Abstract
Mycobacterium asiaticum was first reported as a cause of human disease in 1982, with only a few cases in the literature to date. This study aims to review the clinical significance of M. asiaticum isolates in Queensland, Australia. A retrospective review (1989 to 2008) of patients with M. asiaticum isolates was conducted. Data were collected through the Queensland TB Control Centre database. Disease was defined in accordance with the American Thoracic Society criteria. Twenty-four patients (13 female) had a positive culture of M. asiaticum, many residing around the Tropic of Capricorn. M. asiaticum was responsible for pulmonary disease (n = 2), childhood lymphadenitis (n = 1), olecranon bursitis (n = 1), 6 cases of possible pulmonary disease, and 2 possible wound infections. Chronic lung disease was a risk factor for pulmonary infection, and wounds/lacerations were a risk factor for extrapulmonary disease. Extrapulmonary disease responded to local measures. Pulmonary disease responded to ethambutol-isoniazid-rifampin plus pyrazinamide for the first 2 months in one patient, and amikacin-azithromycin-minocycline in another patient. While M. asiaticum is rare in Queensland, there appears to be an environmental niche. Although often a colonizer, it can be a cause of pulmonary and extrapulmonary disease. Treatment of pulmonary disease remains challenging. Extrapulmonary disease does not mandate specific nontuberculous mycobacterium (NTM) treatment.
Nontuberculous mycobacteria (NTM) have been recognized as human pathogens since the 1950s (17, 30). Over recent decades the taxonomy has continued to evolve, and new species are being recognized. This is attributable to improved methods of identification, including chemotaxonomic and molecular methods (11).
Mycobacterium asiaticum was first named by Weiszfeiler et al., who recognized it as a new slow-growing mycobacterium species with characteristics separate from those of Mycobacterium simiae and the other slow growers, Mycobacterium kansasii and Mycobacterium marinum (31). It was first reported in humans in Queensland (QLD), Australia, by Blacklock et al. in 1982, where M. asiaticum was thought to be responsible for pulmonary disease in 2 of 5 human cases (5). The first case report of pulmonary disease secondary to M. asiaticum in the United States was in 1990 (27). These reported patients with progressive pulmonary disease secondary to M. asiaticum mostly had an underlying chronic respiratory problem such as chronic obstructive pulmonary disease (COPD). M. asiaticum has also been reported to be responsible for extrapulmonary disease in humans, namely, flexor tenosynovitis (8), olecranon bursitis (6), and keratitis (7).
Previous studies, with their limitations, have suggested climate as a factor responsible for the distribution of M. asiaticum, with an association noted between prevalence of M. asiaticum and subtropical climates, such as Queensland's, particularly an area of northern Queensland near the Tropic of Capricorn (5). This association was noted in both animal and human studies (31).
There are limited data about the antimicrobial susceptibilities of M. asiaticum and response to treatment. Weiszfeiler et al. reported that M. asiaticum in monkeys is resistant to streptomycin, isoniazid, p-aminosalicylate, and rifampin but susceptible to cycloserine (32). However the use of antituberculosis therapy, including agents such as isoniazid, rifampin, ethambutol, pyrazinamide, streptomycin, and capreomycin, yielded more-encouraging results in humans (5, 27), with clinical response not correlating with in vitro susceptibilities. Older fluoroquinolones, such as ofloxacin, have also been used, although M. asiaticum has higher mean inhibitory concentrations than other slow-growing mycobacteria (15).
The aim of this study was to evaluate isolates of M. asiaticum in Queensland and describe the incidence and epidemiology of disease, risk factors, clinical and radiological spectrum, treatments, and outcome.
MATERIALS AND METHODS
We conducted a retrospective review of all cases in which Mycobacterium asiaticum was isolated in QLD between November 1989 and December 2007. In QLD all NTM isolates are speciated by a central reference laboratory, the QLD Mycobacterial Reference Laboratory (QMRL), and are notifiable to the Queensland Tuberculosis Control Center (QTBCC). Letters and questionnaires are sent to the treating physicians. Data requested include patient characteristics such as age, race, occupation, and exposures and clinical data such as risk factors, comorbidities, clinical features, site of infection, microbiology and laboratory features, radiological features, treatment, and outcome. Physicians are asked whether they consider the organism to be responsible for clinical disease and are referred to guidelines to aid in this assessment. These guidelines were developed by the QTBCC; they are in accordance with standard American Thoracic Society (ATS) criteria (11) and were used to define disease for the purposes of the study. Participation in this survey is voluntary.
Sputum samples were processed by a number of regional private laboratories, and mycobacterial isolates were referred onto the QMRL for speciation. All public hospital sputum specimens from the greater Brisbane area were transported directly to the QMRL and processed using 4% NaOH and phosphoric acid and concentrated using centrifugation. A mycobacterium growth indicator tube (MGIT) (Becton Dickinson [BD]) was inoculated using 0.5 ml of sediment, and 0.25 ml was used to inoculate solid Lowenstein-Jensen slopes. Inoculated MGIT media were incubated at 35°C in a BD Bactec 960 machine until a positive signal was detected. Solid media were incubated and screened weekly for the presence of growth.
