Abstract
Clostridium botulinum type E has been associated with botulism in adults but never in infants. Infant botulism type E cases have been associated with neurotoxigenic strains of C. butyricum. We report the first infant botulism case due to C. botulinum type E worldwide.
CASE REPORT
On 1 June 2007, a 9-day-old girl was brought to the Central DuPage Hospital, Winfield, IL, with decreased oral intake, weak cry, lethargy, and hypotonia. On the same day, the patient was admitted to the pediatric intensive care unit due to progressive decreased oral intake, and a nasogastric feeding tube was inserted. The neurologic examination revealed general hypotonia and lethargy but maintenance of deep tendon reflexes in the lower extremities. The infant received ceftriaxone, ampicillin, and ampicillin-cefotaxime during her hospitalization. On 4 June, infant botulism was suspected, and the Infant Botulism Treatment and Prevention Program was consulted. Botulism immune globulin intravenous (human) (BIG-IV) was administered the same day. The patient was discharged from the hospital on 7 June.
Serum and stool samples (both collected on 4 June) were submitted to the Centers for Disease Control and Prevention (CDC) for laboratory testing. An additional stool sample, collected just prior to discharge on 7 June, was also submitted to the CDC. The two fecal samples and the serum sample were tested for botulinum neurotoxin (BoNT) by standard methods (6). The fecal sample collected on 4 June was positive for BoNT type E, with approximately 113 50% lethal doses (LD50)/ml. This level of toxin was lower than the levels reported for infant botulism types A and B but similar to those reported for type E cases (9). The serum sample collected on 4 June and the stool sample collected on 7 June were both negative for BoNT. The two fecal samples were examined for the presence of neurotoxin-producing clostridia in accordance with previously reported methods (6), with some modifications. Plates streaked directly from the 4 June stool sample showed several lecithinase-positive (Lec+) colonies and lipase-negative/lecithinase-negative (Lip−/Lec−) colonies. All of these colonies were negative for production of BoNT by a mouse bioassay and negative for the presence of the bont/E gene by PCR. Lip+ colonies were also present but heavily mixed with other colony types. An alcohol treatment for spore selection (6) was then performed for the 4 June fecal sample. This plate contained both Lip+ colonies and Lec+ colonies; however, only the Lip+ colonies were positive for the presence of the bont/E gene by PCR. Both colony types were inoculated into broth media and incubated for 5 days under anaerobic conditions at 35°C. Only the Lip+ colonies produced BoNT type E. Plates streaked directly from the 7 June stool sample showed only Lip−/Lec− colonies. The two fecal samples were also inoculated into broth media, including heat-treated and non-heat-treated chopped-meat broth media (6). These enrichment cultures were incubated under anaerobic conditions for 5 days at 35°C. BoNT type E was detected only in the supernatant of the unheated chopped-meat broth from the stool sample collected on 4 June. Two distinct colony types (Lip+ colonies and Lec+ colonies) were observed following inoculation onto agar media. However, only Lip+ colonies produced BoNT type E, and these colonies were positive for the presence of bont/E gene by PCR.
The BoNT type E-producing strain (strain CDC52256) was further characterized by biochemical properties (Table 1). These characteristics corresponded to those of Clostridium botulinum group II, as listed by Holdeman et al. (12). In addition, the 16S rRNA and the bont/E genes of strain CDC52256 were sequenced. The 16S rRNA partial gene nucleotide sequence corresponded to nonproteolytic C. botulinum (group II), and the bont/E gene nucleotide sequence (GenBank accession number GQ294552) was 100% identical to the previously reported subtype E3 sequence (11) (Fig. 1).
TABLE 1.
