Abstract
The prevalence of Leishmania infantum-specific antibodies and asymptomatic infection was assessed in a randomized sample of 526 healthy adults from a continental area of Northwestern Italy where L. infantum is not endemic and where autochthonous cases of visceral leishmaniasis (VL) were recently reported. L. infantum-specific antibodies were detected by Western blotting (WB) in 39 subjects (7.41%), while L. infantum kinetoplast DNA was amplified from buffy coat in 21 out of 39 WB-positive subjects, confirming asymptomatic infection in 53.8% of seropositives. Risk factors significantly associated with WB positivity were uninterrupted residence since childhood in a local rural environment (odds ratio [OR], 3.5; 95% confidence interval [CI], 1.7 to 7.3), daily contact with animals though not exclusively with dogs (OR, 3.7; 95% CI, 1.3 to 10.7), older age (OR, 2.31; 95% CI, 1.2 to 4.5), and agricultural/other outdoor activities (OR, 3.8; 95% CI, 0.99 to 3.7.) Logistic regression analysis showed that uninterrupted residence in a local rural environment and an age of >65 years were the only independent predictors of seropositivity assessed by WB. Follow-up at 24 months did not show evidence of VL in either seropositive or PCR-positive subjects. The detection of a high seroprevalence rate, confirmed as asymptomatic infection by PCR in more than half of the cases, among healthy residents in a continental area of northwestern Italy makes local L. infantum transmission very likely. In a region where VL is considered nonendemic, these findings warrant further epidemiological investigations as well as interventions with respect to both the canine reservoir and vectors, given the possible risks for immunosuppressed patients.
The incidence of human visceral leishmaniasis (VL) has displayed a sharp increase since the early 1990s in Mediterranean countries of southern Europe, mainly under the influence of human immunodeficiency virus (HIV) coinfection (50). This phenomenon occurred particularly in the coastal regions of southern Spain (2, 3), France (40), and southern Italy (25), where the incidence of officially reported VL cases almost doubled between 1987 and 2004 (9, 34). However, in the above regions, where the infection was known to be endemic for decades, the incidence of HIV-unrelated VL increased as well (34) as a result of better reporting following the establishment of a WHO surveillance network in 1994 (14) and also of the expansion of an already large canine reservoir and the spread of sandflies (26). In contrast, northern regions of the same countries traditionally have not been considered areas of endemicity because of their continental climate and the absence of the vector. In recent years, however, both the presence of sandflies and the emergence of autochthonous canine foci were reported in continental regions of northern Italy, near Verona in the northeast (35), and around Torino and other areas in the northwestern Piedmont region (17), as well as in the alpine region of Valle d'Aosta (19). More recent surveys confirm the spread of sandflies as well as of canine infection in many areas of northern Italy (36). Though the few sporadic cases of human VL reported yearly in the northwestern Italian region of Piedmont are considered imported, data on disease incidence are discordant, given that the 14 cases officially reported in the Piedmont region in the years 2004 to 2007 do not match a survey we performed of hospital discharge forms, which allowed us to find at least 35 diagnoses of visceral leishmaniasis in the same period (unpublished data). Recently, three autochthonous cases of human VL, with patients showing the same peripheral blood-amplified kinetoplast DNA (kDNA) restricted fragment length polymorphism (RFLP) pattern found in their dogs, have been reported in a rural area on the hills surrounding the town of Asti, 45 miles east of Torino (20, 21); two more cases of Leishmania kDNA identity between dogs and owners were observed in 2007 in the same locality (A. Biglino, unpublished data). Furthermore, in the above area canine seroprevalence rose to 11% in recent years while the presence of the vector was confirmed in 40% of capture stations (21). For these reasons, we set out to assess the prevalence of asymptomatic L. infantum human infection among healthy, HIV-negative adults living in a continental region of northwestern Italy which is traditionally not considered an area of endemicity and where a possible focus of autochthonous human transmission is ongoing.
MATERIALS AND METHODS
The study was approved by the Ethics Committee of the Piedmont Region in 2005. The investigation concerned an area of about 300 km2 located 45 miles east of Torino, including the town of Asti and 20 rural municipalities where both canine seroprevalence and Phlebotomus presence increased steadily in the last 10 years and where the first autochthonous human cases of VL had occurred since 2003 (20, 21). Considering a population size of 95,300 subjects in the above area and assuming a prevalence rate of 2.5% (40) with a worst acceptable value of 1.5%, we estimated with a 95% confidence level a sample size of 927 subjects, which was selected using general practitioners' records obtained from local health authorities (including all residents in the area) as a sampling frame, with a randomized start based on random number tables and a sampling ratio of 1:100. Selected subjects over 18 years old were interviewed by an infectious diseases specialist using a standard questionnaire at the Infectious Diseases Unit, Cardinal Massaja Hospital, Asti, about their medical history (including previous visceral/cutaneous leishmaniasis) and exposure to possible risk factors for VL including geographic origin, present/previous places of residence, occupation, outdoor activities, ownership of dogs/other pets, and holiday destinations. Written informed consent to undergo physical examination, complete blood count, blood chemistry, and Leishmania and HIV type I/II serology was obtained from 526 adults. Peripheral blood was also collected into sterile Vacutainer-EDTA tubes as well as in plain sterile tubes, centrifuged at +4° in order to obtain buffy coat and allowed to clot at +4° in order to obtain serum samples, which were stored at −80°C until processed. None of the subjects was taking immunosuppressive medications or had an immunosuppressive condition. Three subjects underwent intermittent short courses of oral prednisone for asthma or other respiratory allergies in the preceding 12 months; four subjects had type 2 diabetes (two of them on oral medications).
