Abstract
Vesicular stomatitis virus (VSV) has long been regarded as a promising recombinant vaccine platform and oncolytic agent but has not yet been tested in humans because it causes encephalomyelitis in rodents and primates. Recent studies have shown that specific tropisms of several viruses could be eliminated by engineering microRNA target sequences into their genomes, thereby inhibiting spread in tissues expressing cognate microRNAs. We therefore sought to determine whether microRNA targets could be engineered into VSV to ameliorate its neuropathogenicity. Using a panel of recombinant VSVs incorporating microRNA target sequences corresponding to neuron-specific or control microRNAs (in forward and reverse orientations), we tested viral replication kinetics in cell lines treated with microRNA mimics, neurotoxicity after direct intracerebral inoculation in mice, and antitumor efficacy. Compared to picornaviruses and adenoviruses, the engineered VSVs were relatively resistant to microRNA-mediated inhibition, but neurotoxicity could nevertheless be ameliorated significantly using this approach, without compromise to antitumor efficacy. Neurotoxicity was most profoundly reduced in a virus carrying four tandem copies of a neuronal mir125 target sequence inserted in the 3′-untranslated region of the viral polymerase (L) gene.
Vesicular stomatitis virus (VSV) is a nonsegmented, negative-strand rhabdovirus widely used as a vaccine platform as well as an anticancer therapeutic. While VSV is predominantly a pathogen of livestock (34), it has a very broad species tropism. The cellular tropism of VSV is determined predominantly at postentry steps, since the G glycoprotein of the virus mediates entry into most tissues in nearly all animal species (10).
Though viral entry can take place in nearly all cell types, in vivo models of VSV infection have revealed that the virus is highly sensitive to the innate immune response, limiting its pathogenesis (4). VSV is intensively responsive to type I interferon (IFN), as the double-stranded RNA (dsRNA)-dependent PKR (2), the downstream effector of pattern recognition receptors MyD88 (32), and other molecules mediate shutdown of viral translation and allow the adaptive immune response to clear the virus. The vulnerability of the virus to the type I IFN response, typically defective in many cancers, has been exploited to generate tumor-selective replication (49), such that the virus is now poised to enter phase I trials. However, the virus remains potently neurotoxic, causing lethal encephalitis not only in rodent models (7, 22, 53) but also in nonhuman primates (25).
VSV very often infiltrates the central nervous system (CNS) through infection of the olfactory nerves (41). When administered intranasally, the virus replicates rapidly in the nasal epithelium and is transmitted to olfactory neurons, from which it then moves retrograde axonally to the brain and replicates robustly, causing neuropathogenesis. While intranasal inoculation does cause neuropathy in mice, neurotoxicity following viral administration also occurs when the virus is delivered intravascularly (47), intraperitoneally (42), and (not surprisingly) intracranially (13). Previously, other groups have modified the VSV genome to be more sensitive to cellular IFNs (49) and have actually encoded IFN in the virus (36). However, the former can result in attenuation of the virus, such that it has reduced anticancer potential, while the latter still results in lethal encephalitis (unpublished results). In order to mitigate the effects of VSV infection on the brain without perturbing the potent oncolytic activity of the virus, we utilized a microRNA (miRNA) targeting paradigm, whereby viral replication is restricted in the brain without altering the tropism of the virus for other tissues.
To redirect the tissue tropism of anticancer therapeutics, we (26) and others (11, 14, 55) have previously exploited the tissue-specific expression of cellular miRNAs. miRNAs are ∼22-nucleotide (nt) regulatory RNAs that regulate a diverse and expansive array of cellular activities. Through recognition of sequence-complementary target elements, miRNAs can either translationally suppress or catalytically degrade both cellular (6) and viral (50) RNAs. We have determined that cellular miRNAs can potentially regulate numerous steps of a virus life cycle and that this regulation of the virus by endogenous miRNAs can then abrogate toxicities of replication-competent viruses (27; E. J. Kelly et al., unpublished data).
miRNAs are known to be highly upregulated in many different tissues, including (but not limited to) muscle (40), lung (44), liver (15, 44), spleen (44, 46), and kidney (51). In addition, the brain has a number of upregulated miRNAs, with each different subtype of cell having a unique miRNA profile. miR-125 is highly upregulated in all cells in the brain (neurons, astrocytes, and glia cells), while miR-124 is found predominantly in neuronal cells (48). Glial cells and glioblastomas are thought to have decreased expression of miR-128 compared to neurons (17), while miR-134 is particularly abundant in dendrites of neurons in the hippocampus (43). In addition to these miRNAs, the tumor suppressor miRNA let-7 and miRs 9, 26, and 29 (51) are also found to be enriched in the brain, with expression varying not only between different cell types and regions of the brain but also temporally (48).