To confirm the presence of mycobacterial species, a Ziehl-Neelsen stain was performed, followed by a multiplexed PCR targeting the 16S rRNA region. This method is used routinely to discriminate between Mycobacterium tuberculosis, Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium abscessus, and unspecified Mycobacterium species (33). For those identified as unspecified Mycobacterium species, prior to 2004, further identification was performed using a Mycobacterium avium complex (MAC) GenProbe or phenotypic identification. In 2004, both 16S RNA sequencing and the Hain Life Sciences GenoType Mycobacterium kit were introduced. The Hain Life Sciences GenoType Mycobacterium AS (additional species) kit was used to identify some isolates between 2004 and 2008. However, species identification was confirmed on all isolates (including those pre-2004) by amplifying hypervariable regions a and b outside the 16S region of the genome. This amplified product was sequenced using BigDye Terminator chemistry. Analysis was performed using both GenBank and RIDOM databases.
Susceptibility testing in 1991 was performed using the proportion method on 7H10 agar, with resistance defined as ≥1% of the bacterial population being resistant to the drug. Subsequently the BD Bactec MGIT 960 method was used for streptomycin, rifampin, isoniazid, and ethambutol by following the manufacturer's instructions. Clarithromycin was tested at concentrations of 4 μg/ml, 16 μg/ml, and 64 μg/ml, in line with CLSI guidelines.
RESULTS
Patient characteristics and distribution.
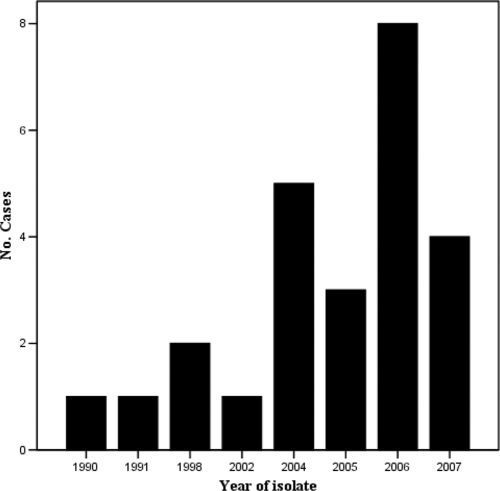
M. asiaticum was isolated from 24 patients (11 male and 13 female) between 1989 and 2007 (Fig. 1). The mean age was 54.8 years. The biggest cluster of isolates (n = 8) was centered on Townsville in northern Queensland (Fig. 2). Fifty-eight percent of isolates were from sputum specimens, 21% were found in bronchial washings, 4% were found in both sputum and bronchial washings (Table 1), and 17% were from extrapulmonary sites (Table 2). Underlying chronic lung disease was present in 65% of patients with pulmonary isolates, namely, COPD (40%), bronchiectasis (20%), lung cancer, and cystic fibrosis (CF). One patient had possible past tuberculosis, and another was on treatment for tuberculosis. Other NTM were isolated from 5 of 20 patients with pulmonary M. asiaticum prior to, and from 4 patients after, isolation of M. asiaticum. NTM organisms were isolated from three patients at the same time as M. asiaticum was isolated. The most common NTM were members of MAC and unspeciated slow growers. Isolation of non-NTM organisms, the most common being Pseudomonas aeruginosa and Staphylococcus aureus, was more frequent after isolation of M. asiaticum.
FIG. 1.
Numbers of patients with isolates according to year of first isolation.
FIG. 2.
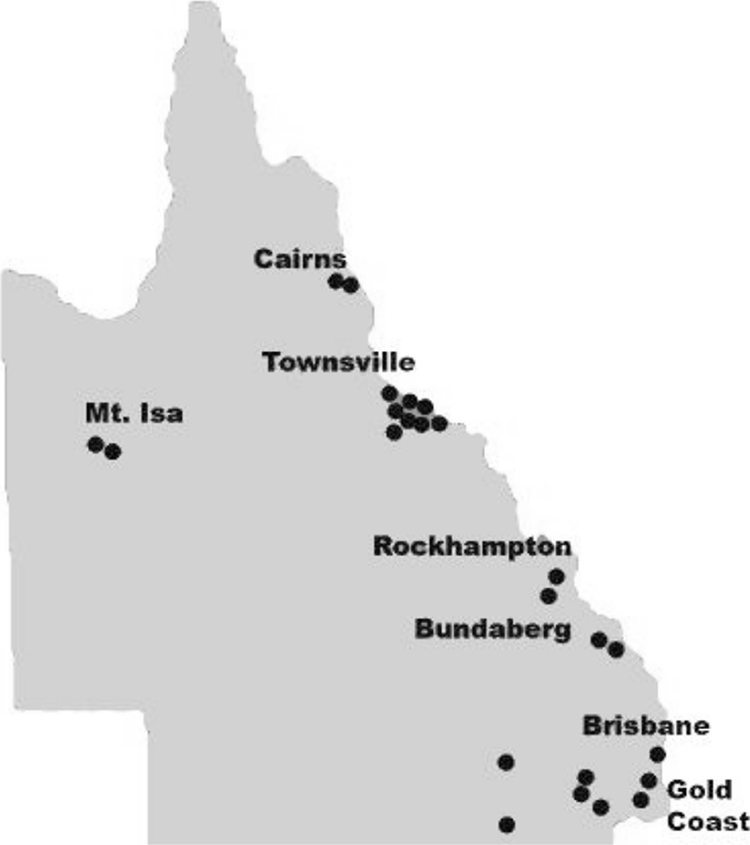
Distribution of patient isolates of M. asiaticum in Queensland (copyright Queensland Health, Brisbane, Queensland, Australia).