Comparison of biochemical properties of strain CDC52256 with those of C. botulinum type E and C. butyricum type E
| Reaction | Presencea in: |
||
|---|---|---|---|
| CDC52256 | C. botulinum type E | C. butyricum type E | |
| Acid from: | |||
| Glucose | + | + | + |
| Sucrose | + | + | + |
| Maltose | + | + | + |
| Glycerol | + | v | v |
| Xylose | + | − | + |
| Mannose | + | + | + |
| Trehalose | + | + | + |
| Mannitol | − | − | − |
| Lactose | − | − | + |
| Salicin | − | − | + |
| Rhamnose | − | − | − |
| Starch | − | v | + |
| Cellobiose | − | − | + |
| Arabinose | − | − | v |
| Nitrate reduction | − | − | v |
| Gelatin liquefaction | − | − | − |
| Indol production | − | − | − |
| Catalase production | − | − | − |
| Lecithinase production | − | − | − |
| Esculin hydrolysis | − | − | + |
| Meat digestion | − | − | − |
| Lipase production | + | + | − |
| Milk coagulation | − | − | + |
+, present; −, absent; v, variable.
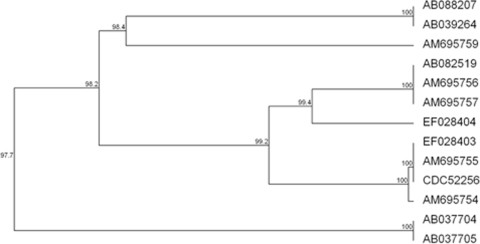
FIG. 1.
Comparison of bont/E gene nucleotide sequences. The bont/E genes of strain CDC52256 sequenced in this study and 12 bont/E gene sequences previously reported were compared (GenBank accession numbers are shown). The six previously described subtypes (E1 to E6) are apparent. C. botulinum strain CDC52256 clustered within subtype E3.
C. botulinum and rare strains of C. butyricum and C. baratii are Gram-positive anaerobic bacterial groups that produce an extremely potent toxin, the BoNT. There are seven serologically distinct types of BoNT (A through G). Botulism may result from either the ingestion of preformed toxin (food-borne) or the growth of C. botulinum in either the intestinal tract or a wound. Infant botulism is caused by in vivo production of BoNT by germinating spores of C. botulinum in the intestinal tract of an infant. The main clinical features of infant botulism are constipation (defined by 3 or more days without defecation), hypotonia, listlessness, lethargy, difficulty in suckling and swallowing, weak cry, pooled oral secretions, general muscle weakness, and loss of head control. Neurological findings include ptosis, ophthalmoplegia, sluggish pupillary reaction to light, flaccid expression, dysphagia, weak gag reflex, and poor anal sphincter tone.
Since infant botulism was first described in 1976, more than 2,900 cases have been reported worldwide. C. botulinum types A and B accounted for 98.7% of the cases in which the toxin type is known (14). In addition, C. baratii type F has been recovered from infant botulism patients in the United States (5, 10, 16) and Hungary (18), and C. butyricum type E has been isolated from infant patients in the United States (8), Italy (4, 9, 15), and Japan (1).
In June 2007, the DuPage County Health Department, the Illinois Department of Public Health, and the CDC investigated a suspected case of infant botulism in a 9-day-old infant. The CDC identified C. botulinum type E in a clinical specimen from this patient. To the best of our knowledge, this is the first report worldwide of an infant botulism case due to C. botulinum type E.
This case, with an onset at 9 days of age, is unusual since the mean age at the onset of infant botulism in the United States is 13.8 weeks (14). However, other infant botulism cases in extremely young patients have been reported (5, 13). The mean length of hospitalization for four type E infant botulism cases in Italy was 2.8 weeks (9), and a patient with type E infant botulism in Japan was hospitalized for 40 days (1). Therefore, another important characteristic of this case was the rapid recovery of the patient, i.e., after 6 days of hospitalization. However, none of the patients from Italy and Japan mentioned above received specific treatment with botulism immune globulin. In the United States, the mean length of hospital stay in infants with botulism caused by type A or type B toxin who receive BIG-IV is 2.6 weeks (2). Treatment with BIG-IV is indicated for infant botulism types A and B. However, this drug is produced with plasma samples of donors immunized with pentavalent botulinum toxoid (A to E) (2). The levels of IgG against the other types have not been reported. To the best of our knowledge, this is the first report on a type E infant botulism case treated with BIG-IV, and the rapid recovery of the patient could be due to the treatment with antitoxin. However, it is possible as well that C. botulinum type E organisms were rapidly cleared from the intestinal tract.
