Abstract
Herpes simplex virus (HSV) immediate-early (IE) protein ICP0 is a multifunctional regulator of HSV infection. ICP0 that is present in the tegument layer has not been well characterized. Protein compositions of wild-type and ICP0 null virions were similar, suggesting that the absence of ICP0 does not grossly impair virion assembly. ICP0 has a RING finger domain with E3 ubiquitin ligase activity that is necessary for IE functions. Virions with mutations in this domain contained greatly reduced levels of tegument ICP0, suggesting that the domain influences the incorporation of ICP0. Virion ICP0 was resistant to removal by detergent and salt and was associated with capsids, features common to inner tegument proteins.
Herpes simplex virus (HSV) particles are composed of three morphologically distinct structural components. The icosahedral capsid is surrounded by the tegument layer, which is in turn surrounded by a host-derived lipid envelope (49). After the viral envelope fuses with the host cell membrane, the bulk of the tegument appears to remain with the cell membrane (38). Direct delivery of tegument proteins, such as VP16, can rapidly stimulate viral gene expression (2). The release of another tegument protein, vhs, leads to degradation of mRNAs (48). Tegument components can also affect pre-immediate-early (pre-IE) events, including processes that occur after penetration but prior to IE gene expression (30). The tegument layer comprises more than 19 viral proteins and trace amounts of cellular proteins (33). Tegument infected cell protein 0 (ICP0) (58) and ICP4 (57) are present in 100 to 200 copies per virion. Tegument ICP0 and ICP4 are detected by Western blotting (13, 14, 35, 45, 52, 56, 58, 59) and mass spectrometry (35) but not by protein staining (53).
ICP0 is a 110-kDa phosphoprotein that is a promiscuous transactivator of viral and cellular genes (20, 21, 47). ICP0 is expressed with IE kinetics and is needed for efficient progression to lytic infection (20, 27, 51, 54). It is required for growth during low-multiplicity infections (7) and is needed for efficient reactivation from latency (9, 28, 29, 34). ICP0 also inhibits the antiviral response to cellular interferons (42) and may help the virus bypass innate cellular repression pathways (26). Newly synthesized ICP0 induces proteasome-dependent degradation of components of nuclear subdomains called ND10, including promyelocytic leukemia antigen (PML) and Sp100 (8, 23). The N-terminal RING finger zinc-binding motif of ICP0 is required for ND10 disruption. The RING finger functions as an E3 ubiquitin ligase in vitro and induces colocalization of conjugated ubiquitin at ND10 (3, 19). The disruption of ND10 structures is thought to facilitate HSV gene expression (6, 37). Little is known about the ICP0 that is brought in with the infecting virion. It is not clear whether tegument ICP0 functions in a manner similar to its IE counterpart. IE protein ICP27 is required for the cytoplasmic localization and the incorporation of ICP0 and ICP4 into mature virions (52). VP22 is also necessary for the efficient incorporation of ICP0. Virions assembled in the absence of VP22 have reduced amounts of ICP0, ICP4, glycoprotein E (gE), and gD (13, 14). Here, we characterize fundamental properties of ICP0 that is present in HSV virions.