TABLE 1.
Clinical characteristics of 20 patients with pulmonary M. asiaticum isolatesa
| ID | Age/sex | Specimen type | M. asiaticum microbiology | Risk factors/comorbidities | Isolates pre-M. asiaticum | Isolates post-M. asiaticum | Pulmonary disease |
|---|---|---|---|---|---|---|---|
| 1 | 52/M | Sputum | S+ C+, 4/5; S− C+, 1/5 | Asthma/COPD | Nil | Nil | Present |
| 2 | 73/F | Sputum | S++, 3/3; C+; subsequent multiple positive cultures | BE, COPD | P. aeruginosa | Other NTM, Aspergillus, Acinetobacter, A. xylosoxidans | Present |
| 3 | 79/M | Sputum | S−; C+, 10/04; S− C+, 2/5 | COPD, previous Dukes C bowel cancer | NTM | S. aureus, S. maltophilia, P. aeruginosa | Possible |
| 4 | 55/M | Sputum | S− C+ | COPD | Other NTMb | P. aeruginosa | Possible |
| 5 | 28/F | Sputum | S− C+ | CF, ABPA | Other NTM, P. aeruginosa, Aspergillus sp., S. aureus | P. aeruginosa, S. aureus | Possible |
| 6 | 77/F | Sputum | S, NA; C+ | BE, GERD | Other NTM, H. influenzae | P. aeruginosa, Proteus mirabilis | Possible |
| 7 | 85/M | Br wash | S− C+ | NSCLC | Nil | Nil | Possible |
| 8 | 72/M | Br wash | S− C+ | HBV, NSCLC | Nil | Nil | Possible |
| 9 | 52/M | Br wash | S− C+ | None | Nil | Nil | No |
| 10 | 38/M | Sputum | S, NA; C+ | Non-IV drug use | Nil | Nil | No |
| 11 | 68/M | Sputum | S− C+ | DM, COPD | Nil | NTM | No |
| 12 | 62/F | Sputum | S− C+ | TB | M. tuberculosis | Nil | No |
| 13 | 46/F | Sputum | S− C+ | Nil | Nil | No | |
| 14 | 77/F | Br wash | S− C+ | BE, DM | Nil | Nil | No |
| 15 | 83/M | Br wash | S− C+ | Asthma/COPD | Nil | MAC, S. aureus, Aspergillus sp. | No |
| 16 | 76/F | Sputum | S++ C+ | DM | NTM | NA | |
| 17 | 52/M | Br wash/sputum | S− C+, 2/04; S− C+, 10/04 | COPD | NTM | S. maltophilia, P. aeruginosa, S. aureus | No |
| 18 | 37/F | Sputum | S− C+ | Alcoholism, COPD, BE | Nil | Nil | No |
| 19 | 25/M | Sputum | S+ C+ | Nil | Nil | No | |
| 20 | 84/M | Sputum | S− C+ | Previous TB? | Nil | Nil | No |
ABPA, allergic bronchopulmonary aspergillosis; BE, bronchiectasis; Br wash, bronchial washings; C, culture; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; Cz, ceftazidime; DM, diabetes mellitus; F, female; GERD, gastroesophageal reflux disease; HBV, hepatitis B virus; ID, patient identification number; IV, intravenous; M, male; MAC, Mycobacterium avium complex; NA, not available; NSCLC, non-small cell lung cancer; NTM, nontuberculous mycobacteria; S, smear; S. maltophilia, Stenotrophomonas maltophilia; 2/04, February 2004; 10/04, October 2004; 2/05, February 2005.
The most common other NTM were MAC and unspeciated slow growers. Others included M. smegmatis, M. gordonae, M. kubicae, and M. peregrinum.
TABLE 2.
Clinical characteristics, treatment, and outcome of 4 patients with nonpulmonary NTM isolatesa
| ID | Age/sex | Risk factor | Specimen type | M. asiaticum microbiology | Other isolates | Disease | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 21 | 24/M | Elbow laceration | Elbow aspirate | S− C+ | Nil | Olecranon bursitis | Aspiration, local steroid, flucloxacillin | Developed sinus infection, healed with local Rx |
| 22 | 4/F | Nil | Submandibular node | S, NA; C+ | Nil | Lymphadenitis | Excised | Healed |
| 23 | 45/M | Preexisting wound | R foot swab | S− C+ | S. aureus on tissue culture | Possible wound infection | Bx; BCC excised | Good response |
| 24 | 22/M | Preexisting wound | Wound swab, L shin | S, NA; C+ | Nil | Possible wound infection | Lost to f/u | Lost to f/u |
BCC, basal cell carcinoma; Bx, biopsy; C, culture; F, female; f/u, follow up; ID, patient identification number; L, left; M, male; NA, not available; R, right; Rx, treatment.
