Abstract
A multiplex PCR-ligation detection reaction (PCR-LDR) assay was developed for rapid detection of methicillin, tetracycline, and vancomycin resistance, as well as toxic shock toxin and Panton-Valentine leukocidin. The assay was tested on 470 positive blood culture bottles containing Staphylococcus aureus or enterococci. PCR-LDR exhibited a sensitivity and specificity of ≥98% for all components except tetracycline resistance, which had a sensitivity of 94.7%. Rapid and sensitive detection of antimicrobial resistance and virulence genes could help guide therapy and appropriate infection control measures.
Previous studies have demonstrated the ability of multiplex PCR-ligation detection reaction (PCR-LDR) assays to detect and identify a variety of clinically significant bacteria and flaviviruses (4, 12, 14). We have now developed a multiplex PCR-LDR assay to directly determine antibiotic resistance profiles, as well as the toxic shock syndrome (Tsst-1) and Panton-Valentine leukocidin (PVL) toxins, of Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium present in positive blood culture bottles. Rapid identification of antimicrobial resistance and virulence determinants could have a significant impact on reducing morbidity, mortality, and health care costs. Methicillin and vancomycin resistance genes were included in the assay because of the clinical importance of vancomycin-resistant enterococci (VRE), methicillin-resistant S. aureus (MRSA), and most recently vancomycin-resistant S. aureus (VRSA) infections (5, 15, 18). Tetracycline resistance genes were included because of renewed interest in this group of antibiotics due to their activity against several biothreat agents and the increase in community-acquired MRSA (7, 8, 21, 22).
Positive blood cultures containing S. aureus, E. faecalis, and/or E. faecium were obtained from patients at Weill Cornell Medical Center, New York Presbyterian Hospital (WCMC-NYPH). Blood cultures were collected and incubated in a BacT/Alert system (bioMérieux, Durham, NC) in accordance with the manufacturer's instructions. When a blood culture was read as positive by the BacT/Alert, 100-μl aliquots of the blood culture were transferred in quadruplicate to a 96-deep-well plate for subsequent extraction of DNA; negative controls containing only Tris-EDTA buffer were incorporated into each 96-well plate, and the plates were stored at −70°C. An additional 400 μl of the positive blood culture was stored for retesting of any discordant results. Bacteria were identified using standard methods. Additional clinical isolates of S. aureus, E. faecalis, and E. faecium were collected from wound, urine, respiratory, or autopsy samples. These isolates were spiked into negative clinical blood culture bottles as described previously (12). The use of human clinical samples in this research has complied with all relevant federal guidelines and WCMC-NYPH institutional policies.
Bacterial isolates previously shown by sequencing to contain the mecA, vanA, vanB, tetK, tetL, tetM, or lukS-lukF (PVL) gene(s) were obtained from the WCMC-NYPH clinical microbiology collection. E. faecium N97-0330 (vanD3) and E. faecium N03-0072 (vanD5) (1) were provided by Michael Mulvey. E. faecium NEF1 (vanD1) (9) was provided by Albert Sotto and Jean Phillippe Lavigne.
DNA from clinical specimens was extracted using an ABI 6100 nucleic acid prep system (Applied Biosystems, Foster City, CA) as described previously (12). The procedure used fine grade charcoal to remove sodium polyanetholesulfonate (SPS), an essential component of blood culture media and a potent inhibitor of PCR.
PCR and LDR primers were designed based on sequences obtained from the GenBank database (http://www.ncbi.nlm.nih.gov) as previously described (12). In instances where sequences did not share 100% identity, multiple PCR/LDR primers were designed over the selected region. To prevent failure of PCR amplification, two PCR primer pairs were designed for each of the gene amplicons. The 44 PCR primers used in the multiplex PCR assay are presented in Table SA1 in the supplemental material. The 71 LDR primers used in the multiplex LDR assay are presented in Table SA2 in the supplemental material. Two to three LDR primer pairs were designed for each of the PCR amplicons. Identification of at least two LDR signals for any gene was required for a positive result. The assay also incorporated two previously defined positive amplification controls specific to the 16S rRNA genes, identifying the bacteria as Staphylococcus species, E. faecium, or E. faecalis (12). PCRs and LDR were performed as described in Table SA3 in the supplemental material. Capillary electrophoresis (CE) was performed and analyzed as previously described (4, 12, 14) (Fig. 1).
