Abstract
Heat shock is a well-known stress response characterized by a rapid synthesis of a set of proteins which are responsible for protection against stress. We examined the role of temperature on the growth of cricket paralysis virus, a member of the family Dicistroviridae, in insect cells. Heat shock caused an induction of heat shock protein-encoding mRNAs in uninfected cells but not in infected cells. While viral RNA and protein were abundant during heat shock, virion formation was inhibited at higher temperatures. The different susceptibility to pathogens at different temperatures is likely a crucial feature of host-pathogen interaction in cold-blooded animals.
Cricket paralysis virus (CrPV) is a positive-stranded RNA virus belonging to the Dicistroviridae family of viruses (9). The genome of CrPV contains two open reading frames, each preceded by an internal ribosome entry site (IRES) that mediates cap-independent translation (28). Much work has been done on the mechanisms of the two IRES elements (7, 8, 10, 21, 22, 25, 27), but very little work has been done to investigate the biology of the virus and how it interacts with the insect host.
One well-characterized pathway is the insect heat shock response, which includes a robust transcriptional and translational response that leads to the preferential production of chaperone proteins with molecular masses of 22, 23, 26, 27, 70, and 83 kDa, which are known as the heat shock proteins (HSPs) (15-17). These proteins are transcriptionally induced by heat shock factor, a transcription factor that oligomerizes upon temperature elevation (20). These transcribed HSP genes are selectively translated during heat shock via sequences in their 5′ leaders, while non-heat shock mRNAs are poorly translated, leading to an overall decrease in cellular protein synthesis (12, 13).
The cellular responses at high temperature can provide a unique environment for viruses. For example, the inactivation of the cellular protein synthesis machinery at high temperatures in mammals allows the translational machinery to be usurped by certain viral IRES-containing mRNAs (11). The reason for this observation is that IRES-mediated translation requires fewer factors than canonical cap-dependent translation initiation (23), which is modulated by cap binding proteins that are often inactivated during cell stress (23). Of course, when virus growth is dependent on the cap-dependent translational machinery, it is inhibited during heat shock (26). Furthermore, several viruses have been found to use HSPs or heat shock-like proteins to aid in genome replication (1, 5, 14) or capsid formation (2). In these cases, the HSPs are likely to act as chaperones that aid in the folding of macromolecular replication or assembly complexes. In an ongoing program to study the function and regulation of the two IRES elements in the CrPV genome, we examined whether IRES activities and viral growth are affected by temperature. Such effects could be especially significant because the environmental temperature regulates the metabolism of cold-blooded crickets, the natural hosts of CrPV.
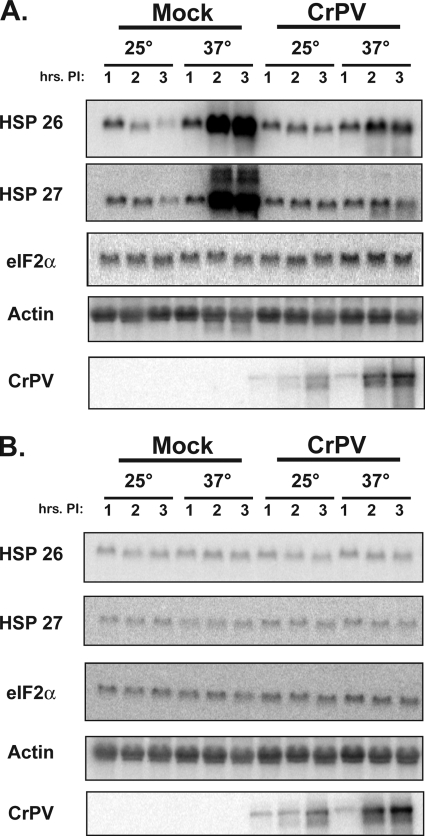
To examine the effects of temperature on the replication of CrPV in insect cells, cultured Drosophila S2 cells were mock infected or infected with CrPV at a multiplicity of infection of 20. Briefly, S2 cells were incubated with phosphate-buffered saline (PBS) or a CrPV-PBS inoculum for 30 min at room temperature. Prewarmed medium was added, and cells were incubated for the indicated times. RNA was extracted from cells at different times after infection using the TRIzol reagent (Invitrogen) and selected RNA abundances were examined in Northern blot assays. Figure 1A shows that mock-infected S2 cells displayed a robust increase in HSP RNA abundance after 2 h at 37°C (Fig. 1A, HSP26 and HSP27), compared to cells grown at 25°C. In contrast, CrPV-infected S2 cells failed to increase HSP26 and HSP27 mRNA abundance at the higher temperature. The abundance of both the actin and eIF2α mRNAs remained relatively unaffected by temperature or the presence of virus (Fig. 1A). To monitor whether prolonged heat shock treatment resulted in loss of viability of S2 cells, we heat treated cells at 37°C for 5 h and determined the number cells that took up trypan blue, which only interacts with dead cells. Results showed that heat treatment reduced cell viability to approximately 89%, compared to 97% in non-heat-treated cells (data not shown). Therefore, S2 cell viability was only marginally affected by prolonged heat treatment. These findings indicate that incubation of insect cells at 37°C results in a specific induction of heat shock mRNAs, which was inhibited by viral infection. Curiously, viral RNA abundance was enhanced at the higher temperature (Fig. 1A), suggesting that efficiency of viral translation, replication, or both is modulated by temperature.