Significance.
One patient had cavitary pulmonary disease, another had disease in the setting of bronchiectasis, 2 had extrapulmonary disease (olecranon bursitis and lymphadenitis), 6 had possible pulmonary disease, and 2 had possible extrapulmonary disease (wound/soft tissue infections). The remaining isolates were not felt to be associated with NTM disease by the treating physician or there was insufficient information available to make a judgment.
Pulmonary disease.
Two patients satisfied the QTBCC and ATS criteria for definite NTM disease due to M. asiaticum.
Patient 1 was a 52-year-old male from far north Queensland, presenting in 1991 with a productive cough and weight loss, on a background of smoking-related COPD. A chest X ray (CXR) showed left upper lobe cavitation. Four of five sputa were smear positive, and all were culture positive for M. asiaticum. He received ethambutol, rifampin, and isoniazid for 12 months, with pyrazinamide added for the first 2 months. In vitro susceptibilities showed susceptibility to clarithromycin, rifampin (20 μmol/ml), rifabutin, ethambutol, clofazimine, and ciprofloxacin and resistance to rifampin (5 and 10 μmol/ml). CXR showed clearing by 3 months and further clearing at 6 months. Sputum conversion occurred after 4 months of treatment.
The second patient presented in late 1998 at the age of 73 years with a lifetime history of chronic productive cough and an episode of hemoptysis. Two of three sputum samples were smear positive for acid-fast bacilli (AFBs), 1 was smear negative, and all 3 grew M. asiaticum. CXR showed nonspecific bronchial wall thickening, and computed tomography (CT) of the chest revealed evidence of bronchiectasis involving the right middle lobe and the right lower lobe. Between 1998 and 2000, she had intermittent episodes of small-volume hemoptysis and recurrent exacerbations partially responding to courses of ciprofloxacin. She was managed with sputum clearance techniques, chest physiotherapy, and nebulized hypertonic saline during exacerbations, but no specific NTM therapy. Sputum cultures from February 2000 and June 2001 were heavily smear positive for AFBs, but no further identification was available. A repeat CT, however, showed progression of the bronchiectasis with mild to moderate bronchiectasis in both lower lobes and the lingula. Sputum specimens continued to grow M. asiaticum.
In 2003 treatment was commenced with ethambutol, rifampin, and clarithromycin. She developed a rash, attributed to ethambutol after rechallenge, and ciprofloxacin was substituted. Clarithromycin was changed to azithromycin, as it was perceived that the former was ineffective. In 2004 prothionamide was added because of progressive symptoms, and intermittent courses of ceftazidime were commenced for Pseudomonas infection. In 2005, rifampin was ceased to simplify treatment. Nebulized amikacin was tried but resulted in dysphonia. Intravenous amikacin was therefore given thrice weekly for 8 weeks, in combination with minocycline (100 mg/day) and azithromycin (250 mg/day). In vitro susceptibilities were done retrospectively for the purpose of this study. The isolate was susceptible to ethambutol and clarithromycin and resistant to rifampin, isoniazid, and streptomycin. The patient remained on oral treatment for 2 years, with good symptomatic response and sputum conversion. Treatment was ceased in 2007; however, cough and sputum increased. The October 2007 CT showed no extension of the bronchiectasis compared to 2006. As there had been 18 months of negative sputum cultures (except for one isolate of Mycobacterium gordonae, regarded as a contaminant), it was felt her symptoms were most likely due to her underlying bronchiectasis. At the patient's request azithromycin was recommenced thrice weekly. Sputum specimens now regularly culture Achromobacter xylosoxidans.
Possible pulmonary disease.
All patients with possible pulmonary disease had underlying lung pathology, including COPD (n = 2), CF (n = 1), bronchiectasis (n = 1), and lung cancer (n = 2). All the patients with underlying COPD and lung cancer were current or past smokers. The CF and bronchiectasis patients were nonsmokers (Table 3).
TABLE 3.
Clinical features, treatment, and outcome of 6 patients with possible pulmonary diseasea
| ID | Smoker | Clinical features | Radiology | Treatment | Outcome/comments |
|---|---|---|---|---|---|
| 3 | Yes | SOB on exertion, minimal sputum | Nodules >5 mm, small RUL nodular opacity, unchanged on serial X rays; presumed granuloma | Not treated | Alive, ICU admission with type 1 respiratory failure in 2008 |
| 4 | Yes | NA | NA | 18-mo Rx for NTM; no details specified | Deceased (NTM disease + COPD) |
| 5 | No | Recurrent infective exacerbations | Extensive bilateral BE | Not treated for M. asiaticum | Alive |
| 6 | No | Cough, anorexia, wt loss | BE RML, progressed to fibrotic and airspace change in lingula, LUL, RUL, and RML | Not treated for M. asiaticum; previous treatment for other NTM | M. asiaticum isolated late in course of NTM disease; developed cor pulmonale |
| 7 | Yes | NA | Lung mass | XRT | Alive |
| 8 | Yes | NA | RUL mass | Chemoradiation, excision of cerebellar metastasis | Repeat FOB: S− C− |
BE, bronchiectasis; C, culture; COPD, chronic obstructive pulmonary disease; FOB, fiber optic bronchoscopy; ID, patient identification number; LUL, left upper lobe; NA, not available; NTM, nontuberculous mycobacteria; RML, right middle lobe; RUL, right upper lobe; Rx, treatment; S, smear; SOB, shortness of breath; XRT, radiation treatment.