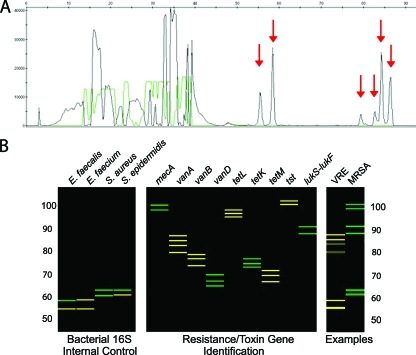
FIG. 1.
(A) Capillary electrophoresis (CE) trace for the antibiotic resistance, and toxin PCR-LDR assay of a vancomycin-resistant E. faecium isolate. The x axis shows the LDR product size, and the y axis shows fluorescence intensity of the bands. Red arrows indicate signals that are unique to the 16S rRNA genes of E. faecium and the vanA gene. (B) The CE data are displayed as a reconstructed gel image generated by a software program developed in our laboratory. The left panel represents the size and fluorescence of the 16S rRNA gene LDR products which act as a positive control and differentiate between the bacteria E. faecalis, E. faecium, and Staphylococcus species. The middle panel represents the LDR signals that identify the presence of methicillin resistance (mecA), vancomycin resistance (vanA, vanB, vanD), tetracycline resistance (tetL tetK, tetM), the toxic shock toxin gene (tst), and the PVL toxin genes (lukS-lukF). Data in the right panel represent two blood cultures. In one, the 16S rRNA gene control identified E. faecium, and the vanA gene was detected, indicating a VRE; in the other the 16S rRNA gene control identified S. aureus, and the mecA and lukS-lukF genes were detected, indicating an MRSA gene encoding the PVL toxin.
The antibiotic susceptibility of each organism was determined by VITEK2 (bioMerieux, Durham, NC). If an intermediate or discrepant call was made by the VITEK2, secondary/confirmatory testing was performed by Etest (AB Biodisk, Solna, Sweden). MICs were interpreted based on CLSI guidelines (3). Production of Tsst-1 was confirmed by reverse passive latex agglutination, using the TST-RPLA kit (Oxoid, United Kingdom). S. aureus ATCC 51651 and ATCC 13566 were used as positive and negative controls, respectively. The presence of the PVL toxin genes was confirmed by amplification and sequencing by using the primers luk-PV-1 and luk-PV-2 (10).
The resistance and toxin genotypes of 470 blood culture samples (193 positive blood cultures and 277 clinical isolates spiked into negative blood cultures) were examined using the multiplex PCR-LDR assay. Twenty of the positive blood cultures were polymicrobial; fourteen of these contained at least one S. aureus, E. faecium, or E. faecalis resistance gene.
Correlation of the multiplex PCR-LDR assay with standard susceptibility testing for pure cultures is presented in Table 1. The PCR-LDR assay detected methicillin resistance in S. aureus with 99.2% sensitivity and 99.1% specificity. One isolate was mecA positive and oxacillin susceptible, and one was mecA negative and oxacillin resistant. Vancomycin resistance was detected in E. faecium and E. faecalis with 98.6% sensitivity and specificity compared to susceptibility testing. Almost all (98.4%) vancomycin resistance in E. faecium was due to vanA, whereas vanB was responsible for an appreciable portion (44.4%) of resistance in E. faecalis (see Table SA4 in the supplemental material). Two isolates of E. faecalis were vancomycin susceptible but were positive for vanA or vanB. Low-level expression of the van genes may have failed to raise the MIC above the breakpoint for resistance. One isolate of E. faecium was vancomycin resistant but was negative for vanA, vanB, and vanD. Resistance may have been due to gene sequences not incorporated into this assay, such as vanE or vanG (5).
TABLE 1.