The presence of asymptomatic Leishmania infection was assessed by Western blotting (WB) as well as by parasite kDNA amplification, taking into account the different sensitivity and specificity of these techniques in detecting asymptomatic infection in immunocompetent subjects (24, 32).
Western blot assay.
Western blotting was carried out on nitrocellulose sheets blotted with fractionated proteins from a lysate of late-log-phase promastigotes of L. infantum ZMON-1 (IPT-1 Roma), according to a previous report (22). Samples were considered positive when at least two bands of 169, 115, 66, or 33 kDa were found. This protocol has been previously evaluated in parallel with an immunofluorescent antibody technique (IFAT; a reference test) on canine serum (18), finding a high index of agreement between IFAT and WB results (κ = 0.92, 95% confidence interval [CI], 0.82 to 1.00). The same protocol recently showed a corresponding agreement with IFAT on human serum (E. Ferroglio, unpublished data) while analogous immunoblotting techniques demonstrated a sensitivity and a specificity of 100% in detecting VL in HIV-negative patients (13, 47). Positive and negative control sera (respectively, from patients with visceral leishmaniasis and from healthy subjects living in a mountain village located in the Aosta Valley, Italy) were included in every assay.
PCR assay.
Total genomic DNA was extracted from freshly thawed buffy coats using the commercial kit GenomeElute (Sigma-Aldrich, St. Louis, MO), following the manufacturer's directions. The PCR protocol previously described by Lachaud et al. (31), employing the primers RV1 and RV2 to amplify a fragment of 145 bp present on the highly reiterated minicircles of Leishmania kDNA, was adapted according to Ferroglio et al. (21). The above primers have been shown to be both highly sensitive and highly specific as they detected parasitemia in 100% of symptomatic as well as asymptomatic seropositive dogs but in none of the healthy seronegative dogs (31).
Statistical analysis.
Data were entered in Microsoft Excel databases on two separate computers by two independent operators performing single entry and visual checking and under the final supervision of a third operator. Data were analyzed by using SPSS for Windows, version 14.0. The associations between Leishmania WB seropositivity and possible predictors (risk factors) were analyzed by a univariate Pearson's chi-squared test. The odds ratio (OR) and relative 95% confidence interval have been estimated using a logistic regression model. In order not to overestimate the significance of the level of association, the model has been performed using the enter method: all variables were considered in the model, independently from the significance level; therefore, all ORs were adjusted for all possible confounder effects with other variables.
RESULTS
Serology and DNA amplification.
Circulating anti-Leishmania antibodies were detected by WB in 39 (7.41%) out of 526 healthy, HIV-negative subjects (301 male; 225 female; median age, 48 years; age range, 19 to 84 years).
L. infantum kDNA was detected in buffy coats from 22 out of 39 WB-positive (56.4%) subjects and in none of the 487 WB-negative subjects; parasitemia was therefore present in 4.2% of the overall population.
Risk factor association.
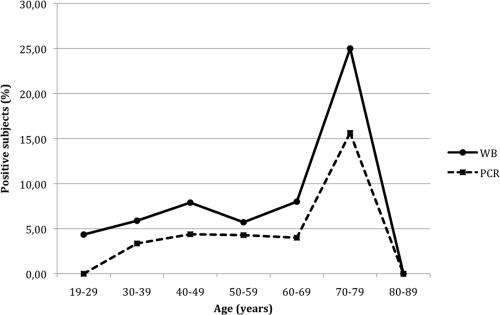
Antibodies to Leishmania, detected by WB, were found in 17 out of 139 subjects aged >56 years (12.2%), compared to 22 out of 387 younger subjects (5.7%); in 31 out of 297 residents in the country (10.4%) and in 8 out of 229 residents in urban areas (3.5%); in 28 out of 222 residents in the surveyed area since their childhood (12.6%) but in only 11 out of 304 subjects (3.6%) who, before moving to the area under investigation, lived in other Italian or foreign regions, including those at higher risk for Leishmania infection (central-southern Italy/islands); in 22 out of 218 (10.1%) subjects whose occupation was characterized by prolonged contact with the rural environment (i.e., farmers, night watchmen, road guards, housewives, and the unemployed) or with dogs and other animals (35 out of 376, or 9.3%) but in only 17 out of 308 (5.5%) employees/professionals working regularly in urban areas and in 4 out of 150 (2.7%) individuals denying contacts with animals (Table 1). Among the 222 residents who had always lived in the surveyed area since childhood (median length of residence history, 48 years; range, 25 to 84 years), 15 out of 76 subjects aged >56 years—most of them local farmers who traditionally spent holidays at home—showed WB positivity (19.7%), compared to only 13 out of 146 younger subjects (8.9%) (χ2 = 7.14; P = 0.0075); in contrast, no association between older age and seropositivity emerged among the 304 residents who moved from other Italian or foreign Mediterranean regions into the area under scrutiny. No significant association was found between seropositivity and gender, vacationing in high-risk areas (such as Mediterranean coasts/islands), or practicing outdoor activities such as gardening, hunting, fishing, camping, or trekking. Logistic regression analysis showed that uninterrupted residence in a local rural environment versus any other place (OR, 3.84; CI, 1.87 to 7.9; Wald test, 7.7; P = 0.005) and older (>56 years) versus younger age (OR, 2.31; CI, 1.18 to 4.49; Wald test, 4.1; P = 0.043) were the only independent predictors for seropositivity assessed by WB. Age-prevalence curves were in accordance with an increasing risk of asymptomatic infection, diagnosed using either WB or kDNA PCR, in older subjects (Fig. 1). Finally, positivity for both WB and kDNA was evidenced in 11 out of 139 subjects aged >56 years (7.9%) compared to 11 out of 387 younger subjects (2.8%) (χ2 = 7.5; P = 0.006; OR, 3.23; CI, 1.34 to 7.8) and in 20 out of 376 subjects reporting daily contact with animals (5.3%) but in only 2 out of 150 subjects denying such contacts (1.3%) (χ2 = 3.8; P = 0.04; OR, 3.94; CI, 0.90 to 17.12). No significant association was found between positivity for both WB and kDNA and the remaining risk factors.