Patients with the established risk factors of COPD and bronchiectasis had other NTM isolates prior to M. asiaticum, making it hard to determine whether M. asiaticum contributed to the pulmonary disease. The most common other NTM isolated was M. intracellulare. Others included M. avium, Mycobacterium smegmatis, Mycobacterium peregrinum, Mycobacterium kubicae, and M. gordonae.
Clinical features were nonspecific and included cough, hemoptysis, weight loss, and recurrent infective exacerbations. Radiological changes were mostly consistent with underlying disease and included progressive bronchiectasis, nodules >5 mm, and upper lobe opacity. None of the patients had cavitary changes.
Two of these six patients were treated for NTM disease presumed due to MAC, with M. asiaticum considered either a copathogen or contaminant. Drugs used included ethambutol, rifampin, clofazimine, clarithromycin, and ciprofloxacin. The duration of treatment was 18 to 24 months initially, followed by further treatment if there was evidence of disease progression. One patient died despite treatment. NTM disease together with COPD was thought to be a contributory factor to his death. The other patient had received treatment for NTM disease prior to isolation of M. asiaticum, with no further treatment given afterwards. She had evidence of progressive disease, with continued weight loss, and developed cor pulmonale. Both lung cancer patients received treatment for their lung cancer but no treatment for NTM disease.
Extrapulmonary isolates.
The patient with olecranon bursitis has been previously reported (6). He (patient 21) sustained a left elbow laceration in August 1988 and presented with olecranon bursitis in October 1989. It was initially deemed to be a low-grade bursitis, and no treatment was given. By November 1989 there was persistent elbow inflammation. It was therefore aspirated and injected with methylprednisolone. In December 1989, it was again aspirated and treatment with flucloxacillin was begun. Radiology showed no evidence of osteomyelitis. The aspirate was smear negative for acid-fast bacilli, but M. asiaticum was grown. This was sensitive to cycloserine and prothionamide and resistant to isoniazid, rifampin, and ethambutol. Around this time the patient moved from Townsville to a town just north of Melbourne. By January 1990 an infection had developed. He received a second course of flucloxacillin, and the infection was treated locally with H2O2 irrigation, povidone-iodine dressings, and immobilization. No specific antimycobacterial treatment was given. The wound started to heal slowly, and further swabs did not isolate mycobacteria.
A 4-year-old girl presented with submandibular lymphadenitis (patient 22), and an excision biopsy demonstrated multiple caseating granulomata. M. asiaticum was cultured. No further treatment was given.
In both of the other 2 extrapulmonary cases M. asiaticum was cultured from nonhealing wounds. The first case (patient 23) presented with a nonhealing right foot wound thought to be from a coral cut. A wound swab grew M. asiaticum, but biopsy revealed a basal cell carcinoma infiltrating the deep dermis, and tissue culture grew a heavy growth of S. aureus. Histology of the surgically excised specimen 2 months after isolation of M. asiaticum did not reveal evidence of mycobacterial infection.
The second patient, an Irish tourist in north QLD, presented with a left shin ulcer, and a wound swab grew M. asiaticum. No further follow-up was available, as the patient returned to Ireland.
DISCUSSION
Although data in the literature are limited, it has already been established that M. asiaticum is a pathogen that can be associated with human disease. However, the nature, extent, and spectrum of disease have not been very well described. Data on treatment and response to treatment are also lacking, and physicians often have to rely on “expert opinion” when treating disease due to this slow-growing mycobacterium. To our knowledge this is the largest series of M. asiaticum isolates described in the literature to date.
Although M. asiaticum is rare, our study reveals a relatively high incidence of M. asiaticum isolates in Queensland. NTM disease as a group seems to be more prevalent in warmer climates. In the 2000 national NTM survey, Queensland and the Northern Territory rated highest among all Australian states and territories for the incidence of NTM disease, with 3.8 and 4.1 reported cases per 100,000 people, respectively (12). NTM causing nonpulmonary disease was also found to be prevalent in Queensland (4, 13). The ideal growth conditions for many NTM include a temperature between 10 and 45°C. With the Tropic of Capricorn running through the center of QLD, an ideal climate for NTM growth exists year round. Rates of disease in Victoria and New South Wales, more-southern states of Australia, in 2000 were 1.3 and 0.78 per 100,000, respectively (12). Distribution data for M. asiaticum from our study confirm the observations of Blacklock et al. (5), where 3 of 5 patients in that study lived around the Tropic of Capricorn, 1 was born near there and moved to southern Queensland, and 1 lived 600 km north. Most of our isolates came from in and around this area, mostly from Townsville.