FIG. 1.
Effects of CrPV infection on host mRNA abundance during heat stress. Virus infections of S2 cells were performed (see text) in the absence (A) or presence (B) of ActD. Five micrograms of total RNA was loaded into each lane. A phosphorimage of a representative Northern blot assay for each RNA is shown. Times postinfection (hrs. PI) at which RNA was prepared are indicated.
Next, it was examined whether the decrease in heat shock mRNA abundance and the increase in viral RNA at 37°C were due to turnover of host RNA or altered rates of host RNA synthesis. Thus, the experiments described in Fig. 1A were repeated in the presence of 1 mg/ml actinomycin D (ActD), which inhibits RNA polymerase II, and which was added at the beginning of infection. Figure 1B shows that host mRNAs did not increase in abundance at low or high temperature in the presence of ActD. In contrast, viral RNA still accumulated to a higher level at 37°C in the presence of ActD, suggesting that HSPs (Fig. 2) or other newly synthesized host factors did not contribute to the increase in viral RNA at 37°C.
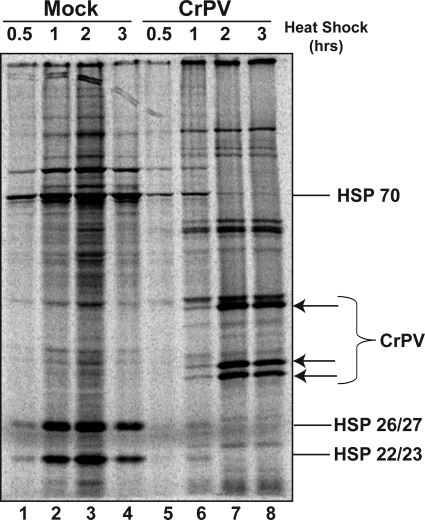
FIG. 2.
Effects of CrPV infection on protein expression during heat stress. S2 cells were labeled with [35S]methionine and [35S]cysteine for 10 min, and lysates were separated on SDS-polyacrylamide gels. A phosphorimage of a representative SDS-polyacrylamide gel electrophoresis is shown. The migration positions of CrPV proteins and selected HSPs are indicated.
To examine the effects of enhanced viral RNA abundance on viral protein abundance at 37°C, S2 cells were metabolically labeled with a mixture of [35S]methionine and [35S]cysteine. Briefly, mock- and CrPV-infected cells were incubated for 20 min prior to the indicated time point in serum-free medium at the indicated temperatures and then 150 μCi of protein label mix (Perkin-Elmer) was added for 10 min of incubation. Cells were washed with PBS, and proteins were recovered in protein lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, protease inhibitors [Roche]). Protein lysates were cleared by centrifugation, and protein concentrations were determined by the Bradford assay (Bio-Rad). Equivalent amounts of protein were separated in 10% sodium dodecyl sulfate (SDS)-containing polyacrylamide gels, and images of the radiolabeled proteins were obtained by a phosphorimaging analysis (Molecular Dynamics). As expected from the data in Fig. 1A, a variety of HSPs were synthesized in mock-infected cells but not in CrPV-infected cells at 37°C (Fig. 2). This observation supports the findings of Moore and colleagues (18). In contrast, significant amounts of viral proteins were synthesized at 37°C, suggesting that internal initiation-mediated translation of the viral RNA and translation elongation were not impaired at this temperature.