TABLE 1.
Association between Leishmania Western blotting positivity and possible risk factors
| Predictive variable (no. of subjects) | Western blotting result |
OR (95% CI) | Pearson's χ2 (P value)a | |
|---|---|---|---|---|
| No. positive (%) | No. negative (%) | |||
| Age | ||||
| <57 (387) | 22 (5.7) | 365 (94.3) | 2.31 (1.18-4.49) | 6.38 (P= 0.012) |
| >56 (139) | 17 (12.2) | 122 (87.8) | ||
| Present place of residence | ||||
| Rural (297) | 31 (10.4) | 266 (89.6) | 3.21 (1.45-7.14) | 9.1 (P= 0.002) |
| Urban (229) | 8 (3.5) | 221 (96.5) | ||
| Previous place of residence | ||||
| Surveyed area since childhood (222) | 28 (12.6) | 194 (87.4) | 3.84 (1.87-7.9) | 15.1 (P= 0.0001) |
| Other Italian/foreign regions (304) | 11 (3.6) | 293 (96.4) | ||
| Occupation | ||||
| Farming/other outdoor jobsb (218) | 22 (10.1) | 196 (89.9) | 1.92 (0.99-3.71) | 3.88 (P= 0.048) |
| Employees, professionals (308) | 17 (5.5) | 291 (94.5) | ||
| Contact with animals (frequency) | ||||
| Daily (376) | 35 (9.3) | 341 (90.7) | 3.74 (1.3-10.73) | 6.9 (P= 0.008) |
| Seldom/no contact (150) | 4 (2.7) | 146 (97.3) | ||
| Contact with animals (type) | ||||
| Dogs (293) | 26 (8.9) | 267 (91.1) | 1.64 (0.82-3.28) | 2.05 (P = n.s.) |
| Other (233) | 13 (5.6) | 220 (94.4) | ||
| Holiday destinations | ||||
| Italy/abroad (302) | 17 (5.6) | 285 (94.4) | 0.54 (0.28-1.05) | 3.29 (P = n.s.) |
| Home (surveyed area) (224) | 22 (9.8) | 202 (90.2) | ||
| Outdoor leisure activitiesc | ||||
| Yes (365) | 30 (8.2) | 335 (91.8) | 1.51 (0.7-3.26) | 1.12 (P = n.s.) |
| No (161) | 9 (5.6) | 152 (94.4) | ||
Significant associations are in boldface. n.s., not significant.
Other jobs include night watchmen and security/road guards.
Fishing, hunting, camping, trekking, etc.
FIG. 1.
Age-prevalence curves of asymptomatic infection detected using WB and kDNA PCR as indicated.
DISCUSSION
This study describes the results of a survey of asymptomatic L. infantum human infection in the central Piedmont, a northwestern Italian region not traditionally considered an area of endemicity, characterized by continental climate and by a low incidence of officially reported VL cases. Canine leishmaniasis, which represents the only domestic reservoir for human disease, has indeed being increasing in northern Italy, spreading to rural and periurban areas in recent years. Traditionally, only southern, central, and insular regions of Italy-particularly along the Tyrrhenian littoral—were considered stable foci of endemicity for both human and canine leishmaniasis (44). Recently new foci, with autochthonous clinical cases reported in dogs, have occurred in northern areas of the country, near Verona (35) and Torino (17, 19). Our investigation was prompted by further evidence of both increasing canine seropositivity and the spread of sandflies in the last decade, as well as by the occurrence of autochthonous human VL cases in the surveyed area.