Antibiotic resistance phenotypes and PCR-LDR gene identification in blood cultures containing S. aureus or enterococci
| Phenotypea | No. of samples | No. of organisms with indicated genotype |
|
|---|---|---|---|
| mecA positive | mecA negative | ||
| MRSA | 124 | 123 | 1 |
| MSSA | 111 | 1 | 110 |
| vanA or -B positive | vanA or -B negative | ||
| E. faecium Vanr | 64 | 63 | 1 |
| E. faecium Vans | 14 | 0 | 14 |
| vanA or -B positive | vanA or -B negative | ||
| E. faecalis Vanr | 9 | 9 | 0 |
| E. faecalis Vans | 128 | 2 | 126 |
| tetK, -L, or -M positive | tetK, -L, or -M negative | ||
| S. aureus Tetr | 22 | 22 | 0 |
| S. aureus Tets | 213 | 2 | 211 |
| E. faecalis Tetr | 105 | 99 | 6 |
| E. faecalis Tets | 32 | 0 | 32 |
| E. faecium Tetr | 15 | 13 | 2 |
| E. faecium Tets | 63 | 1 | 62 |
r, resistance; s, susceptibility; Van, vancomycin; Tet, tetracycline.
Tetracycline resistance was detected in staphylococci and enterococci with 94.6% sensitivity and 99.0% specificity compared to standard susceptibility testing. The assay detected resistance in 22 isolates of S. aureus with 100.0% sensitivity and 99.0% specificity. The mechanism of resistance was divided between efflux pumps (tetK, 68.2%) and ribosomal protection proteins (tetM, 31.8%) (see Table SA4 in the supplemental material). In contrast, 98.2% of the resistance in E. faecium and E. faecalis was due to ribosomal protection proteins (tetM). Two S. aureus and one E. faecium were positive for tetK or tetM but susceptible in the phenotypic assay. This may result from low-level expression of the tet genes. Eight enterococci were tetracycline resistant but negative for tetK, tetL, and tetM. This is likely due to tetracycline resistance conferred by other gene sequences not incorporated into this assay (e.g., tetO, tetQ, or tetS) (13).
Table 2 shows the antibiotic phenotypes and PCR-LDR results from mixed cultures. Although the resistance genes detected by the assay could not be precisely assigned to a specific organism because of the ability of staphylococci and enterococci to harbor these resistance elements, the PCR-LDR assay was able to identify and detect the predicted resistance genes for each mixed culture.
TABLE 2.
Antibiotic resistance phenotypes and PCR-LDR gene identification in mixed cultures
| Organism identification and resistance phenotypea | PCR-LDR gene identification |
|---|---|
| E. faecalis Tetr, E. faecium | tetM |
| E. faecalis, E. faecium Vanr | vanA |
| E. faecalis Tetr, E. faecium Vanr | tetM vanA |
| E. faecalis Tetr Vanr, E. faecium | tetM vanA |
| E. faecalis, E. faecium Tetr Vanr, CNStaph | tetM vanB |
| E. faecalis Tetr, MRSA Oxar | tetM mecA |
| E. faecalis Tetr, MRSA Oxar | tetM mecA |
| E. faecalis, S. aureus, K. pneumoniaeb | tetM |
| E. faecalis Tetr, S. aureus, K. pneumoniae | tetM |
| E. faecalis Tetr, CNStaphc | tetL |
| E. faecalis Tetr, CNStaph | tetL tetM |
| E. faecalis Tetr, CNStaphc | tetM |
| E. faecalis Tetr Vanr, CNStaphc | tetM vanA |
| E. faecium Vanr, MRSA Oxar | vanA mecA |
There were six other mixed cultures that did not contain resistant organisms. CNStaph, coagulase-negative staphylococcus; Oxa, oxacillin.
E. faecalis and S. aureus were phenotypically Tets. K. pneumoniae was not tested.
These CNStaph isolates were oxacillin resistant, but PCR-LDR did not detect mecA or staphylococcal 16S rRNA gene sequences, presumably due to small numbers of organisms.
The tst gene encoding the Tsst-1 toxin was identified by the multiplex PCR-LDR assay in 15/242 samples containing S. aureus (6.2%; nine methicillin-susceptible S. aureus [MSSA] and six MRSA isolates). Tsst-1 activity was confirmed in 14 of the positive samples by using the TST-RPLA kit (one sample failed to grow from the archived material). Lack of Tsst-1 activity was confirmed in 25/25 S. aureus samples randomly selected from those organisms that were negative for the tst gene. The lukS and lukF PVL genes were detected in 24.0% of MRSA and 7.0% of MSSA samples. There was a higher incidence of PVL in non-bloodstream-infection (non-BSI) MRSA samples (27.0%) than that in BSI MRSA samples (18.0%).