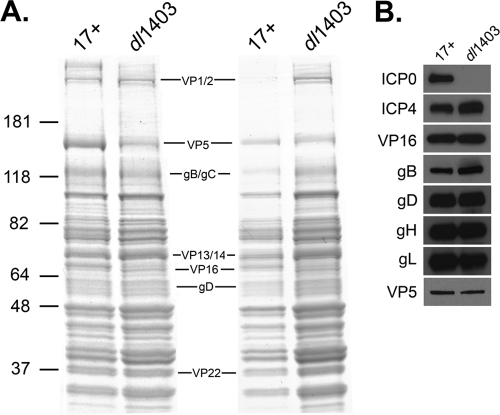
We investigated whether ICP0 influences the protein composition of mature extracellular virions. All viruses were provided by R. Everett. Cell-free extracellular virions were propagated on U2OS cells and isolated as described previously (44). One microgram (Fig. 1A, left) or equivalent VP5 units (Fig. 1A, right) of virions of parental virus HSV type 1 (HSV-1) Glasgow strain 17 syn+ (4) (herein referred to as 17+) or virions of ICP0 null mutant dl1403 (Table 1) were analyzed by SDS-PAGE followed by Coomassie blue staining. For each structural protein of the 17+ virions that was detectable by Coomassie blue staining, there was a counterpart protein detected in dl1403. Although several proteins were detected at various levels, the overall protein profile of virions released from U2OS cells was not grossly altered by the absence of ICP0 (Fig. 1A). Equivalent VP5 units of extracellular virions were analyzed by Western blotting with antibodies against ICP0 (mouse monoclonal antibody [MAb] 11060, a gift of R. Everett) (22), gB (MAb H1817), ICP4 (MAb HIA021), VP5 (MAb HA018), and VP16 (MAb 1-21), all from Virusys (Sykesville, MD), and gD (MAb DL6) (10) and gH-gL (rabbit polyclonal antibody R137) (46), gifts of R. Eisenberg and G. Cohen. The envelope glycoproteins and tegument proteins tested did not appear to be reduced in the absence of ICP0 (Fig. 1B). By these measures, ICP0 does not appear to grossly affect the protein content of mature virions. Based on this analysis, however, we cannot rule out that one or more structural proteins in addition to ICP0 may be missing from the ICP0 null virus.
FIG. 1.
Protein composition of herpes simplex virions in the absence of ICP0. (A) The HSV-1 wild type (17+) or ΔICP0 strain (dl1403) was analyzed by SDS-PAGE followed by Coomassie blue staining. One microgram (left) or equivalent VP5 units (right) of each virus were loaded. In the center, locations of several HSV structural proteins are indicated. Numbers to the left are molecular mass markers in kilodaltons. (B) Equivalent VP5 units of extracellular virions were analyzed by SDS-PAGE followed by Western blotting with antibodies against the indicated tegument protein or glycoprotein.
TABLE 1.
Details of ICP0 proteins expressed by viruses
| Virus | ICP0 structuref | Description |
|---|---|---|
| 17+a | 1-775 | Wild type |
| dl1403b | 1-105 | ICP0 null mutant |
| dl1403Rc | 1-775 | ICP0+ rescuant |
| FXEd | 1-105::150-775 | RING finger domain deletion mutant |
| FXERc | 1-775 | Rescuant with wild-type RING finger |
| K144E mutante | Point mutation at amino acid 144 | Mutant with substitution in RING finger helix |
| N151D mutante | Point mutation at amino acid 151 | Mutant with substitution in RING finger helix |
Parental virus HSV-1 Glasgow strain 17 syn+ (4).
Has a 2-kb lesion in both inverted repeat copies of the ICP0 gene (54).
Rescued virus was obtained by cotransfection with mutant virion DNA and a plasmid containing a fragment comprising the ICP0 gene (18, 50).
Has a defined lesion in the ICP0 RING finger domain yielding a deletion of 45 amino acids (16-18, 24).
Has a single amino acid substitution in the alpha helix of the ICP0 RING finger (15).
Amino acids included or relevant mutation in ICP0 variant.
ICP4 is expressed with IE kinetics and is a major transactivator of early and late genes (12). IE ICP0 and ICP4 interact and have a synergistic effect on gene expression (25, 47). We used Western blot analysis to determine whether the absence of ICP0 affected the incorporation of ICP4. ICP4 was detected in dl1403 virions (Fig. 1B), suggesting that it can be incorporated into virions independently of ICP0. Note that these results do not rule out a role for ICP0 in proper virion assembly. Tegument assembly involves a complex series of protein-protein interactions, and individual tegument proteins may serve redundant functions (40). Other tegument proteins in dl1403 virions may substitute for a structural or assembly role of tegument ICP0.
