Abstract
After allogeneic stem cell transplantation, a 49-year-old man developed fever and inflammation at the site of a plant puncture on a finger. A hyalohyphomycete was recovered by incubating the plant spine fragment following surgery. Amplification of the internal transcribed spacer region and 5.8S rRNA, β-tubulin, and translation elongation factor coding genes identified Fusarium proliferatum, which was confirmed later by culture.
CASE REPORT
A febrile 49-year-old Ecuadorian man was evaluated at the National Institutes of Health Clinical Center 8 days after allogeneic hematopoietic stem cell transplantation for pain, erythema, and swelling in the right third finger. The patient had been neutropenic for 8 days and had been started empirically on ceftazidime and vancomycin for fevers that began 4 days earlier. Finger pain and swelling had also begun 4 days earlier, but he did not report the symptoms until they became severe. The patient described a puncture injury to the finger from the spine of a Chinese palm tree (Trachycarpus fortunei) 8 months before. Since the injury, he had experienced two flares of erythema and swelling at the site, most recently 3 months ago; each episode resolved spontaneously. There had been no skin breakdown or drainage from the finger.
The patient underwent hematopoietic stem cell transplantation for myelodysplastic syndrome (refractory anemia with excess blasts) diagnosed 4 months earlier. The patient lived in Quito, Ecuador, where he owned an avocado farm but did not operate it directly. He had an urban home with a plant-filled patio and no pets.
Upon examination, the patient was found to have rigors and appeared to be very uncomfortable. His temperature was 38.4°C, his blood pressure was 113/59 mm Hg, his heart rate was 82 beats per min, his respiratory rate was 20 breaths per min, and his pulse oximetry reading was 100% on room air. The volar surface of the right third finger slightly distal to the proximal interphalangeal joint was erythematous, warm, focally indurated and tender, but with intact skin (Fig. 1a). He was able to flex but not fully extend the finger. There were no other cutaneous lesions and no onychomycosis.
FIG. 1.
(a) The volar aspect of the proximal interphalangeal joint of the patient's right third finger was inflamed. (b) Spin-spin relaxation time (T2)-weighted MRI of the hand shows a tissue defect corresponding to the plant spine (arrow).
Laboratory studies revealed a white blood cell count of 14 cells/mm3, a hemoglobin level of 9.7 g/dl, a platelet count of 26,000/mm3, and a C-reactive protein level of 1.97 mg/liter. Magnetic resonance imaging (MRI) of the finger (Fig. 1b) revealed enhancement of the superficial and peritendinous soft tissues, as well as a punctate foreign body in the superficial volar aspect of the finger. Fungal and bacterial blood cultures were negative.
Intravenous voriconazole at 4 mg/kg every 12 h was initiated as empirical therapy for prolonged fever and persistent neutropenia. The patient was taken to the operating room, where incision and drainage of the finger lesion yielded gray fluid and a tiny (∼2-mm) plant spine fragment. The spine and abscess material were sent for microbiological studies. At surgery, there was no evidence that the infection extended into the joint space.
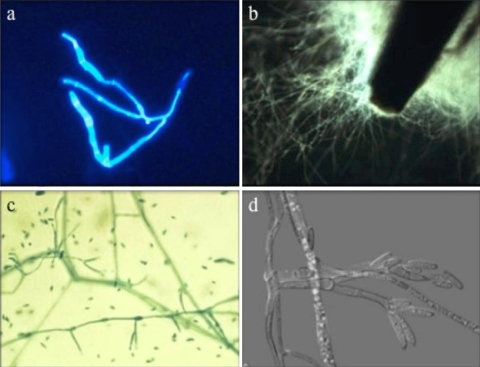
Microscopic examination of Fungi-Fluor-stained abscess material revealed septate hyphae with parallel walls and hyphae that were irregular in diameter, with some rounded forms. Culture of the abscess material (Fig. 2) and plant spine grew Fusarium proliferatum (see below). Following surgical drainage, the pain, erythema, and swelling in the finger resolved and the incision healed uneventfully.
FIG. 2.