Several real-time PCR assays have been developed that can detect S. aureus and MRSA in positive blood cultures (17, 23). A multiplex assay utilizing bead hybridization was able to distinguish several species of staphylococci and detect PVL, mecA, and other resistance genes (20). PCR-LDR assays detected methicillin and vancomycin resistance in S. aureus, E. faecalis, and E. faecium with >98% sensitivity and specificity compared to phenotypic susceptibility testing. The specificity may be an underestimate, since in several situations, such as determining methicillin resistance, gene detection is the more appropriate gold standard (3). The lower sensitivity we observed for detecting tetracycline resistance is probably due to our assay targeting only 3 of 38 known tet genes. Even large-scale multiplex assays might need to be restricted to detecting resistance to a limited number of “front-line” antibiotics. Our data also illustrate the difficulty of interpreting genotype data from mixed cultures; nonetheless, detection of resistance genes in these specimens could help guide empirical antibiotic therapy.
Tsst-1 and PVL toxins are commonly associated with community-acquired strains of Staphylococcus aureus (10); however, not all community-acquired strains carry the PVL toxin, and Tsst-1 can be found in nosocomial strains (2, 15, 16). Community-acquired MRSA strains, which are more likely to have these virulence genes, are more commonly found in skin or soft-tissue infections than in bloodstream infections (8, 11, 15, 16). Of the 15 isolates carrying tst in our study, 73% came from non-BSIs. Similarly, of the 45 isolates carrying PVL toxin genes, 76% were from non-BSIs, and 71% were methicillin resistant. Although these virulence factors are not stable markers for distinguishing between community-acquired and nosocomial Staphylococcus strains, they are nevertheless important for molecular epidemiology and monitoring virulence factors and antibiotic resistance patterns. Combined with conventional culturing methods, a multiplex molecular assay has the potential to provide accurate, timely information, resulting in improved patient care and a reduction in broad-spectrum antibiotic usage while also providing epidemiological data for infection control and antimicrobial/toxin surveillance systems (6, 19).
Supplementary Material
Acknowledgments
We thank Benjamin See, Carmen Azurin, and the technical staff of the clinical microbiology laboratory at WCMC for collecting and characterizing clinical isolates; Jianmin Huang for providing the AK16D ligase enzyme; and Yu-wei Cheng and Hanna Pincas for helpful discussion.
This work was supported by Public Health Service grant UC1-AI062579 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 28 October 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Boyd, D. A., P. Kibsey, D. Roscoe, and M. R. Mulvey. 2004. Enterococcus faecium N03-0072 carries a new VanD-type vancomycin resistance determinant: characterization of the VanD5 operon. J. Antimicrob. Chemother. 54:680-683. [DOI] [PubMed] [Google Scholar]
- 2.Cercenado, E., O. Cuevas, M. Marin, E. Bouza, P. Trincado, T. Boquete, B. Padilla, and A. Vindel. 2008. Community-acquired methicillin-resistant Staphylococcus aureus in Madrid, Spain: transcontinental importation and polyclonal emergence of Panton-Valentine leukocidin-positive isolates. Diagn. Microbiol. Infect. Dis. 61:143-149. [DOI] [PubMed] [Google Scholar]
- 3.CLSI. 2007. Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Das, S., M. R. Pingle, J. Munoz-Jordan, M. S. Rundell, S. Rondini, K. Granger, G. J. Chang, E. Kelly, E. G. Spier, D. Larone, E. Spitzer, F. Barany, and L. M. Golightly. 2008. Detection and serotyping of dengue virus in serum samples by multiplex reverse transcriptase PCR-ligase detection reaction assay. J. Clin. Microbiol. 46:3276-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Depardieu, F., B. Perichon, and P. Courvalin. 2004. Detection of the van alphabet and identification of enterococci and staphylococci at the species level by multiplex PCR. J. Clin. Microbiol. 42:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema, D. J., K. J. Dodgson, B. Sigurdardottir, and M. A. Pfaller. 2004. Rapid detection of antimicrobial-resistant organism carriage: an unmet clinical need. J. Clin. Microbiol. 42:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frean, J., K. P. Klugman, L. Arntzen, and S. Bukofzer. 2003. Susceptibility of Yersinia pestis to novel and conventional antimicrobial agents. J. Antimicrob. Chemother. 52:294-296. [DOI] [PubMed] [Google Scholar]