We determined whether the RING finger domain influences ICP0 incorporation into the tegument layer. First, the relative amounts of VP5 in various ICP0 mutant virions (Table 1) propagated on U2OS cells were determined for each preparation of virus (Fig. 2A). The ICP0 contents of virions were then analyzed by SDS-PAGE and Western blotting with MAb 11060 to ICP0. As expected, the wild-type strain 17+ contained tegument ICP0 (Fig. 2A, lane 17+) while the dl1403 mutant did not (Fig. 2A, lane dl1403). Strain FXE virions did not contain ICP0 (Fig. 2A, lane FXE), suggesting that the RING finger domain is important for the association of ICP0 with mature virions. ICP0 was detected in dl1403 and FXE rescuants (Fig. 2A, lanes dl1403R and FXER), both of which bear the restored ICP0 gene. Interestingly, K144E virions had greatly reduced levels of tegument ICP0 whereas N151D virions had wild-type levels (Fig. 2A, lanes K144E and N151D). Trace amounts of ICP0 were reproducibly detected in K144E virions upon longer exposure to film. To confirm that the reduction in tegument ICP0 in FXE and K144E viruses was not due to lack of expression of mutant ICP0 in infected cells, the expression of ICP0 during mutant virus infection was compared to that during wild-type 17+ infection. U2OS cells were infected with equivalent VP5 units, corresponding to a multiplicity of infection (MOI) of 1 for 17+. Cell lysates were prepared 18 h postinfection. Samples were analyzed by SDS-PAGE and Western blotting with antibody to ICP0 (MAb H1A027; Virusys, Sykesville, MD) or to β-actin (MAb AC-74; Sigma, St. Louis, MO) to demonstrate equivalent levels of loading. Mutant ICP0 was readily detected in cells infected with FXE or the K144E mutant (Fig. 2B) as reported previously (15, 18, 54). Thus, the RING finger plays an important role in the incorporation of ICP0 into virions.
FIG. 2.
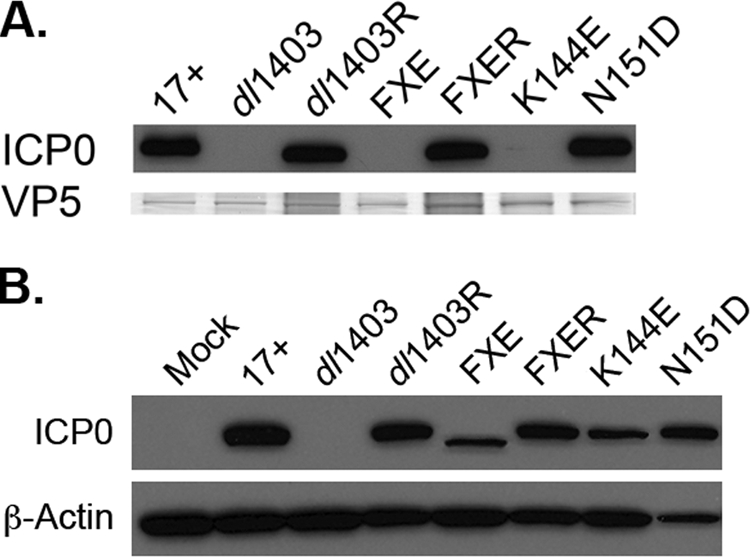
Effect of RING finger mutations on the incorporation of ICP0 into virions. (A) Incorporation of tegument ICP0 into HSV-1 mutants. The indicated extracellular virions (identified by relevant mutations where applicable) were analyzed by SDS-PAGE followed by Western blotting with the 11060 antibody to ICP0. In parallel, VP5 was detected by Coomassie staining to demonstrate equivalent levels of particle loading. dl1403R and FXER, dl1403 and FXE rescuants carrying a restored ICP0 gene. (B) Expression of ICP0 in cells infected with wild-type or mutant viruses. U2OS cells were infected with the indicated virus (MOI = 1) for 18 h. Cell lysates were analyzed by SDS-PAGE followed by Western blotting with MAb H1A027 to ICP0.