(a) Wet mount of abscess fluid, stained with Fungi-Fluor, showing septate branching hyphae with parallel walls and hyphae that are irregular in diameter (original magnification, ×200). (b) Tip of the plant spine, which had been inoculated directly into a 12B Bactec bottle upon removal from the abscess, covered with mold after 2 days of growth. (c) Smear of plant spine culture, stained with lactophenol aniline blue, showing abundant clavate to pyriform microconidia and rare, slightly bent, sickle-shaped macroconidia (original magnification, ×400). (d) A differential interference contrast microscopy image of F. proliferatum demonstrates slender, branched septate hyphae with conidiophores arising laterally from hyphae (original magnification, ×640); conidiogenous cells bear apical falcate or nearly straight, septate macroconidia.
We considered that the likelihood of recovery of the etiologic agent from this tiny fragment by simply plating onto agar would be low. Therefore, we employed a more unconventional approach, incubating the specimen in liquid medium in the automated Bactec system.
Three surgical specimens were submitted to the Microbiology Service. The plant spine was inoculated into a 12B Bactec bottle (Becton Dickinson, Sparks, MD), and the bottle was incubated at 35°C. After 3 days, the Bactec bottle was subjected to a vortex and the solution was subcultured on Emmons Sabouraud agar (SAB) and potato dextrose agar (PDA). Soft tissue biopsy material and abscess fluid from the finger were also submitted for bacterial, mycobacterial, and fungal cultures, with subsequent subculture of the fungal culture on SAB and PDA. The abscess material was examined microscopically using the Fungi-Fluor fluorescent fungal stain (Polysciences, Inc., Warrington, PA). Susceptibility testing was performed at the Pathology and Laboratory Medicine Service, Audie L. Murphy Memorial Veterans Hospital, San Antonio, TX, using Clinical and Laboratory Standards Institute/NCCLS methodology (4).
Fungal DNA was extracted from mycelium grown for 5 days on PDA by using the UltraClean microbial DNA isolation kit (MoBio Laboratories, Solana Beach, CA) with manufacturer modifications of the original protocol to optimize it for molds. Molecular identification of the isolate was performed using the internal transcribed spacer (ITS) region (ITS1-5.8S rRNA gene-ITS2) and β-tubulin and translation elongation factor 1α (EF-1α) coding genes. PCR amplification of these targets was accomplished using previously described reagents and cycling conditions with the primer pairs ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (26), T1 (5′-AACATGCGTGAGATTGTAAGT-3′) and T22 (5′-TCTGGATGTTGTTGGGAATCC-3′) (17), and EF-1 (5′-ATGGGTAAGGAGGACAAGAC-3′) and EF-2 (5′-GGAAGTACCAGTGATCATGTT-3′) (19), respectively. The resultant ITS PCR products were sequenced using ITS1 and ITS4 primers, and β-tubulin PCR products were sequenced using T1 and T2 (5′-TAGTGACCCTTGGCCCAGTTG-3′) primers. The EF-1α PCR products were sequenced using one forward primer, EF-3 (5′-GTAAGGAGGASAAGACTCACC-3′), and one reverse sequencing primer, EF-22 (5′-AGGAACCCTTACCGAGCTC-3′) (19). All sequencing reactions used the ABI Prism BigDye Terminator cycle sequencing ready reaction kit, version 1.1 (Applied Biosystems). Sequencing reactions were performed at 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min for a total of 25 cycles. Sequencing products were purified with a CleanSEQ sequencing reaction cleanup system (Agencourt, Beverly, MA) and were analyzed with the 3100 genetic analyzer according to the instructions of the manufacturer (Applied Biosystems). The Lasergene program (version 7; DNASTAR [www.dnastar.com]) was used for sequence assembly and alignment. Sequences obtained with each primer set were compared to GenBank nucleotide sequences by using nucleotide-nucleotide BLAST (www.ncbi.nlm.nih.gov/BLAST) and realigned relative to best-matched sequences by using MegAlign (DNASTAR) with neighbor-joining analysis. As many Fusarium spp., including F. proliferatum, do not have a type strain, sequences were compared with closest matches in GenBank. Sequencing of the ITS region assigned the isolates to the genus Fusarium but could not provide further intrataxon discrimination. ITS sequences revealed 100% alignment homology to sequences from numerous Fusarium species. The β-tubulin sequences demonstrated 100% alignment homology to F. proliferatum (GenBank accession no. AF336910 and AF060389) and F. annulatum (GenBank accession no. FAU61540) sequences. DNA database comparison of the EF-1α sequence showed 100% identity to sequences from F. proliferatum strains (GenBank accession no. FJ538242, FJ538244, and EF452998) (Fig. 3).