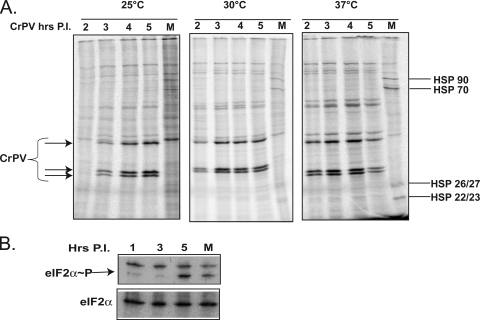
To investigate whether the temperature effects on viral and host mRNA translation seen were specific for cellular heat shock-inducing responses at 37°C, effects on viral and host protein production were examined at lower temperatures. Infected cells incubated at the standard temperature of 25°C did not synthesize significant amounts of viral structural proteins until around 3 h after infection (Fig. 3A). Furthermore, at 25°C, a global inhibition of host cell translation was observed after infection with CrPV (Fig. 3A, compare mock-infected to infected cells), suggesting that CrPV infection results in global inhibition of translation. This inhibition was accompanied by phosphorylation of translation initiation factor eIF2α (Fig. 3B). It is noteworthy that phosphorylation of eIF2α during heat stress is only marginally induced (4), which is in contrast to what can be seen in mammalian cells (19). At a slightly elevated temperature of 30°C, viral proteins were detected at 2 h after infection (Fig. 3A, center panel). An overall cessation of host protein synthesis occurred at this temperature in mock-infected cells (Fig. 3A, compare lanes M in the left and center panels), concomitant with a slight induction of HSPs. At 37°C, viral structural proteins were already robustly produced at 2 h postinfection (Fig. 3A, right panel). Importantly, the rates of viral protein synthesis seem to be similar at all three temperatures, suggesting that viral RNA abundance, and not a step in translation, limits viral mRNA translation at the different temperatures.
FIG. 3.
Effects of temperature and viral infection on protein synthesis and initiation factor eIF2 phosphorylation. (A) Infected S2 cells were radiolabeled and processed as described in the legend to Fig. 2. A phosphorimage of the gel is shown. The migration positions of CrPV proteins (closed arrowheads) and selected HSPs are indicated. (B) Lysates from uninfected cells (lanes M) and cells infected at 25°C were prepared at different times after infection and separated on an SDS-polyacrylamide gel. Western blot analysis was performed to detect phosphorylated (eIF2α∼P) and total (eIF2α) abundances of eIF2α by using antibodies directed against eIF2α-P (Cell Signaling, Danvers, MA) or eIF2α (gift from Martin Bushell, University of Nottingham, Nottingham, United Kingdom).
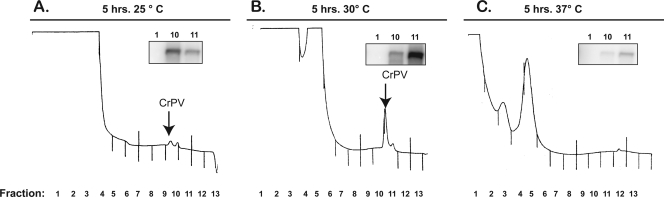
The robust production of viral RNA and protein at 37°C may predict that increasing temperature would result in increased virus production in infected insect cells. To test this hypothesis, the production of CrPV virions in infected cells was examined by sucrose gradient analysis. Briefly, S2 cells were infected with CrPV for 5 h and lysates were prepared in virion lysis buffer (0.3 M NaCl, 15 mM Tris-HCl [pH 7.5], 15 mM MgCl2, 1% Triton X-100, 100 mg/ml cycloheximide, 1 mg/ml heparin). Lysates were incubated in 30 mM EDTA and analyzed in sucrose gradients (Fig. 4). Inclusion of EDTA disrupts polysomes and avoids sedimentation of ribosomal subunits beyond fraction 5 in the gradient (Fig. 4). We noted a small, putative virion peak sedimenting at approximately 170S from 25°C infected lysates (Fig. 4A), a much larger 170S peak from 30°C infected lysates (Fig. 4B), and a tiny, hardly visible 170S peak from 37°C infected lysates (Fig. 4C). To examine whether the 170S peaks represented infectious assembled virions, aliquots of fractions 1, 10, and 11 from each gradient were used to infect S2 cells. After overnight incubation at 25°C, RNAs were harvested from the infected cells with TRIzol reagent and detected by Northern blot analyses. In each case, the sizes of the observed peaks correlated with the amount of viral RNA detected following the overnight infection (Fig. 4A, B, and C, insets). Thus, even though a temperature of 37°C greatly increased the amounts of viral protein and RNA, very little infectious virus was produced at this temperature. It is possible that a heat shock-induced factor, as opposed to