While K144E and N151D mutations in the RING alpha helix both decrease the ability of ICP0 to activate gene expression, the K144E phenotype is more pronounced (3, 15). Also, K144E and N151D mutants both fail to induce the colocalization of conjugated ubiquitin, but ICP0 with the N151D mutation may still interact with the ubiquitin machinery (19). It is not clear whether these activities relate to the inclusion of the N151D mutant protein and the exclusion of the K144E mutant protein from the tegument. Late in infection, newly synthesized ICP0 shuttles from the nucleus to the cytosol (32). ICP0 may be incorporated into the teguments of virions in the cytosol (14, 52). RING finger mutation causes ICP0 to be more readily retained in the nucleus at late time points in infection (36). Future studies will investigate the role that the RING domain plays in the relationship between the cellular localization of ICP0 and the incorporation of ICP0 into the tegument layer.
To determine the position of ICP0 within the tegument layer, a tegument release assay was employed (1, 41, 55). This is a measure of how readily detergent and salt can remove a structural protein from the virion. Typically, outer tegument proteins are partially released from virions following treatment with Triton X-100 and up to 1 M NaCl, but capsid-associated or inner tegument proteins are resistant to such treatment. Extracellular virions (3 × 109 PFU) were resuspended in 0.2 ml lysis buffer (50 mM HEPES [pH 7.4] and 1% Triton X-100) containing 0.1, 0.5, or 1 M NaCl and incubated for 30 min at 37°C. The reaction mixtures were then layered onto 0.5 ml of a 35% sucrose solution (containing 50 mM HEPES [pH 7.4] and 0.1 M NaCl) and centrifuged at 21,000 × g for 10 min. The 200-μl supernatant above the sucrose cushion was recovered. Released proteins in the supernatant were heat precipitated and resuspended in Laemmli buffer. The sucrose cushion was removed, and the pellet, containing capsids and capsid-associated tegument proteins, was resuspended in Laemmli buffer. Pelleted and released samples were separated by SDS-PAGE and blotted onto nitrocellulose. Western blots were probed with antibodies against ICP0 (MAb H1A027), gB (MAb H1817), ICP4 (MAb HIA021), VP5 (MAb HA018), VP16 (MAb 1-21; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or VP1-2 (polyclonal antibody R27B4, a gift of R. Courtney) (39).
As expected, gB was detected in the supernatant, indicating that it was removed along with the virion envelope (Fig. 3). In contrast, ICP0 remained in the pellet along with VP5, the major capsid component, even at high salt concentrations (Fig. 3). ICP0 was not detected in the supernatant even after longer exposures to X-ray film (data not shown). VP1-2 is a known inner tegument protein and remained associated with capsids in the pellet (Fig. 3) (5, 41, 55). In contrast, a fraction of VP16 was readily removed by 1% Triton X-100, consistent with the presence of VP16 in the outer tegument (41, 43, 55). Interestingly, detectable amounts of ICP4 were released by detergent and increasing salt concentrations (Fig. 3), suggesting that ICP4 is an outer tegument component. The presence of tegument ICP4 near the viral envelope may facilitate rapid release into the cytoplasm upon viral penetration and play a pre-IE role in HSV infection. In contrast, ICP0 is capsid associated. Although specific functions have not yet been ascribed to virion ICP0 and ICP4 proteins, it is tempting to speculate that their different positions in the tegument layer reflect distinct roles in pre-IE events.
FIG. 3.