FIG. 3.
A phylogenetic tree based on the EF-1α sequences was constructed using the neighbor-joining method. Bootstrap analysis with 1,000 replications was performed to establish the tree's robustness. Additional clinically significant Fusarium sequences obtained from GenBank were included for comparison. The tree was rooted with F. lunatum CBS 643.76. Numbers in parentheses are GenBank accession numbers.
After 3 days, the 12B Bactec bottle containing the plant spine showed diffuse mold growth around the spine. Growth of mold was also apparent in the abscess fluid cultures at both 30 and 35°C after 3 days. The macroscopic growths of mold on SAB and PDA initially displayed white cottony aerial mycelia, which developed a lilac tinge; the reverse sides of the colonies were maroon. Microscopic examination of subculture growth on PDA revealed densely branched conidiophores with monophialides and polyphialides with short chains of microconidia. No chlamydospores were seen. Short chains of microconidia and the absence of chlamydospores helped to differentiate this species from F. oxysporum, while the presence of polyphialides separated this species from F. verticillioides (Fig. 2). The morphological features were consistent with the molecular diagnosis and compatible with the description by de Hoog et al. (5). Antifungal susceptibility testing of F. proliferatum resulted in MICs of amphotericin B, itraconazole, and voriconazole of 1.0, >8.0, and >8.0 μg/ml, respectively.
Fusarium spp. have emerged as important pathogens in immunosuppressed patients (25). In neutropenic patients, Fusarium spp. can cause soft tissue infections, pneumonia, and disseminated disease with characteristic metastatic skin lesions and a high mortality rate (15, 16). Early detection and identification of fusariosis in immunocompromised patients allows timely intervention, specific therapy, and improved outcome. Herein, we report the case of a patient at the National Institutes of Health Clinical Center with fever and neutropenia and a Fusarium infection related to a puncture wound from a domestic plant source. Recovery of the organism from the clinical specimen was achieved by incubation of resected tissue in liquid medium using the automated Bactec system. Identification of recurrent F. proliferatum infection was made on the basis of morphology and confirmed by molecular techniques.
Fusarium spp. are septate hyaline molds found on plants and in soil in tropical and temperate climates. The most frequent human Fusarium isolates are (in order of decreasing frequency) F. solani, F. oxysporum, and F. proliferatum (7). Several reports, the earliest in 1988 (23), identify F. proliferatum as the etiology of disseminated fusariosis in profoundly immunosuppressed patients with hematologic malignancies (1, 22). Several species, including F. proliferatum, produce mycotoxins that can sicken animals and people who ingest plants that are colonized or infected with those species (13, 20).
Molecular sequencing of the isolate was performed to ensure the correct identification, as Fusarium spp. can exhibit variability in their pathogenicity and susceptibility patterns (9). The isolate of F. proliferatum from this patient was resistant to voriconazole (MIC > 8 μg/ml), which would usually be the preferred treatment agent. The MIC of amphotericin was elevated, at 1.0 μg/ml.
Early resection of the fusarial lesion was definitive treatment for this patient. Had the infection disseminated from the hand, antifungal therapy may not have been successful given the elevated MICs. Identification of isolates of Fusarium to the species level is especially important, as in vitro susceptibility, virulence, and ecology vary widely across this genus.
ITS sequences revealed 100% alignment homology to sequences from numerous Fusarium species (F. proliferatum [15], Giberella fujikuroi [moniliformis] [9], and other Fusarium species [9]). β-tubulin primer sets were used in addition to the ITS universal primers because ITS analysis alone is considered unreliable for the identification strains of Fusarium, such as F. proliferatum strains, that are found within the subgenus Liseola in the G. fujikuroi complex (17). β-tubulin sequence analysis indicated that sequences from our isolate were 100% identical to sequences from F. proliferatum strains (GenBank accession no. AF336910 and AF060389) and F. annulatum (GenBank accession no. FAU61540). F. proliferatum is considered to be a later synonym of F. annulatum (18). The analysis of EF-1α showed sequences from our isolate to be 100% identical to sequences with GenBank accession no. FJ538242, EF452998, and FJ538244.
