolates [60%]), while four of the six type B isolates were considered colonization (two nasal and two pharyngeal colonization isolates [66.6%]) and the single type C isolate was considered pharyngeal colonization. Overall, 11 isolates (50%) were recovered as colonizing isolates, while the other 11 caused infection. From the 11 infecting isolates, 10 (90.9%) were PVL positive, and nine (90%) were PFGE type A and MLVA type I, which seems to indicate a high infective ability in the case of isolates showing PFGE type A and MLVA type I and also harboring the PVL genes.
The recovery of a PVL-positive clone (clone A) only from members of one family and their relatives who had had skin infections strongly suggests pathogenicity of this particular clone. To our knowledge, this is the third description of transmission of PVL-producing MSSA between members of a family and their relatives (1, 16). Previous studies showed identical PFGE profiles in nasal and furuncle PVL-positive isolates from patients with skin infections (6). Gene content respecting virulence factors was variable depending on the isolate. Overall, most isolates from clone A (≥13 of 15 [≥86%]) were positive for PVL; alpha-, beta-, and delta-hemolysins; laminin binding protein; and collagen binding protein, and caused double hemolysis when grown in blood agar plates. These isolates were negative for leukocidin E and D, gamma-hemolysin, a and b exfoliative toxins, fibrinogen binding protein, elastin binding protein, and A and B fibronectin binding factors. The two type A PVL-negative isolates were the only type A LukED-positive isolates. Staphylococcal bicomponent leukotoxins are exotoxins consisting of two nonassociated but synergic class S and class F proteins. In this family of toxins, different S and F protein combinations are encoded by distinct types of transferable genetic elements, e.g., phages or pathogenicity islands. As a consequence, different S. aureus isolates can show dissimilar toxin gene combinations even if they belong to the same clone. In the case of the analyzed isolates in this study, there was no single isolate carrying PVL and LukED; in contrast, only PVL-negative strains were LukED positive.
MLST analysis showed that isolates from PFGE type A and MLVA type I belonged to the S. aureus ST152 clone. Initially, only one isolate belonging to this clone was reported in the MLST database (http://www.mlst.net; identification number 1082, year 2001). This isolate was an MRSA isolate recovered in Norway from abscess from a 2-year-old child. Until 2003, ST152 appeared to be genetically closely related only with another isolate in the MLST database, a single isolate from ST377 (mlst.net identification number 1145). This ST377 isolate was an MRSA isolate recovered in The Netherlands from abscess from a 15-year-old girl. ST377 (MLST alleles 46, 75, 49, 50, 13, 68, 60 [bold number indicates changing allele]) constitutes a single locus variant (SLV), and locus gmk (guanylate kinase) of ST152 (MLST alleles 46, 75, 49, 44, 13, 68, 60) constitutes a single nucleotide variant (SNV). The recovery of ST152/ST377 isolates from abscesses could indicate that these S. aureus clones have a genomic background that confers it a virulence predisposition to cause this type of infection.
Nowadays, ST152 is one of these epidemic PVL-positive CA-MRSA clones which has spread to several countries on various continents (5, 21). Available data have brought to speculate that the PVL-positive clone ST152 originated in Africa and has migrated northwards through the center of Europe and acquired methicillin resistance (19). This idea leads to the very interesting, not-yet-answered question about whether PVL-positive CA-MRSA clones acquired the PVL phage by strains with a methicillin resistance background or, conversely, through acquisition of the SCCmec element by strains with a PVL-positive background. This dichotomy occurs in the case of the herein-described clone, where ST152 isolates recovered in Tenerife are MSSA although ST152 globally distributed is known as an epidemic MRSA clone (5, 21). Since the Canary Islands are very near the west coast of Africa (15 km), our finding of PVL-positive ST152 MSSA fits with the speculation of Ruimy et al. supposing a migration from Africa to Europe, where PVL-positive ST152 MSSA acquired methicillin resistance (19). Not less interesting is that the worldwide spread of PVL-positive CA Staphylococcus aureus clones has been related to international travel. This means that the areas that constitute an international travel hub, as is the case of the Canary Islands, could constitute a source of dissemination of these clones, which reinforces the importance of the development of the kind of studies herein described.