An important reservoir could be Queensland's potable water supplies. In 1980 Tuffley and Holbeche sampled water from rainwater tanks in central Queensland. NTM were isolated in 32 of 141 samples (28). More-recent sampling has identified mycobacteria in >70% of Brisbane sites sampled; however, M. asiaticum was not isolated. Sampling in Townsville and Rockhampton, however, has not been performed. In a Czech study, the authors reported that there were seasonal variations in the occurrence of mycobacteria in drinking water, and they attributed this to a change in water temperature (14). Given Queensland's climate, Queensland's water distribution systems would provide the ideal environment for NTM growth, including M. asiaticum.
Risk factors for M. asiaticum were similar to those observed for other NTM species, with COPD and bronchiectasis being the most commonly observed preexisting conditions in pulmonary cases. The association between bronchiectasis and NTM disease has been well described, and bronchiectasis is now recognized both as a cause and effect of NTM disease (3). In their 2005 series, Koh et al. reported that 1/3 of patients with bilateral bronchiectasis and bronchiolitis on CT had NTM disease (13a). Furthermore, invasive NTM disease has been associated with slow progression of bronchiectasis without cavitation on high-resolution CT (HRCT) (20, 26). The Lady Windermere syndrome first described in 1992 denotes fibronodular bronchiectasis most commonly occurring in thin women >60 years old involving the right middle lobe and lingula (21). This form of bronchiectasis without cavitation is becoming increasingly recognized as an important pattern of NTM pulmonary disease. The M. asiaticum cases described previously by Blacklock et al. both had cavitary disease (5), whereas the case described by Taylor et al. had nodular disease (27). In our series one patient had cavitary disease (patient 1) and at least 2 patients could well fit the pattern of Lady Windermere syndrome (patients 2 and 6). Both of them developed radiological evidence of progression of the bronchiectasis in the presence of NTM including M. asiaticum.
Although lung cancer has not previously been considered a predisposing condition for NTM disease, it is worth noting that 2 patients with possible M. asiaticum disease in our study had non-small cell lung cancer (patients 7 and 8). They were classed as having possible NTM disease on the basis that the specimen was a bronchial washing, which is normally regarded as sterile. The low number of patients in this study precludes us from reaching any conclusions regarding the association between lung cancer and M. asiaticum disease/colonization. It is hard to determine the pathogenicity of NTM in these cases. Radiological changes are not very helpful if the focus of infection is within or in the same lobe as the tumor. Tamura et al. reported on the coexistence of lung cancer and active pulmonary mycobacteriosis. In their series 11/61 patients with lung cancer and coexistent mycobacteriosis had foci in the same lobe. Five of these were NTM infections, and in 3 cases the focus of mycobacterial infection was not separately identifiable (25). It is difficult to make the decision of whether to treat the NTM in these cases. More often than not, as was the case in our series, treatment for the pulmonary malignancy takes priority and the NTM is left untreated. Long-term outcomes are also hard to assess, as survival is often limited.
In our series two patients met the QTBCC/ATS criteria for definite pulmonary disease. Clinical features were nonspecific, in line with what would be expected for NTM disease. In the first case sputum conversion and cure were achieved with standard tuberculosis (TB) treatment extended to 12 months. In the second patient remission was achieved with amikacin thrice weekly for 8 weeks and azithromycin (250 mg/day) and minocycline (100 mg/day) for 2 years. It was hard to assess response to the standard ethambutol-rifampin-clarithromycin regimen as the ethambutol was not tolerated. However clarithromycin-rifampin-ciprofloxacin was not effective in controlling symptoms or converting sputum cultures to negative, even with the addition of prothionamide. The duration of treatment for this patient was difficult to determine. Symptoms recurred when there was an attempt at stopping treatment, but it was felt that the anti-inflammatory properties of azithromycin were controlling symptoms in the last year of treatment, as cultures were repeatedly negative for mycobacteria (1, 10).
It has previously been noted that in vitro sensitivities do not correlate well with clinical response in NTM disease (5, 27). It is therefore difficult to assess the relevance of the susceptibilities performed. In previously published case reports, patients responded to regimens using rifampin-isoniazid-ethambutol-streptomycin, ethambutol-rifampin, and rifampin-ethambutol-capreomycin-pyrazinamide (5, 27). Although these observations are useful, it is hard to give specific treatment recommendations given the relatively small number of M. asiaticum cases in the literature.
It is also interesting to note the change in respiratory flora after isolation of M. asiaticum (Table 1). We hypothesize that the damage done to the lung by active NTM disease would set the stage for colonization by other organisms. This phenomenon appears to be common to pulmonary disease across all NTM species.