Our preliminary results, obtained from a sample of 526 healthy, HIV-negative subjects randomized from the general population, show a strikingly high prevalence of Leishmania-specific antibodies detected with WB (7.4%). These seroprevalence data, consistent with recent surveys on healthy subjects in other European regions of high endemicity such as southern France (32, 38), southern Spain (24), and the Balearic Islands (46) (Table 2), are supported by the detection of circulating L. infantum kDNA in more than half of the seropositive subjects even though subjects were from a region where the pathogen is not considered endemic. Taken together, these data prompt a strong suspicion that significant local transmission of L. infantum to humans has been ongoing for years in the areas surveyed by us; in fact, Leishmania positivity in our population appears to be significantly associated with prolonged residence in the rural zones of the surveyed area rather than in urban or other Italian/foreign locations and with the age of residents (>56 years), suggesting the importance of long-lasting, uninterrupted contact with both the reservoir and the vector. The observation that only WB but not kDNA positivity is strongly associated with increasing age seems to confirm the epidemiological usefulness of immunoblotting in detecting remote exposure to Leishmania. On the other hand, the apparently strong associations between Leishmania seropositivity and agriculture or other outdoor occupations (compared to office work) or daily contact with animals (though not exclusively with dogs) lost their significance with logistic regression analysis, as these risk factors are clearly associated with prolonged residence in a rural environment. Finally, it should be pointed out that our data were obtained from a randomized sample of the general population and, therefore, are probably more representative of the true local epidemiological situation than data obtained from highly selected groups (such as blood donors, where male subjects are dominant).
TABLE 2.
Studies on cryptic L. infantum infection employing different infection markers in areas of endemicity
| Study information |
Country/locale | Serological test | No. of subjects | No. seropositive (%) | No. kDNA positive (%) | |
|---|---|---|---|---|---|---|
| Author (year) | Reference | |||||
| P. Marty et al. (1994) | 38 | Southern France (Alpes Maritimes) | WB | 50 | 16 (32%) | |
| Y. Le Fichoux et al. (1999) | 32 | Southern France (Monaco) | WB | 565 | 76 (13%) | 9/73 WB positive (12%) |
| J. I. Garrote et al. (2004) | 24 | North-Central Spain | ELISA | 4.825 (225 HIV+) | 241 (4.9%) 139 (64%) | |
| C. Riera et al. (2004) | 46 | Balearic Islands (Eivissa) | ELISA, WB | 656 | 16 (2.4%) 50 (7.6%) | 27/122 (22%) |
| J. Martin-Sanchez et al. (2004) | 37 | Southern Spain (Sevilla) | IFAT (titer ≥1:20) | 170 | 95 (56%) | 23/95 IFAT positive (24%) |
| C. Colomba et al. (2005) | 11 | Southern Italy (Sicily) | ELISA, IFAT | 500 | 0 | |
| F. Scarlata et al. (2008) | 48 | Southern Italy (Sicily) | IFAT | 1449 | 11 (0.75%) | 4/11 IFAT positive (36.4%) |
| A. Alborzi et al. (2008) | 1 | Southern Iran | IFAT | 388 | 212 (54%) | 95/212 IFAT positive (45%) |
| M. Fakhar et al. (2008) | 16 | Iran (Fars Province) | DAT | 802 | 13 (1.62%) | 100/802 (12.5%) |
| A. Biglino et al. | This study | Northwestern Italy (Asti Province) | WB | 526 | 39 (7.41) | 22/39 WB positive (56.4%) |
Great caution is required in diagnosing subclinical Leishmania infection by means of indirect methods, such as serological/delayed-type hypersensitivity (DTH) tests, on peripheral blood of healthy individuals in the absence of a true gold standard, considering that marrow/spleen aspiration for culture and PCR are unwarranted in these subjects. In fact, though enzyme-linked immunosorbent assays (ELISAs), immunofluorescent antibody tests (IFAT), and direct agglutination tests (DAT) are highly specific and sensitive in diagnosing active VL in immunocompetent subjects (10, 30), titers decline more or less rapidly in a significant portion of patients after disease resolution, following the reduction of parasitized cell number (12, 30). Moreover, the above tests frequently give false-negative results in immunocompromised patients even in the presence of positive kDNA amplification (23), making their sensitivity unsatisfactory for detecting asymptomatic Leishmania infection, which is characterized by low or intermittent parasitemia. Consequently, it is not surprising that data from IFAT, DAT, or ELISA have shown low seroprevalence among healthy subjects living in Mediterranean areas of high endemicity (11, 48) or even in Middle Eastern areas, where parasitemia, evidenced by DNA amplification, may be present in 16% of the healthy population (16) (Table 2). On the other hand, the high sensitivity of qualitative techniques such as immunoblotting (24, 32, 39, 41) makes them excellent epidemiological tools for detecting past contact with the parasite, as well as ideal candidates for diagnosing subclinical infections. It should be noted, however, that WB specificity for this particular purpose may be reduced by the long-lasting persistence of circulating antibodies after parasite clearance in some patients (12). Indeed, though asymptomatic/oligosymptomatic leishmaniasis is common in humans, less than 5% of infected immunocompetent adults-and about twice that number among children—develop clinically relevant visceral disease while the remaining subjects either clear the infection or become asymptomatic carriers for years (29). Furthermore, in areas where the infection is not considered endemic, diagnosis may be delayed because of a low index of suspicion or by uncommon presentation in immunosuppressed subjects. For these reasons we chose to employ WB in order to trace patients with possible subclinical infection as this test is expected to be positive in the presence of parasite replication in 100% of immunocompetent and in more than 70% of immunocompromised subjects (13, 47). Moreover, WB may be an epidemiological marker for identifying all exposed individuals; in fact, it often remains positive after successful treatment of VL (44, 48) and, very likely, after spontaneous resolution of asymptomatic infection as well. In contrast, DTH techniques such as the leishmanin skin test (LST), usually marking cell-mediated parasite control or clearance, could be less able to identify asymptomatic, kDNA-positive subjects (1, 37), given the variability of standardization, potency, and stability of leishmanin antigens (6). Finally, we chose to employ L. infantum kDNA amplification to confirm subclinical infection in seropositive cases since its specificity may be as high as that of traditional culture techniques, which, in turn, are flawed by an unsatisfactory sensitivity compared to molecular techniques in the presence of very low/intermittent parasitemias (42).