- 8.Gorwitz, R. J. 2008. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr. Infect. Dis. J. 27:1-7. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne, J. P., H. Marchandin, N. Bouziges, and A. Sotto. 2005. First infection with VanD-type glycopeptide-resistant Enterococcus faecium in Europe. J. Clin. Microbiol. 43:3512-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 11.Palavecino, E. 2007. Clinical, epidemiological, and laboratory aspects of methicillin-resistant Staphylococcus aureus (MRSA) infections. Methods Mol. Biol. 391:1-19. [DOI] [PubMed] [Google Scholar]
- 12.Pingle, M. R., K. Granger, P. Feinberg, R. Shatsky, B. Sterling, M. Rundell, E. Spitzer, D. Larone, L. Golightly, and F. Barany. 2007. Multiplexed identification of blood-borne bacterial pathogens by use of a novel 16S rRNA gene PCR-ligase detection reaction-capillary electrophoresis assay. J. Clin. Microbiol. 45:1927-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts, M. C. 2005. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 245:195-203. [DOI] [PubMed] [Google Scholar]
- 14.Rondini, S., M. R. Pingle, S. Das, R. Tesh, M. S. Rundell, J. Hom, S. Stramer, K. Turner, S. N. Rossmann, R. Lanciotti, E. G. Spier, J. Munoz-Jordan, D. Larone, E. Spitzer, F. Barany, and L. M. Golightly. 2008. Development of multiplex PCR-ligase detection reaction assay for detection of West Nile virus. J. Clin. Microbiol. 46:2269-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossney, A. S., C. M. Herra, G. I. Brennan, P. M. Morgan, and B. O'Connell. 2008. Evaluation of the Xpert methicillin-resistant Staphylococcus aureus (MRSA) assay using the GeneXpert real-time PCR platform for rapid detection of MRSA from screening specimens. J. Clin. Microbiol. 46:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossney, A. S., A. C. Shore, P. M. Morgan, M. M. Fitzgibbon, B. O'Connell, and D. C. Coleman. 2007. The emergence and importation of diverse genotypes of methicillin-resistant Staphylococcus aureus (MRSA) harboring the Panton-Valentine leukocidin gene (pvl) reveal that pvl is a poor marker for community-acquired MRSA strains in Ireland. J. Clin. Microbiol. 45:2554-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamper, P. D., M. Cai, T. Howard, S. Speser, and K. C. Carroll. 2007. Clinical validation of the molecular BD GeneOhm StaphSR assay for direct detection of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in positive blood cultures. J. Clin. Microbiol. 45:2191-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratidis, J., F. J. Bia, and S. C. Edberg. 2007. Use of real-time polymerase chain reaction for identification of methicillin-resistant Staphylococcus aureus directly from positive blood culture bottles. Diagn. Microbiol. Infect. Dis. 58:199-202. [DOI] [PubMed] [Google Scholar]
- 19.Sundsfjord, A., G. S. Simonsen, B. C. Haldorsen, H. Haaheim, S. O. Hjelmevoll, P. Littauer, and K. H. Dahl. 2004. Genetic methods for detection of antimicrobial resistance. APMIS 112:815-837. [DOI] [PubMed] [Google Scholar]
- 20.Tang, Y. W., A. Kilic, Q. Yang, S. K. McAllister, H. Li, R. S. Miller, M. McCormac, K. D. Tracy, C. W. Stratton, J. Han, and B. Limbago. 2007. StaphPlex system for rapid and simultaneous identification of antibiotic resistance determinants and Panton-Valentine leukocidin detection of staphylococci from positive blood cultures. J. Clin. Microbiol. 45:1867-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbull, P. C., N. M. Sirianni, C. I. LeBron, M. N. Samaan, F. N. Sutton, A. E. Reyes, and L. F. Peruski, Jr. 2004. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J. Clin. Microbiol. 42:3626-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urich, S. K., and J. M. Petersen. 2008. In vitro susceptibility of isolates of Francisella tularensis types A and B from North America. Antimicrob. Agents Chemother. 52:2276-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolk, D. M., M. J. Struelens, P. Pancholi, T. Davis, P. Della-Latta, D. Fuller, E. Picton, R. Dickenson, O. Denis, D. Johnson, and K. Chapin. 2009. Rapid detection of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) in wound specimens and blood cultures: multicenter preclinical evaluation of the Cepheid Xpert MRSA/SA skin and soft tissue and blood culture assays. J. Clin. Microbiol. 47:823-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.