The bont/E gene nucleotide sequence of strain CDC52256 was 100% identical to the previously reported subtype E3 sequence (11). Based on the amino acid sequences of the neurotoxin, BoNT type E-producing strains of C. botulinum and C. butyricum have been classified into six subtypes (7). Subtypes E1, E2, E3, and E6 include C. botulinum strains, and subtypes E4 and E5 include C. butyricum strains. Subtype E3 has been identified in strains isolated from clinical and food specimens associated with food-borne botulism outbreaks in the United States and from fish in Germany and Finland (7, 11).
Two of the most recognized potential sources of BoNT-producing clostridium spores in infant botulism cases are honey and dust (14). However, the source of the spores is rarely determined. In the case reported here, there was no history of honey consumption, but some construction activity in the home area was noted. In the United States, C. botulinum type E has been found in marine life and sediment from the Pacific Northwest and the Great Lakes (6). There was no evidence that the infant in this case was exposed to soil or marine life from these areas. In addition, there have been no reports of the presence of C. botulinum type E in non-water-associated soils in the United States. However, isolation of this organism from the fecal sample from this infant required the use of a specific treatment, suggesting that published reports on distribution of type E spores in the United States could be underestimated due to the difficulty in recovering this organism. In that sense, the detection of the bont/E gene by PCR in culture supernatants was useful for directing subsequent testing, including a targeted search for type E-producing Clostridia spp.
We report here the first case worldwide of infant botulism due to C. botulinum type E. C. botulinum type E is a psychotropic bacterium, with an optimum growth temperature of 25°C (17), and has been related more frequently to food-borne botulism cases in northern regions. However, the description of a wound botulism case due to C. botulinum type E (3) and the infant botulism case reported here indicate that this organism could grow and produce BoNT at a normal body temperature.
The reported incidence of infant botulism may reflect both the distribution of the C. botulinum spores in the environment and the ability of physicians to identify and report the disease (14). The infant botulism case reported here suggests that other infants may be at risk, particularly those exposed to environments with high prevalences of C. botulinum type E spores. Because the signs of botulism in infants can be slight or, as in the case reported here, the progress and recovery can be very rapid, some infant botulism cases may go unidentified. Certainly, improving clinical diagnosis and laboratory confirmation of type E infant botulism will allow a better understanding of the epidemiology of the disease.
Acknowledgments
We acknowledge the assistance of the DuPage County Health Department and the Illinois Department of Public Health. We thank the Division of Food-borne, Bacterial, and Mycotic Diseases Sequencing Facility (CDC) for DNA sequencing and the Biotechnology Core Facility (CDC) for oligonucleotide synthesis. We also thank Steve Arnon and Jessica Payne of the California Department of Public Health, Infant Botulism Treatment and Prevention Program, for thoughtful discussions concerning this case.