The documentation of a significant prevalence of asymptomatic Leishmania infection in continental areas of central/southern Europe is intriguing and may be relevant from the epidemiological as well as from the clinical point of view. On strictly epidemiological grounds, our data are consistent with several reports concerning the northward spread of other vector-borne diseases, announced in the 1990s by outbreaks of West Nile fever in eastern Europe (28) and culminating in a recent outbreak of Chikungunya fever in Italy (45). Vector displacement, as a possible consequence of climate change, trade, and globalization, is thought to be among the main reasons underlying the above phenomena. In the case of L. infantum, however, the importance of persistent local transmission is paramount given the constant expansion of poorly controlled canine reservoirs in addition to the spread of sandfly vectors throughout continental Europe. In geographic areas such as Northern Italy where both issues have probably been underestimated for years, our findings may have direct clinical implications both for immunocompetent and for immunocompromised subjects. In the first case, the main problem is represented by delayed diagnosis of VL, with a possible increase in patient morbidity (and, exceptionally, mortality). Indeed, a low index of suspicion is to be expected among nonspecialist physicians in central/northern Europe, particularly with respect to patients without a history of travel or residence in known areas of endemicity. For instance, the median time between symptom onset and correct diagnosis was reported to be 85 days for VL patients in Germany (49). On the other hand, VL is being reported with increasing frequency in transplant recipients, with 80 cases reported thus far (4, 7), while the originally high risk for HIV-infected patients appears to be greatly reduced since the turn of the century, thanks to immune reconstitution mediated by effective antiretroviral treatments. The most likely pathogenetic mechanisms of VL in transplant patients are either primary infection acquired by immunosuppressed subjects traveling in regions of endemicity (27) or reactivation of a preexisting asymptomatic infection in the recipient (5), while iatrogenic acquisition through either an infected graft or blood transfusion probably represents an uncommon situation. In fact, only 12 posttransfusion VL cases have been reported, to our knowledge, since 1948 (15, 43); 11 of the above occurred in infants or children, who are highly receptive to Leishmania, while the relevance of blood transfusion in the immunocompetent adult host is controversial (8). Moreover, leukodepletion of donated blood at collection, as well as of packed red cells afterwards, significantly reduces the number of both amastigotes and promastigotes by 4 to 8 logs (8). Nevertheless, the risk of transfusion-transmitted leishmaniasis should not be dismissed since a significantly higher seroprevalence has been observed among multiply-transfused patients than among voluntary blood donors in regions of high endemicity (33). Yet one must also bear in mind the impossibility of separating the role of transfusion from that of natural transmission in areas of high endemicity in the Western hemisphere without proving Leishmania kDNA identity between donor and recipient. Indeed, in the presence of widespread asymptomatic infection carriage, the possible role of a human rather than exclusively canine reservoir, with anthroponotic transmission, must be taken into consideration (33) and actively investigated though this aspect is more commonly observed in areas of high endemicity (51).
Given the foregoing considerations, our findings undoubtedly raise clinical issues concerning, on the one hand, the possible screening of both transplant patients and organ/bone marrow donors for asymptomatic leishmaniasis and, on the other hand, the need of longitudinal follow-up studies on transfused immunosuppressed and pediatric patients in order to assess the residual risk (if any) from deleukocytized blood. This approach appears particularly advisable in the areas of continental Europe traditionally not considered areas of endemicity, where clinical suspicion of VL is low and where recent findings of unexpectedly high human prevalence of asymptomatic L. infantum infection are not being followed by public health interventions targeted to both the vector and the reservoir.
Acknowledgments
We are grateful to Giuseppe Migliaretti, Statistical Unit, Department of Public Health and Microbiology, University of Torino, Italy, for critical review of the manuscript and for statistical analysis.