Acknowledgments
This study was partially supported by grants FUNCIS 05/50 and FIS 06/0002 to S.M.-A. S.M.-A. was partially supported by Public Health Research Foundation (FIS contract 99/3060 and Stabilization Program FUNCIS-CES05/002). E.P.-R. and C.L.-A. were partially supported by grants from Consejería de Educación, Cultura y Deportes, and FUNCIS, respectively, Gobierno de Canarias Autonomous Government, Spain.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Adler, A., V. Temper, C. S. Block, N. Abramson, and A. E. Moses. 2006. Panton-Valentine leukocidin-producing Staphylococcus aureus. Emerg. Infect. Dis. 12:1789-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth, M. C., K. L. Hatter, D. Miller, J. Davis, R. Kowalski, D. W. Parke, J. Chodosh, B. D. Jett, M. C. Callegan, R. Penland, and M. S. Gilmore. 1998. Molecular epidemiology of Staphylococcus aureus and Enterococcus faecalis in endophthalmitis. Infect. Immun. 66:356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chini, V., E. Petinaki, A. Foka, S. Paratiras, G. Dimitracopoulos, and I. Spiliopoulou. 2006. Spread of Staphylococcus aureus clinical isolates carrying Panton-Valentine leukocidin genes during a 3-year period in Greece. Clin. Microbiol. Infect. 12:29-34. [DOI] [PubMed] [Google Scholar]
- 4.Dailiana, Z. H., N. Rigopoulos, S. E. Varitimidis, L. Poultsides, E. Petinaki, and K. N. Malizos. 2008. Clinical and epidemiological features of upper-extremity infections caused by Staphylococcus aureus carrying the PVL gene: a four-year study in Greece. Med. Sci. Monit. 14:511-514. [PubMed] [Google Scholar]
- 5.Garnier, F., A. Tristan, B. François, J. Etienne, M. Delage-Corre, C. Martin, N. Liassine, W. Wannet, F. Denis, and M. C. Ploy. 2006. Pneumonia and new methicillin-resistant Staphylococcus aureus clone. Emerg. Infect. Dis. 12:498-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwatsuki, K., O. Yamasaki, S. Morizane, and T. Oono. 2006. Staphylococcal cutaneous infections: invasion, evasion and aggression. J. Dermatol. Sci. 42:203-214. [DOI] [PubMed] [Google Scholar]
- 7.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Hook, J. Etienne, F. Vandenesch, and M. G. Bowden. 2007. Staphylococcus aureus Panton Valentine leukocidin causes necrotizing pneumonia. Science 315:1130-1133. [DOI] [PubMed] [Google Scholar]
- 8.Laifer, G., R. Frei, H. Adler, and U. Fluckiger. 2006. Necrotising pneumonia complicating a nasal furuncle. Lancet 367:1628. [DOI] [PubMed] [Google Scholar]
- 9.Lindqvist, M., B. Isaksson, A. Samuelsson, L. E. Nilsson, and A. Hallgren. 2009. A clonal outbreak of methicillin-susceptible Staphylococcus aureus with concomitant resistance to erythromycin, clindamycin and tobramycin in a Swedish county. Scand. J. Infect. Dis. 41:324-333. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Aguilar, C., E. Perez-Roth, A. Moreno, M. C. Duran, C. Casanova, A. Aguirre-Jaime, and S. Mendez-Alvarez. 2007. Association between the presence of the Panton-Valentine leukocidin-encoding gene and a lower rate of survival among hospitalized pulmonary patients with staphylococcal disease. J. Clin. Microbiol. 45:274-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, X. X., T. Ito, P. Chongtrakool, and K. Hiramatsu. 2006. Predominance of clones carrying Panton-Valentine leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J. Clin. Microbiol. 44:4515-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Aguilar, G., A. Avalos-Mishaan, K. Hulten, W. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2004. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr. Infect. Dis. J. 23:701-716. [DOI] [PubMed] [Google Scholar]
- 13.McLaws, M. L., and P. C. Taylor. 2003. The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two-year pilot. J. Hosp. Infect. 53:259-267. [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra, M., G. Wang, and W. M. Johnson. 2000. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 38:1032-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monday, S. R., and G. A. Bohach. 1999. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J. Clin. Microbiol. 37:3411-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obed, A., A. A. Schnitzbauer, T. Bein, N. Lehn, H. J. Linde, and H. J. Schlitt. 2006. Fatal pneumonia caused by Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus (PVL-MRSA) transmitted from a healthy donor in living-donor liver transplantation. Transplantation 81:121-124. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Roth, E., F. Claverie-Martin, J. Villar, and S. Mendez-Alvarez. 2001. Multiplex PCR for simultaneous identification of Staphylococcus aureus and detection of methicillin and mupirocin resistance. J. Clin. Microbiol. 39:4037-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Roth, E., F. Lorenzo-Diaz, N. Batista, A. Moreno, and S. Mendez-Alvarez. 2004. Tracking methicillin-resistant Staphylococcus aureus clones during a 5-year period (1998 to 2002) in a Spanish hospital. J. Clin. Microbiol. 42:4649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruimy, R., A. Maiga, L. Armand-Lefevre, I. Maiga, A. Diallo, A. K. Koumaré, K. Ouattara, S. Soumaré, K. Gaillard, J. C. Lucet, A. Andremont, and E. J. Feil. 2008. The carriage population of Staphylococcus aureus from Mali is composed of a combination of pandemic clones and the divergent Panton-Valentine leukocidin-positive genotype ST152. J. Bacteriol. 190:3962-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabat, A., J. Krzyszton-Russjan, W. Strzalka, R. Filipek, K. Kosowska, W. Hryniewicz, J. Travis, and J. Potempa. 2003. New method for typing Staphylococcus aureus strains: multiple-locus variable-number tandem repeat analysis of polymorphism and genetic relationships of clinical isolates. J. Clin. Microbiol. 41:1801-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tristan, A., M. Bes, H. Meugnier, G. Lina, B. Bozdogan, P. Courvalin, M. E. Reverdy, M. C. Enright, F. Vandenesch, and J. Etienne. 2007. Global distribution of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus, 2006. Emerg. Infect. Dis. 13:594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]