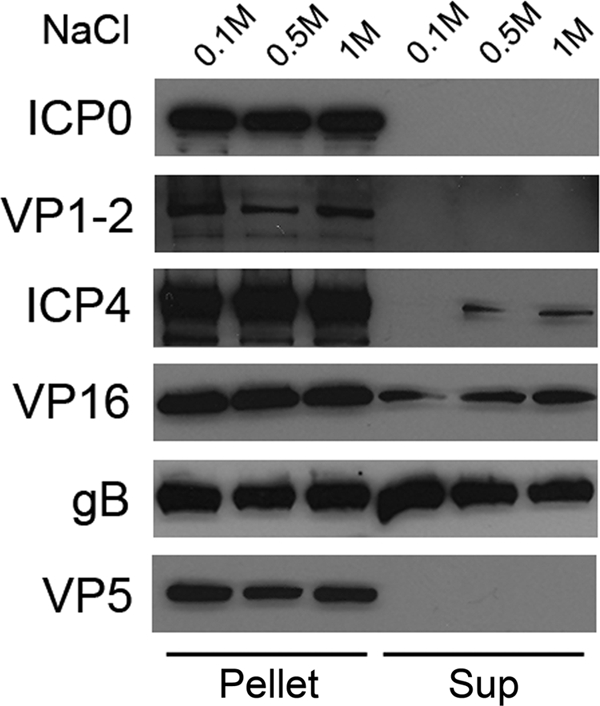
Sensitivity of tegument ICP0 to detergent lysis and salt treatment. Extracellular virions were treated with 1% Triton X-100 in the presence of 0.1, 0.5, or 1 M NaCl. The reaction mixtures were then centrifuged through a 35% sucrose cushion. Pelleted (pellet) and released (sup) fractions were analyzed by SDS-PAGE and immunoblotting with antibodies to ICP0, VP1-2, ICP4, VP16, gB, or VP5.
We previously showed that HSV entry requires 26S proteasome activity at a postpenetration step, likely the transport of incoming capsids to the nucleus (11). We are currently investigating whether tegument ICP0 and its E3 ubiquitin ligase domain play a role in proteasome-dependent entry of HSV. There is also potential for functional interaction of ICP0 with the inner tegument protein VP1-2, which has an N-terminal ubiquitin-specific protease domain (31). Ongoing studies are aimed at delineating the fate and function of tegument ICP0 during viral entry.
Acknowledgments
This investigation was supported by Public Health Service grants AI-083850 (to A.V.N.) and AI-07617 (to M.G.D.) from the National Institute of Allergy and Infectious Diseases and a grant from the Jeffress Memorial Trust.
We are grateful to Gary H. Cohen, Richard J. Courtney, Roselyn J. Eisenberg, and Roger D. Everett for the kind gifts of reagents.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batterson, W., and B. Roizman. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J. Virol. 46:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, S. M., D. A. Ritchie, and J. H. Subak-Sharpe. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329-346. [DOI] [PubMed] [Google Scholar]
- 5.Bucks, M. A., K. J. O'Regan, M. A. Murphy, J. W. Wills, and R. J. Courtney. 2007. Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids. Virology 361:316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkham, J., D. M. Coen, and S. K. Weller. 1998. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J. Virol. 72:10100-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 9.Clements, G. B., and N. D. Stow. 1989. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J. Gen. Virol. 70(Pt. 9):2501-2506. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, G. H., B. Dietzschold, M. Ponce de Leon, D. Long, E. Golub, A. Varrichio, L. Pereira, and R. J. Eisenberg. 1984. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J. Virol. 49:102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delboy, M. G., D. G. Roller, and A. V. Nicola. 2008. Cellular proteasome activity facilitates herpes simplex virus entry at a postpenetration step. J. Virol. 82:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLuca, N. A., and P. A. Schaffer. 1985. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol. 5:1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy, C., J. H. Lavail, A. N. Tauscher, E. G. Wills, J. A. Blaho, and J. D. Baines. 2006. Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea. J. Virol. 80:8664-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott, G., W. Hafezi, A. Whiteley, and E. Bernard. 2005. Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J. Virol. 79:9735-9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett, R., P. O'Hare, D. O'Rourke, P. Barlow, and A. Orr. 1995. Point mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J. Virol. 69:7339-7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, R. D. 1987. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J. 6:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett, R. D. 1988. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol. 202:87-96. [DOI] [PubMed] [Google Scholar]
- 18.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70(Pt. 5):1185-1202. [DOI] [PubMed] [Google Scholar]
- 19.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 21.Everett, R. D. 1984. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D., A. Cross, and A. Orr. 1993. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology 197:751-756. [DOI] [PubMed] [Google Scholar]