This study was supported by grants from the Compagnia di San Paolo, Torino, Italy, and by the Fondazione Cassa di Risparmio, Progetto SIRIO, Asti, Italy.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Alborzi, A., B. Pourabbas, F. Shahian, J. Mardaneh, G. R. Puladfar, and M. Ziyaeyan. 2008. Detection of Leishmania infantum kinetoplast DNA in the whole blood of asymptomatic individuals by PCR-ELISA and comparison with other infection markers in endemic areas, southern Iran. Am. J. Trop. Med. Hyg. 79:839-842. [PubMed] [Google Scholar]
- 2.Alvar, J., C. Canavate, B. Gutierrez-Solar, M. Jimenez, F. Laguna, R. Lopez-Velez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar, J., P. Aparicio, A. Aseffa, M. Den Boer, C. Canavate, J. P. Dedet, L. Gradoni, R. Ter Horst, R. Lopez-Vélez, and J. Moreno. 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin. Microbiol. Rev. 21:334-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori, S., A. Cascio, C. Parravicini, R. Bianchi, and M. Corbellino. 2008. Leishmaniasis among organ transplant recipients. Lancet Infect. Dis. 8:191-199. [DOI] [PubMed] [Google Scholar]
- 5.Basset, D., F. Faraut, P. Marty, J. Dereure, E. Rosenthal, C. Mary, F. Pratlong, L. Lachaud, P. Bastien, and J. P. Dedet. 2005. Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes Infect. 7:1370-1375. [DOI] [PubMed] [Google Scholar]
- 6.Bern, C., J. Amann, R. Haque, R. Chowdhury, M. Ali, K. M. Kurkjian, L. Vaz, Y. Wagatsuma, R. F. Breiman, W. E. Secor, and J. H. Maguire. 2006. Loss of leishmanin skin test antigen sensitivity and potency in a longitudinal study of visceral leishmaniasis in Bangladesh. Am. J. Trop. Med. Hyg. 75:744-748. [PubMed] [Google Scholar]
- 7.Campos-Varela, I., O. Len, L. Castells, N. Tallada, E. Ribera, C. Dopazzo, V. Vargas, J. Gavaldà, and R. Charco. 2008. Visceral leishmaniasis among liver transplant recipients: an overview. Liver Transpl. 14:1816-1819. [DOI] [PubMed] [Google Scholar]
- 8.Cardo, L. J. 2006. Leishmania: risk to the blood supply. Transfusion 46:1641-1645. [DOI] [PubMed] [Google Scholar]
- 9.Cascio, A., L. Gradoni, F. Scarlata, M. Gramiccia, S. Giordano, R. Russo, et al. 1997. Epidemiological surveillance of visceral leishmaniasis in Sicily. Am. J. Trop. Med. Hyg. 57:75-78. [DOI] [PubMed] [Google Scholar]
- 10.Chappuis, F., S. Rijal, A. Soto, J. Menten, and M. Boelaert. 2006. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 333:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colomba, C., L. Saporito, V. F. Polara, T. Barone, A. Corrao, and L. Titone. 2005. Serological screening for Leishmania infantum in asymptomatic blood donors living in an endemic area (Sicily, Italy). Transfus. Apher. Sci. 33:311-314. [DOI] [PubMed] [Google Scholar]
- 12.De Almeida Silva, L., H. D. Romero, A. Prata, R. T. Costa, E. Nascimento, S. F. Carvalho, and V. Rodrigues. 2006. Immunologic tests in patients after clinical cure of visceral leishmaniasis. Am. J. Trop. Med. Hyg. 75:739-743. [PubMed] [Google Scholar]
- 13.Deniau M., C. Canavate, F. Faraut-Gambarelli, and P. Marty. 2003. The biological diagnosis of leishmaniasis in HIV-infected patients. Ann. Trop. Med. Parasitol. 97(Suppl. 1):S115-S133. [DOI] [PubMed] [Google Scholar]
- 14.Desjeux P., J. P. Meert, B. Piot, J. Alvar, F. J. Medrano, M. Portus, C. Munoz, et al. 2000. Leishmania/ HIV co-infection in south-western Europe 1990-1998. Retrospective analysis of 965 cases. Document WHO/LEISH/2000.42. World Health Organization, Geneva, Switzerland.