Extrapulmonary infections in this group included lymphadenitis in a child and olecranon bursitis following an elbow laceration. NTM have long been recognized as a cause of cervical lymphadenitis in children (17, 30). The commonest NTM species involved is the M. avium complex. Others include M. kansasii, Mycobacterium scrofulaceum, Mycobacterium xenopi, and Mycobacterium chelonae. Lymphadenitis due to M. asiaticum has not been previously reported in humans, although there is a case report of lymphadenitis due to M. asiaticum in a red-handed tamarin (24). NTM lymphadenitis is commonly treated with local excision, with excellent response, as was the case with our patient. Bursitis has been previously reported with M. kansasii, Mycobacterium szulgai, M. avium-M. intracellulare, and M. marinum (2, 9, 16, 18, 19, 22, 23, 29). Cases are usually treated with a combination of surgery and appropriate antimycobacterial agents. To our knowledge the patient with olecranon bursitis is the only reported case of bursitis secondary to M. asiaticum (6). He responded to nonspecific treatment including drainage, regular dressings, and arm rest.
Our study had several limitations. (i) It is a relatively small series, with only one definite case of pulmonary disease attributable to infection with M. asiaticum. (ii) Because our data collection relied on physicians returning questionnaires, some of the data were missing or incomplete. (iii) Although physicians had access to guidelines to assess the clinical significance of M. asiaticum, the assessment was made by the individual physicians, who would have various levels of exposure to and experience with patients with NTM infections.
Despite the limitations of a small case series, we have made a number of epidemiologically and clinically relevant observations. M. asiaticum seems to be more prevalent in a warm climate such as that of Queensland. It is often a colonizer/contaminant, but can be responsible for pulmonary disease including noncavitary disease. It can also be responsible for childhood lymphadenitis and soft tissue infections. Risk factors and clinical and radiological features are largely similar to those observed for other NTM species. Treatment for pulmonary disease is challenging, and larger series are needed to enable specific treatment recommendations. Local excision seems sufficient for childhood lymphadenitis, as observed for other NTM species. Bursitis may also respond to local measures, and the isolation of M. asiaticum does not mandate specific treatment.
Footnotes
Published ahead of print on 28 October 2009.
REFERENCES
- 1.Anwar, G., S. Bourke, G. Afolabi, P. Middleton, C. Ward, and R. Rutherford. 2008. Effects of long-term azithromycin in patients with non-CF bronchiectasis. Respir. Med. 102:1494-1496. [DOI] [PubMed] [Google Scholar]
- 2.Barham, G. S., and D. G. Hargreaves. 2006. Mycobacterium kansasii olecranon bursitis. J. Med. Microbiol. 55:1745-1746. [DOI] [PubMed] [Google Scholar]
- 3.Barker, A. F. 2002. Bronchiectasis. N. Engl. J. Med. 346:1383-1393. [DOI] [PubMed] [Google Scholar]
- 4.Blacklock, Z. M., and D. J. Dawson. 1979. Atypical mycobacteria causing non-pulmonary disease in Queensland. Pathology 11:283-287. [DOI] [PubMed] [Google Scholar]
- 5.Blacklock, Z. M., D. J. Dawson, D. W. Kane, and D. McEvoy. 1983. Mycobacterium asiaticum as a potential pulmonary pathogen for humans. A clinical and bacteriologic review of five cases. Am. Rev. Respir. Dis. 127:241-244. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, D. J., Z. M. Blacklock, L. R. Ashdown, and E. C. Bottger. 1995. Mycobacterium asiaticum as the probable causative agent in a case of olecranon bursitis. J. Clin. Microbiol. 33:1042-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford, J. G., A. J. Huang, S. C. Pflugfelder, E. C. Alfonso, R. K. Forster, and D. Miller. 1998. Nontuberculous mycobacterial keratitis in south Florida. Ophthalmology 105:1652-1658. [DOI] [PubMed] [Google Scholar]
- 8.Foulkes, G. D., J. C. Floyd, and J. L. Stephens. 1998. Flexor tenosynovitis due to Mycobacterium asiaticum. J. Hand Surg. Am. 23:753-756. [DOI] [PubMed] [Google Scholar]
- 9.Friedman, N. D., and D. J. Sexton. 2001. Bursitis due to Mycobacterium goodii, a recently described, rapidly growing mycobacterium. J. Clin. Microbiol. 39:404-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giamarellos-Bourboulis, E. 2008. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int. J. Antimicrob. Agents 31:12-20. [DOI] [PubMed] [Google Scholar]