Fusarium spp. are an increasingly important cause of infections in humans, particularly those with impaired immunity (25). A type of hyalohyphomycosis, fusariosis can occur in normal as well as immunocompromised human hosts. In normal hosts, the mold typically infects soft tissues through traumatic inoculation (14). These infections take the form of onychomycosis (10), keratitis and endophthalmitis (6), and mycetoma (7). Fusarium species may also superinfect third-degree burns and other deep wounds (7).
In patients with neutropenia due to cytotoxic chemotherapy, Fusarium species cause a range of invasive infections, from localized cellulitis extending from an infected toenail to pneumonia and disseminated disease. Systemic fusariosis typically occurs in patients with prolonged or recurrent profound neutropenia and is characterized by metastatic skin lesions, positive blood cultures for 50% of patients, and a high mortality rate (14). In such cases, the source of the infection may be the skin or sinopulmonary tract but is often elusive (3, 7).
Identification of Fusarium as a cause of infection in immunocompromised patients is important. The differential diagnosis of a soft tissue infection includes many serious bacterial etiologies. The implications of failing to make a diagnosis are potentially grave. The infection may progress locally, causing extensive tissue destruction. It may disseminate hematogenously, causing overwhelming sepsis and multiorgan failure. Finally, the use of empirical broad antimicrobial therapy mandated by the absence of a specific microbiological diagnosis may result in unnecessary drug toxicities. The molecular species-level identification of F. proliferatum was also clinically important, given the variation in virulence, antifungal susceptibility, and natural history among the species of Fusarium.
Host factors are the critical determinants of vulnerability to Fusarium infections. Patients with hematologic malignancies undergoing myeloablative chemotherapy are particularly at risk for Fusarium infections because of several features of their disease and therapy: acute leukemia, profound neutropenia (<5 × 109 neutrophils/ml), prolonged neutropenia (particularly that lasting more than 14 days), and fluconazole prophylaxis (12).
Environmental factors outside the hospital clearly contribute to exposure to Fusarium. The mold is essentially ubiquitous, colonizing a wide variety of plants throughout the world, including palm trees (21). The plant in this case, T. fortunei, does not grow in the wild. Though we were not able to obtain a specimen of the patient's plant, botanical study of the species has shown that it is colonized by several fungi, including Fusarium spp., in the diverse climates in which it is cultivated (24).
While there exists extensive literature on fungal infections acquired through trauma from plants colonized with molds (2, 8, 27), the present account is, to our knowledge, the only report of a case involving F. proliferatum. The more common F. solani was implicated in a recurrent soft tissue abscess in a normal host after introduction by trauma from a small bamboo chip (11). Retained nonorganic foreign bodies may also harbor molds that are normally kept in check by host defenses.
The environmental ubiquity of Fusarium spp. and their association with plants coincide with the cutaneous inoculation from the plant spine in this patient's history. Prior to the development of neutropenia, his intrinsic host defenses likely contained the organism. With the onset of neutropenia, the Fusarium hyphae could invade local tissue and create a painful erythematous lesion. Because the skin is a portal of entry for Fusarium and other molds, cutaneous infections should be managed aggressively. This patient was taken to the operating room for surgical drainage not because the centimeter-wide finger infection itself was life threatening but because of its potential to propagate and cause a highly lethal syndrome. Left unchecked, this lesion might have become the focus for hematogenous dissemination.
With the use of liquid and solid culture media and the availability of direct sequencing, the causes of soft tissue lesions resected for suspected invasive fungal infection can now be identified more rapidly.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers for our isolate, designated NIH1047M, are GQ243744 for the β-tubulin sequence and GU168776 for the EF-1α sequence.
Acknowledgments
This work was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases, the National Heart, Lung, and Blood Institute, the NIH Clinical Center, and the National Cancer Institute.
We thank Frank Witebsky for his critical review and thoughtful comments.
Footnotes
Published ahead of print on 18 November 2009.