- 15.Dey, A., and S. Singh. 2006. Transfusion transmitted leishmaniasis: a case report and review of the literature. Indian J. Med. Microbiol. 24:165-170. [PubMed] [Google Scholar]
- 16.Fakhar, M., M. H. Motazedia, G. R. Hatam, Q. Asgari, M. Kalantari, and M. Mohebali. 2008. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann. Trop. Med. Parasitol. 102:577-583. [DOI] [PubMed] [Google Scholar]
- 17.Ferroglio, E., L. Rossi, W. Mignone, and M. Maroli. 2000. Sandfly vectors investigation at an unstable focus of canine leishmaniasis in Italy (Piedmont) and the risk of permanent infection transmission. Parassitologia 42(Suppl. 1):114-115. [Google Scholar]
- 18.Ferroglio, E., A. Trisciuoglio, S. Gastaldo, W. Mignone, and M. Delle Piane. 2002. Comparison of ELISA, IFAT and Western blot for the serological diagnosis of Leishmania infantum infection in dog. Parassitologia 44:64. [Google Scholar]
- 19.Ferroglio, E., M. Maroli, S. Gastaldo, W. Mignone, and L. Rossi. 2005. Canine leishmaniasis, Italy. Emerg. Infect. Dis. 11:1618-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferroglio, E., A. Romano, S. Passera, A. D'Angelo, P. Guiso, E. Ghiggi, C. Bolla, A. Trisciuoglio, and A. Biglino. 2006. Dog's parasite and zoonotic risk: from old to new “emergencies” in the North-West Italy. Parassitologia 48:115-116. [PubMed] [Google Scholar]
- 21.Ferroglio, E., A. Romano, A. Trisciuoglio, M. Poggi, E. Ghiggi, P. Sacchi, and A. Biglino. 2006. Characterization of Leishmania infantum strains in blood samples from infected dogs and humans by PCR-RFLP. Trans. R. Soc. Trop. Med. Hyg. 100:636-641. [DOI] [PubMed] [Google Scholar]
- 22.Ferroglio, E., E. Centaro, W. Mignone, and A. Trisciuoglio. 2007. Evaluation of an ELISA rapid device for the serological diagnosis of Leishmania infantum infection in dog as compared with immunofluorescence assay and Western blot. Vet. Parasitol. 144:162-166. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Garcia, A., J. Martin-Sanchez, M. Gallego, A. Rivero-Roman, A. Camacho, C. Riera, F. Morillas-Marquez, S. Vergara, J. Macias, and J. A. Pineda. 2006. Use of noninvasive markers to detect Leishmania infection in asymptomatic human immunodeficiency virus-infected patients. J. Clin. Microbiol. 44:4455-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrote, J. I., M. P. Gutierrez, R. L. Izquierdo, M. A. Duenas, P. Zarzosa, C. Canavate, M. E. Bali, A. Almaraz, M. A. Bratos, C. Berbel, A. Rodriguez-Torres, and A. O. Domingo. 2004. Seroepidemiologic study of Leishmania infantum infection in Castilla-Leon, Spain. Am. J. Trop. Med. Hyg. 71:403-406. [PubMed] [Google Scholar]
- 25.Gradoni, L., A. Scalone, M. Gramiccia, and M. Troiani. 1996. Epidemiological surveillance of leishmaniasis in HIV-1-infected individuals in Italy. AIDS 10:785-791. [DOI] [PubMed] [Google Scholar]
- 26.Gramiccia, M., and L. Gradoni. 2005. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 35:1169-1180. [DOI] [PubMed] [Google Scholar]
- 27.Halkic, N., R. Ksontini, B. Scholl, C. Blanc, T. Kovacsovics, P. Meylan, C. Muheim, M. Gillet, and F. Mosimann. 2004. Recurrent cytomegalovirus diseases, visceral leishmaniosis, and Legionella pneumonia after liver transplantation: a case report. Can. J. Anaesth. 51:84-87. [DOI] [PubMed] [Google Scholar]
- 28.Hubalek Z. 2008. Mosquito-borne viruses in Europe. Parasitol. Res. 103(Suppl. 1):S29-S43. [DOI] [PubMed] [Google Scholar]
- 29.Jeronimo, S. M. B., M. J. Teixeira, A. Sousa, P. Thielking, R. D. Pearson, and T. G. Evans. 2000. Natural history of Leishmania chagasi infection in Northeast Brazil. Long-term follow-up. Clin. Infect. Dis. 30:608-609. [DOI] [PubMed] [Google Scholar]
- 30.Kumar, R., K. Pai, K. Pathak, and S. Sundar. 2001. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin. Diagn. Lab Immunol. 8:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachaud, L., S. Marchegui-Hammami, E. Chabbert, J. Dereure, J. P. Dedet, and P. Bastien. 2002. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J. Clin. Microbiol. 40:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Fichoux, Y., J.-F. Quaranta, J.-P. Aufeuvre, A. Lelievre, P. Marty, I. Suffia, D. Rousseau, and J. Kubar. 1999. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J. Clin. Microbiol. 37:1953-1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luz, K. G., V. O. da Silva, E. M. Gomes, F. C. Machado, M. A. Araujo, H. E. Fonseca, T. C. Freire, J. B. d'Almeida, M. Palatnik, and C. B. Palatnik-de Sousa. 1997. Prevalence of anti-Leishmania donovani antibody among Brazilian blood donors and multiply transfused hemodialysis patients. Am. J. Trop. Med. Hyg. 57:168-171. [DOI] [PubMed] [Google Scholar]