the higher temperature, inhibited virion formation. We thus performed the experiments in Fig. 4 in the presence of ActD. However, virion formation was not restored at 37°C in the presence of ActD (data not shown), arguing that temperature affected virion formation. It is curious that an elevated temperature of 37°C had an effect on CrPV virion production that is the opposite of that seen in heat-treated baby hamster kidney (BHK) cells infected with rotavirus, a double-stranded RNA virus which infects mammalian cells (14). In the latter case, BHK cells were heat shocked at 43 to 45°C for 20 min, allowed to recover at 37°C for 30 min, and infected with rotavirus for 14 h (14). Results showed that 43 to 45°C pretreatment enhanced virus infection 100-fold compared to control pretreatment at 37°C (14). Because the temperature in cold-blooded insects mimics the surrounding temperature, virus spread is predicted to be inhibited at elevated temperatures. This is in contrast to other activities in crickets, such as chirping and mating (3, 6, 24). The temperature dependence of host susceptibility to infection in cold-blooded animals may be even more striking than in warm-blooded animals.
FIG. 4.
Effects of temperature on the formation of CrPV virions. After 5 h of infection, lysates were incubated on ice for 10 min, cleared by centrifugation at 10,000 × g, and then treated with 30 mM EDTA. Lysates were layered onto an EDTA-containing 10 to 50% sucrose gradient and sedimented at 35,000 rpm in an SW41 rotor (Beckman) for 3 h. Following centrifugation, samples were harvested using a fraction collector attached to a UV detector that monitors absorbance at 254 nm. A representative tracing at 254 nm of the collected gradient is shown. Arrows indicate the locations of infectious CrPV virions. The insets show Northern blot assays of RNA isolated from cells reinfected overnight from aliquots taken from the indicated fractions (numbers above the insets).
Acknowledgments
We thank Karla Kirkegaard for critical reading of the manuscript.
This study was supported by funds from the National Institutes of Health.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Burch, A. D., and S. K. Weller. 2005. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone Hsp90 for proper localization to the nucleus. J. Virol. 79:10740-10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chromy, L. R., J. M. Pipas, and R. L. Garcea. 2003. Chaperone-mediated in vitro assembly of polyomavirus capsids. Proc. Natl. Acad. Sci. U. S. A. 100:10477-10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciceran, M., A. Murray, and G. Rowell. 1994. Natural variation in the temporal patterning of calling song structure in the field cricket Gryllus pennsylvanicus: effects of temperature, age, mass, time of day, and nearest neighbour. Can. J. Zool. 72:38-42. [Google Scholar]
- 4.Duncan, R. F., D. R. Cavener, and S. Qu. 1995. Heat shock effects on phosphorylation of protein synthesis initiation factor proteins eIF-4E and eIF-2 alpha in Drosophila. Biochemistry 34:2985-2997. [DOI] [PubMed] [Google Scholar]
- 5.Glotzer, J. B., M. Saltik, S. Chiocca, A. I. Michou, P. Moseley, and M. Cotten. 2000. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature 407:207-211. [DOI] [PubMed] [Google Scholar]
- 6.Hedrick, A. V., D. Perez, N. Lichti, and J. Yew. 2002. Temperature preferences of male field crickets (Gryllus integer) alter their mating calls. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188:799-805. [DOI] [PubMed] [Google Scholar]
- 7.Jan, E., T. G. Kinzy, and P. Sarnow. 2003. Divergent tRNA-like element supports initiation, elongation, and termination of protein biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 100:15410-15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jan, E., and P. Sarnow. 2002. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 324:889-902. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, K. N., and P. D. Christian. 1998. The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J. Gen. Virol. 79:191-203. [DOI] [PubMed] [Google Scholar]