REFERENCES
- 1.Barrios, N. J., D. V. Kirkpatrick, A. Murciano, K. Stine, R. B. Van Dyke, and J. R. Humbert. 1990. Successful treatment of disseminated Fusarium infection in an immunocompromised child. Am. J. Pediatr. Hematol. Oncol. 12:319-324. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Simon, G. J., I. S. Barequet, and A. Grinbaum. 2002. More than tears in your eyes (Exophiala jeanselmei keratitis). Cornea 21:230-231. [DOI] [PubMed] [Google Scholar]
- 3.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute/NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 1st ed. CLSI/NCCLS document M38-A. Clinical and Laboratory Standards Institute/NCCLS, Wayne, PA.
- 5.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 6.Ferrer, C., J. Alio, A. Rodriguez, M. Andreu, and F. Colom. 2005. Endophthalmitis caused by Fusarium proliferatum. J. Clin. Microbiol. 43:5372-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming, R. V., T. J. Walsh, and E. J. Anaissie. 2002. Emerging and less common fungal pathogens. Infect. Dis. Clin. North Am. 16:915-933, vi-vii. [DOI] [PubMed] [Google Scholar]
- 8.Golledge, C. 1995. An infection from a penetrating plant wound. Aust. Fam. Physician 24:2099. [PubMed] [Google Scholar]
- 9.Guarro, J., M. Nucci, T. Akiti, J. Gene, M. D. Barreiro, and R. T. Goncalves. 2000. Fungemia due to Fusarium sacchari in an immunosuppressed patient. J. Clin. Microbiol. 38:419-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattori, N., A. Shirai, Y. Sugiura, W. Li, K. Yokoyama, Y. Misawa, K. Okuzumi, and K. Tamaki. 2005. Onychomycosis caused by Fusarium proliferatum. Br. J. Dermatol. 153:647-649. [DOI] [PubMed] [Google Scholar]
- 11.Leu, H. S., A. Y. Lee, and T. T. Kuo. 1995. Recurrence of Fusarium solani abscess formation in an otherwise healthy patient. Infection 23:303-305. [DOI] [PubMed] [Google Scholar]
- 12.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 13.Nelson, P. E., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nucci, M., and E. Anaissie. 2002. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35:909-920. [DOI] [PubMed] [Google Scholar]
- 15.Nucci, M., and E. Anaissie. 2007. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nucci, M., K. A. Marr, F. Queiroz-Telles, C. A. Martins, P. Trabasso, S. Costa, J. C. Voltarelli, A. L. Colombo, A. Imhof, R. Pasquini, A. Maiolino, C. A. Souza, and E. Anaissie. 2004. Fusarium infection in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 38:1237-1242. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell, K., and E. Cigelnik. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7:103-116. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell, K., E. Cigelnik, and H. I. Nirenberg. 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465-493. [Google Scholar]
- 19.O'Donnell, K., H. C. Kistler, E. Cigelnik, and R. C. Ploetz. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. U. S. A. 95:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt, J. I. 2000. Toxigenic fungi: which are important? Med. Mycol. 38(Suppl. 1):17-22. [PubMed] [Google Scholar]
- 21.Polizzi, G., and A. Vitale. 2003. First report of Fusarium blight on majesty palm caused by Fusarium proliferatum in Italy. Plant Dis. 87:1149. [DOI] [PubMed] [Google Scholar]
- 22.Richardson, S. E., R. M. Bannatyne, R. C. Summerbell, J. Milliken, R. Gold, and S. S. Weitzman. 1988. Disseminated fusarial infection in the immunocompromised host. Rev. Infect. Dis. 10:1171-1181. [DOI] [PubMed] [Google Scholar]
- 23.Summerbell, R. C., S. E. Richardson, and J. Kane. 1988. Fusarium proliferatum as an agent of disseminated infection in an immunosuppressed patient. J. Clin. Microbiol. 26:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor, J. E., K. D. Hyde, and E. B. G. Jones. 2000. The biogeographical distribution of microfungi associated with three palm species from tropical and temperate habitats. J. Biogeogr. 27:297-310. [Google Scholar]
- 25.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 26.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA sequences for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gefland, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, NY.
- 27.Wilson, M., J. Robson, C. M. Pyke, and J. G. McCormack. 1998. Saksenaea vasiformis breast abscess related to gardening injury. Aust. N. Z. J. Med. 28:845-846. [DOI] [PubMed] [Google Scholar]