This publication was supported by funds made available from the Centers for Disease Control and Prevention, Coordinating Office for Terrorism Preparedness and Emergency Response.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Abe, Y., T. Negasawa, C. Monma, and A. Oka. 2008. Infantile botulism caused by Clostridium butyricum type E toxin. Pediatr. Neurol. 38:55-57. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, S. S., R. Schechter, S. E. Maslanka, N. P. Jewell, and C. L. Hatheway. 2006. Human botulism immune globulin for the treatment of infant botulism. N. Engl. J. Med. 354:462-471. [DOI] [PubMed] [Google Scholar]
- 3.Artin, I., P. Bjorkman, J. Cronqvist, P. Radstrom, and E. Holst. 2007. First case of type E wound botulism diagnosed using real-time PCR. J. Clin. Microbiol. 45:3589-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aureli, P., L. Fenicia, B. Pasolini, M. Gianfranceschi, L. M. McCroskey, and C. L. Hatheway. 1986. Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J. Infect. Dis. 154:207-211. [DOI] [PubMed] [Google Scholar]
- 5.Barash, J. R., T. W. Tang, and S. S. Arnon. 2005. First case of infant botulism caused by Clostridium baratii type F in California. J. Clin. Microbiol. 43:4280-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1998. Botulism in the United States 1899-1996: handbook for epidemiologists, clinicians, and laboratory workers. Centers for Disease Control and Prevention, Atlanta, GA.
- 7.Chen, Y., H. Korkeala, J. Aarnikunnas, and M. Lindstrom. 2007. Sequencing the botulinum neurotoxin gene and related genes in Clostridium botulinum type E strains reveals orfx3 and a novel type E neurotoxin subtype. J. Bacteriol. 189:8643-8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dykes, J. K., C. Luquez, B. H. Raphael, L. M. McCroskey, and S. E. Maslanka. 2008. Laboratory investigation of the first case of Clostridium butyricum type E infant botulism in the United Sates, abstr. P-45. Abstr. 45th Interagency Botulism Res. Coord. Comm. Meet., Philadelphia, PA.
- 9.Fenicia, L., F. Anniballi, and P. Aureli. 2007. Intestinal toxemia botulism in Italy, 1984-2005. Eur. J. Clin. Microbiol. Infect. Dis. 26:385-394. [DOI] [PubMed] [Google Scholar]
- 10.Hall, J. D., L. M. McCroskey, B. J. Pincomb, and C. L. Hatheway. 1985. Isolation of an organism resembling Clostridium baratii which produces type F botulinal toxin from an infant with botulism. J. Clin. Microbiol. 21:654-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill, K. K., T. J. Smith, C. H. Helma, L. O. Ticknor, B. T. Foley, R. T. Svensson, J. L. Brown, E. A. Johnson, L. A. Smith, R. T. Okinaka, P. J. Jackson, and J. D. Marks. 2007. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J. Bacteriol. 189:818-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holdeman, L. V., E. P. Cato, and W. E. C. Moore (ed.). 1977. Anaerobe laboratory manual, 4th ed., p. 79-106. Virginia Polytechnic Institute and State University, Blacksburg, VA.
- 13.Keet, C. A., C. K. Fox, M. Margeta, E. Marco, A. L. Shane, S. J. DeArmond, J. B. Strober, and S. P. Miller. 2005. Infant botulism, type F, presenting at 54 hours of life. Pediatr. Neurol. 32:193-196. [DOI] [PubMed] [Google Scholar]
- 14.Koepke, R., J. Sobel, and S. S. Arnon. 2008. Global occurrence of infant botulism, 1976-2006. Pediatrics 122:e73-e82. [DOI] [PubMed] [Google Scholar]
- 15.McCroskey, L. M., C. L. Hatheway, L. Fenicia, B. Pasolini, and P. Aureli. 1986. Characterization of an organism that produces type E botulinal toxin but which resembles Clostridium butyricum from the feces of an infant with type E botulism. J. Clin. Microbiol. 23:201-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paisley, J. W., B. A. Lauer, and S. S. Arnon. 1995. A second case of infant botulism type F caused by Clostridium baratii. Pediatr. Infect. Dis. J. 14:912-914. [PubMed] [Google Scholar]
- 17.Peck, M. W. 2009. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 55:183-265. [DOI] [PubMed] [Google Scholar]
- 18.Trethon, A., J. Budai, A. Herendi, V. Szabo, and M. Geczy. 1995. Botulism in infancy. Orv. Hetil. 136:1497-1499. [PubMed] [Google Scholar]