- 10.Kieft, J. S. 2009. Comparing the three-dimensional structures of Dicistroviridae IGR IRES RNAs with other viral RNA structures. Virus Res. 139:148-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, Y. K., and S. K. Jang. 2002. Continuous heat shock enhances translational initiation directed by internal ribosomal entry site. Biochem. Biophys. Res. Commun. 297:224-231. [DOI] [PubMed] [Google Scholar]
- 12.Klemenz, R., and W. J. Gehring. 1986. Sequence requirement for expression of the Drosophila melanogaster heat shock protein hsp22 gene during heat shock and normal development. Mol. Cell. Biol. 6:2011-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klemenz, R., D. Hultmark, and W. J. Gehring. 1985. Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J. 4:2053-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López, T., S. Lopez, and C. F. Arias. 2006. Heat shock enhances the susceptibility of BHK cells to rotavirus infection through the facilitation of entry and post-entry virus replication steps. Virus Res. 121:74-83. [DOI] [PubMed] [Google Scholar]
- 15.Michaud, S., R. Marin, and R. M. Tanguay. 1997. Regulation of heat shock gene induction and expression during Drosophila development. Cell. Mol. Life Sci. 53:104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaud, S., R. Marin, J. T. Westwood, and R. M. Tanguay. 1997. Cell-specific expression and heat-shock induction of Hsps during spermatogenesis in Drosophila melanogaster. J. Cell Sci. 110(Pt. 17):1989-1997. [DOI] [PubMed] [Google Scholar]
- 17.Michaud, S., G. Morrow, J. Marchand, and R. M. Tanguay. 2002. Drosophila small heat shock proteins: cell and organelle-specific chaperones? Prog. Mol. Subcell. Biol. 28:79-101. [DOI] [PubMed] [Google Scholar]
- 18.Moore, N. F., J. S. Pullin, and B. Reavy. 1981. The intracellular proteins induced by cricket paralysis virus in Drosophila cells: the effect of protease inhibitors and amino acid analogues. Arch. Virol. 70:1-9. [DOI] [PubMed] [Google Scholar]
- 19.Murtha-Riel, P., M. V. Davies, B. J. Scherer, S. Y. Choi, J. W. Hershey, and R. J. Kaufman. 1993. Expression of a phosphorylation-resistant eukaryotic initiation factor 2 alpha-subunit mitigates heat shock inhibition of protein synthesis. J. Biol. Chem. 268:12946-12951. [PubMed] [Google Scholar]
- 20.Pirkkala, L., P. Nykanen, and L. Sistonen. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15:1118-1131. [DOI] [PubMed] [Google Scholar]
- 21.Roberts, L. O., and E. Groppelli. 2009. An atypical IRES within the 5′ UTR of a dicistrovirus genome. Virus Res. 139:157-165. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki, J., and N. Nakashima. 2000. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc. Natl. Acad. Sci. U. S. A. 97:1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonenberg, N., and A. G. Hinnebusch. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souroukis, K., W. Cade, and G. Rowell. 1992. Factors that possibly influence variation in the calling song of field crickets—temperature, time and male size, age and wing morphology. Can. J. Zool. 70:950-955. [Google Scholar]
- 25.Terenin, I. M., S. E. Dmitriev, D. E. Andreev, E. Royall, G. J. Belsham, L. O. Roberts, and I. N. Shatsky. 2005. A cross-kingdom internal ribosome entry site reveals a simplified mode of internal ribosome entry. Mol. Cell. Biol. 25:7879-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasconcelos, D., E. Norrby, and M. Oglesbee. 1998. The cellular stress response increases measles virus-induced cytopathic effect. J. Gen. Virol. 79(Pt. 7):1769-1773. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, J. E., T. V. Pestova, C. U. Hellen, and P. Sarnow. 2000. Initiation of protein synthesis from the A site of the ribosome. Cell 102:511-520. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, J. E., M. J. Powell, S. E. Hoover, and P. Sarnow. 2000. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol. Cell. Biol. 20:4990-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]