- 34.Mannocci, A., G. La Torre, G. Chiaradia, C. De Waure, M. T. Mainelli, A. Cernigliaro, S. Bruno, and W. Ricciardi. 2007. Epidemiology and direct medical costs of human leishmaniasis in Italy. J. Prev. Med. Hyg. 48:27-36. [PubMed] [Google Scholar]
- 35.Maroli, M., L. Sansoni, F. Bigliocchi, C. Khoury, and M. Valsecchi. 1995. Survey of Phlebotomus neglectus Tonnoir, 1921 (=P. major s.l.) in a leishmanisasis focus in northern Italy province of Verona). Parassitologia 37:241-244. (In Italian.) [PubMed] [Google Scholar]
- 36.Maroli, M., L. Rossi, R. Baldelli, G. Capelli, E. Ferroglio, C. Genchi, M. Gramiccia, M. Mortarino, M. Pietrobelli, and L. Gradoni. 2008. The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop. Med. Int. Health 13:256-264. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Sanchez, J., J. A. Pineda, F. Morillas-Marquez, J. A. Garcia-Garcia, C. Acedo, and J. Macias. 2004. Detection of Leishmania infantum kinetoplast DNA in peripheral blood from asymptomatic individuals at risk for parenterally transmitted infections: relationship between polymerase chain reaction results and other Leishmania infection markers. Am. J. Trop. Med. Hyg. 70:545-548. [PubMed] [Google Scholar]
- 38.Marty, P., A. Lelievre, J. F. Quaranta, A. Rahal, M. Gari-Toussaint, and Y. Le Fichoux. 1994. Use of leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France). Trans. R. Soc. Trop. Med. Hyg. 88:658-659. [DOI] [PubMed] [Google Scholar]
- 39.Marty, P., A. Lelievre, J. F. Quaranta, I. Suffia, M. Eulalio, M. Gari-Toussaint, Y. Le Fichoux, and J. Kubar. 1995. Detection by Western blot of four antigens characterizing acute clinical leishmaniasis due to Leishmania infantum. Trans. R. Soc. Trop. Med. Hyg. 89:690-691. [DOI] [PubMed] [Google Scholar]
- 40.Marty, P., A. Izri, C. Ozon, P. Haas, E. Rosenthal, P. Del Giudice, J. Godenir, E. Coulibaly, M. Gari-Toussaint, P. Delaunay, B. Ferrua, H. Haas, F. Pratlong, and Y. Le Fichoux. 2007. A century of leishmaniasis in Alpes-Maritimes, France. Ann. Trop. Med. Parasitol. 101:563-574. [DOI] [PubMed] [Google Scholar]
- 41.Mary, C., D. Lamouroux, S. Dunan, and M. Quilici. 1992. Western blot analysis of antibodies to Leishmania infantum antigens; potential of the 14-kD antigens for diagnosis and epidemiologic purposes. Am. J. Trop. Med. Hyg. 47:764-771. [DOI] [PubMed] [Google Scholar]
- 42.Mary, C., F. Faraut, L. Lascombe, and H. Dumon. 2004. Quantification of Leishmania infantum DNA by a real time PCR assay with high sensitivity. J. Clin. Microbiol. 42:5249-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathur, P., and J. C. Samantaray. 2004. The first probable case of platelet transfusion-transmitted visceral leishmaniasis. Transfus. Med. 14:319-321. [DOI] [PubMed] [Google Scholar]
- 44.Pozio, E., L. Gradoni, and M. Gramiccia. 1985. La leishmaniose canine en Italie de 1910 a 1983. Ann. Parasitol. Hum. Comp. 60:543-553. [DOI] [PubMed] [Google Scholar]
- 45.Rezza, G., L. Nicoletti, R. Angelini, R. Romi, A. C. Finarelli, M. Panning, P. Cordioli, C. Fortuna, S. Boros, F. Magurano, G. Silvi, P. Angelini, M. Dottori, M. G. Ciufolini, G. C. Majori, A. Cassone, and the CHIKV study group. 2007. Infection with Chikungunya virus in Italy; an outbreak in a temperate region. Lancet 370:1840-1846. [DOI] [PubMed] [Google Scholar]
- 46.Riera, C., R. Fisa, M. Udina, M. Gallego, and M. Portus. 2004. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic islands, Spain) by different diagnostic methods. Trans. R. Soc. Trop. Med. Hyg. 98:102-110. [DOI] [PubMed] [Google Scholar]
- 47.Santos-Gomes, G., S. Gomes-Pereira, L. Campino, M. De Almeida Araújo, and P. Abranches. 2000. Performance of Immunoblotting in diagnosis of visceral leishmaniasis in human immunodeficiency virus-Leishmania sp.-coinfected patients. J. Clin. Microbiol. 38:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scarlata, F., F. Vitale, L. Saporito, S. Reale, V. L. Vecchi, S. Giordano, L. Infurnari, F. Occhipinti, and L. Titone. 2008. Asymptomatic Leishmania infantum/chagasi infection in blood donors of western Sicily. Trans. R. Soc. Trop. Med. Hyg. 102:394-396. [DOI] [PubMed] [Google Scholar]
- 49.Weitzel, T., N. Muhlberger, T. Jelinek, M. Schunk, S. Ehrhardt, C. Bogdan, et al. 2005. Imported leishmaniasis in Germany 2001-2004: data of the SIMPID surveillance network. Eur. J. Clin. Microbiol. Infect. Dis. 24:471-476. (In German.) [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. 1999. Leishmania/HIV co-infection, south-western Europe, 1990-1998. Wkly. Epidemiol. Rec. 74:365-375. [PubMed] [Google Scholar]
- 51.Zijlstra, E. E., and A. M. el-Hassan. 2001. Leishmaniasis in Sudan. Visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 95(Suppl. 1):S27-S58. [DOI] [PubMed] [Google Scholar]