- 11.Griffith, D. E., T. Aksamit, B. A. Brown-Elliott, A. Catanzaro, C. Daley, F. Gordin, S. M. Holland, R. Horsburgh, G. Huitt, M. F. Iademarco, M. Iseman, K. Olivier, S. Ruoss, C. F. von Reyn, R. J. Wallace, Jr., and K. Winthrop. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367-416. [DOI] [PubMed] [Google Scholar]
- 12.Haverkort, F. 2003. National atypical mycobacteria survey, 2000. Commun. Dis. Intell. 27:180-189. [PubMed] [Google Scholar]
- 13.Jackson, E., A. Stewart, E. J. Maguire, and R. E. Norton. 2007. Mycobacterial soft tissue infections in north Queensland. ANZ J. Surg. 77:368-370. [DOI] [PubMed] [Google Scholar]
- 13a.Koh, W. J., K. S. Lee, O. J. Kwon, Y. J. Jeong, S. H. Kwak, and T. S. Kim. 2005. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous pulmonary infection. Radiology 235:282-288. [DOI] [PubMed] [Google Scholar]
- 14.Kubalek, I., and S. Komenda. 1995. Seasonal variations in the occurrence of environmental mycobacteria in potable water. APMIS 103:327-330. [DOI] [PubMed] [Google Scholar]
- 15.Leysen, D. C., A. Haemers, and S. R. Pattyn. 1989. Mycobacteria and the new quinolones. Antimicrob. Agents Chemother. 33:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maloney, J. M., C. R. Gregg, D. S. Stephens, F. A. Manian, and D. Rimland. 1987. Infections caused by Mycobacterium szulgai in humans. Rev. Infect. Dis. 9:1120-1126. [DOI] [PubMed] [Google Scholar]
- 17.Masson, A. M., and F. H. Prissick. 1956. Cervical lymphadenitis in children caused by chromogenic Mycobacteria. Can. Med. Assoc. J. 75:798-803. [PMC free article] [PubMed] [Google Scholar]
- 18.Pien, F. D., D. Ching, and E. Kim. 1991. Septic bursitis: experience in a community practice. Orthopedics 14:981-984. [DOI] [PubMed] [Google Scholar]
- 19.Prasertsuntarasai, T., and E. F. Bello. 2005. Mycobacterium avium complex olecranon bursitis in a patient treated with alefacept. Mayo Clin. Proc. 80:1532-1533. [DOI] [PubMed] [Google Scholar]
- 20.Prince, D. S., D. D. Peterson, R. M. Steiner, J. E. Gottlieb, R. Scott, H. L. Israel, W. G. Figueroa, and J. E. Fish. 1989. Infection with Mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 321:863-868. [DOI] [PubMed] [Google Scholar]
- 21.Reich, J. M., and R. E. Johnson. 1992. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 101:1605-1609. [DOI] [PubMed] [Google Scholar]
- 22.Saadatmand, B., J. K. Poulton, and C. L. Kauffman. 1999. Mycobacterium marinum with associated bursitis. J. Cutan. Med. Surg. 3:218-220. [DOI] [PubMed] [Google Scholar]
- 23.Schickendantz, M. S., and J. T. Watson. 1990. Mycobacterial prepatellar bursitis. Clin. Orthop. Relat. Res. 258:209-212. [PubMed] [Google Scholar]
- 24.Siegal-Willott, J., R. Isaza, C. Fiorello, and M. Reinhard. 2006. Mycobacterium asiaticum infection in a red-handed tamarin (Saguinus midas). J. Zoo Wildl. Med. 37:413-415. [DOI] [PubMed] [Google Scholar]
- 25.Tamura, A., A. Hebisawa, Y. Sagara, J. Suzuki, K. Masuda, H. Nagai, I. Akagawa, N. Nagayama, Y. Kawabe, K. Machida, A. Kurashima, H. Komatsu, and H. Yotsumoto. 2005. Coexistence of lung cancer and active pulmonary mycobacteriosis. Kekkaku 80:413-419. (In Japanese.) [PubMed] [Google Scholar]
- 26.Tanaka, D., H. Niwatsukino, T. Oyama, and M. Nakajo. 2001. Progressing features of atypical mycobacterial infection in the lung on conventional and high resolution CT (HRCT) images. Radiat. Med. 19:237-245. [PubMed] [Google Scholar]
- 27.Taylor, L. Q., A. J. Williams, and S. Santiago. 1990. Pulmonary disease caused by Mycobacterium asiaticum. Tubercle 71:303-305. [DOI] [PubMed] [Google Scholar]
- 28.Tuffley, R. E., and J. D. Holbeche. 1980. Isolation of the Mycobacterium avium-M. intracellulare-M. scrofulaceum complex from tank water in Queensland, Australia. Appl. Environ. Microbiol. 39:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wastiaux, H., H. Maillard, C. Bara, S. Catala, M. Steff, and P. Celerier. 2008. Bursitis due to Mycobacterium intracellulare in an immunocompetent patient. Ann. Dermatol. Venereol. 135:492-495. [DOI] [PubMed] [Google Scholar]
- 30.Weed, L. A., H. M. Keith, and G. M. Needham. 1956. Nontuberculous acid-fast cervical adenitis in children. Proc. Staff Meet. Mayo Clin. 31:259-263. [PubMed] [Google Scholar]
- 31.Weiszfeiler, G., V. Karasseva, and E. Karczag. 1971. A new mycobacterium species: Mycobacterium asiaticum n. sp. Acta Microbiol. Acad. Sci. Hung. 18:247-252. [PubMed] [Google Scholar]
- 32.Weiszfeiler, J. G., V. Karasseva, and E. Karczag. 1981. Mycobacterium simiae and related mycobacteria. Rev. Infect. Dis. 3:1040-1045. [DOI] [PubMed] [Google Scholar]
- 33.Wilton, S., and D. Cousins. 1992. Detection and identification of multiple mycobacterial pathogens by DNA amplification in a single tube. PCR Methods Appl. 1:269-273. [DOI] [PubMed] [Google Scholar]