- 23.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freemont, P. S. 1993. The RING finger. A novel protein sequence motif related to the zinc finger. Ann. N. Y. Acad. Sci. 684:174-192. [DOI] [PubMed] [Google Scholar]
- 25.Gelman, I. H., and S. Silverstein. 1986. Co-ordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J. Mol. Biol. 191:395-409. [DOI] [PubMed] [Google Scholar]
- 26.Gu, H., and B. Roizman. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134-17139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris, R. A., R. D. Everett, X. X. Zhu, S. Silverstein, and C. M. Preston. 1989. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J. Virol. 63:3513-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalejta, R. F. 2008. Functions of human cytomegalovirus tegument proteins prior to immediate early gene expression. Curr. Top. Microbiol. Immunol. 325:101-115. [DOI] [PubMed] [Google Scholar]
- 31.Kattenhorn, L. M., G. A. Korbel, B. M. Kessler, E. Spooner, and H. L. Ploegh. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19:547-557. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J. Virol. 71:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly, B. J., C. Fraefel, A. L. Cunningham, and R. J. Diefenbach. 2009. Functional roles of the tegument proteins of herpes simplex virus type 1. Virus Res. 145:173-186. [DOI] [PubMed] [Google Scholar]
- 34.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loret, S., G. Guay, and R. Lippe. 2008. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 82:8605-8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75(Pt. 6):1223-1233. [DOI] [PubMed] [Google Scholar]
- 37.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67-75. [DOI] [PubMed] [Google Scholar]
- 38.Maurer, U. E., B. Sodeik, and K. Grunewald. 2008. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proc. Natl. Acad. Sci. U. S. A. 105:10559-10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNabb, D. S., and R. J. Courtney. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 66:7581-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy, M. A., M. A. Bucks, K. J. O'Regan, and R. J. Courtney. 2008. The HSV-1 tegument protein pUL46 associates with cellular membranes and viral capsids. Virology 376:279-289. [DOI] [PubMed] [Google Scholar]
- 44.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orlando, J. S., J. W. Balliet, A. S. Kushnir, T. L. Astor, M. Kosz-Vnenchak, S. A. Rice, D. M. Knipe, and P. A. Schaffer. 2006. ICP22 is required for wild-type composition and infectivity of herpes simplex virus type 1 virions. J. Virol. 80:9381-9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlan, M. P., and D. M. Knipe. 1985. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 5:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. Herpes simplex viruses, p. 2501-2602. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 50.Russell, J., N. D. Stow, E. C. Stow, and C. M. Preston. 1987. Herpes simplex virus genes involved in latency in vitro. J. Gen. Virol. 68(Pt. 12):3009-3018. [DOI] [PubMed] [Google Scholar]
- 51.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sedlackova, L., and S. A. Rice. 2008. Herpes simplex virus type 1 immediate-early protein ICP27 is required for efficient incorporation of ICP0 and ICP4 into virions. J. Virol. 82:268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67(Pt. 12):2571-2585. [DOI] [PubMed] [Google Scholar]
- 55.Wolfstein, A., C. H. Nagel, K. Radtke, K. Dohner, V. J. Allan, and B. Sodeik. 2006. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7:227-237. [DOI] [PubMed] [Google Scholar]
- 56.Yang, T. Y., and R. J. Courtney. 1995. Influence of the host cell on the association of ICP4 and ICP0 with herpes simplex virus type 1. Virology 211:209-217. [DOI] [PubMed] [Google Scholar]
- 57.Yao, F., and R. J. Courtney. 1989. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J. Virol. 63:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao, F., and R. J. Courtney. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 66:2709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, Y., and J. L. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 67:1482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]