MicroRNAs have previously been exploited to modulate the tissue tropism of nonreplicating lentiviral vectors (8, 9), as well as curbing known toxicities of replication-competent picornaviruses (5, 26), adenoviruses (11), herpes simplex virus 1 (33), and influenza A virus (39). In addition, a recombinant VSV encoding a tumor suppressor target was found to be responsive to sequence-complementary miRNAs in vitro, possibly by affecting expression of the matrix (M) protein (14), and evidence from Dicer-deficient mice suggests that endogenously expressed microRNA targets within the P and L genes of VSV could restrict enhanced pathogenicity of the virus (37). However, in vivo protection from neuropathogenesis by this means has not been demonstrated for VSV.
Here we evaluate the efficiencies of different brain-specific miRNAs for shutting down gene expression and extensively characterize the ability of miRNA targeting to attenuate the neurotoxicity of vesicular stomatitis virus in vivo. We constructed and evaluated recombinant VSVs with miRNA target (miRT) insertions at different regions of the viral genome, with special focus upon those affecting viral L expression. In addition, we looked at the regulatory efficiency of different brain-specific miRNAs and the impact of miRT orientation on VSV replication and determined the impact of the virus on oncolytic activity in vivo.
MATERIALS AND METHODS
Cell culture and lentiviral vector production.
H1-HeLa, 293T, and BHK cells were obtained from American Type Culture Collection (ATCC), Manassas, VA, and were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in 5% CO2. Cath.A was purchased from ATCC and maintained in RPMI plus 4% FBS and 8% horse serum. NB41A3 was purchased from ATCC and maintained in Ham's F12 medium plus 3% FBS and 15% horse serum. Primary rat astrocytes were purchased from Lonza Corporation (Basel, Switzerland) and cultured according to the manufacturer's instructions. Lentiviral vectors were obtained by transfection of 10 μg each of lentiviral transfer plasmid (pHR-sin-F.Luc miRT), generously provided by Y. Ikeda, and lentiviral packaging plasmid (CMV ΔR8.91), with 3 μg VSV-G packaging construct pMD.G, in a T75 flask. Supernatant was harvested at 72 h posttransfection and filtered through a 0.45-μm syringe filter.
Viruses.
Plasmid DNA for VSV encoding luciferase was kindly provided by Glen Barber (University of Miami, Miami, FL). miRTs were cloned into novel NotI restriction sites. Restriction sites were generated in the 3′-untranslated region (3′UTR) of the M or L gene by overlap extension PCR with the following primers: M (S), CTAACTTCTAGCTTCTGCGGCCGCGAACAATCCCCGGTTTACTC; M (AS), GAGTAAACCGGGGATTGTTCGCGGCCGCAGAAGCTAGAAGTTAG; L (S), GAGATTAAAAAATCATGCGGCCGCGAGGAGACTCCAAACTTTAAG; and L (AS), CTTAAAGTTTGGAGTCTCCTCGCGGCCGCATGATTTTTTAATCTC.
MicroRNA target sites (see Fig. 2) were flanked with NotI sites. miRNAs were purchased as oligonucleotides from Integrated DNA Technologies (Coralville, IA) and were annealed in STD buffer as previously described (26).
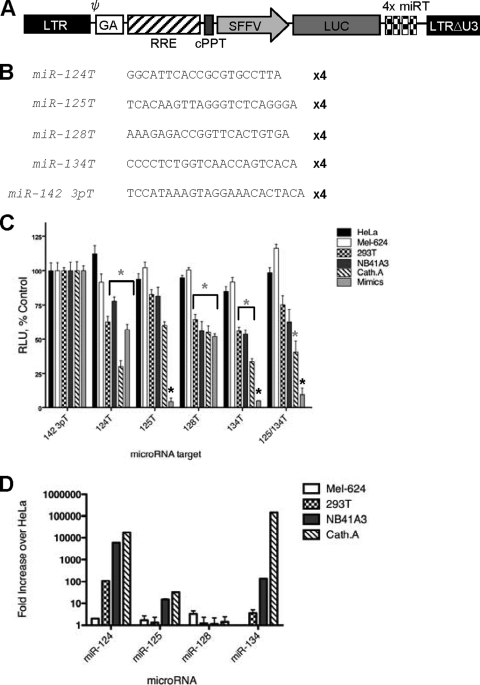
FIG. 2.
Analysis of lentiviral vectors incorporating neuron-specific microRNA targets in a panel of cell lines. (A) Schematic diagram of lentiviral vector containing microRNA targets. LTR, long terminal repeat; GA, Gag; SFFV, spleen focus-forming virus (promoter); RRE, Rev response element; cPPT, central polypurine tract. (B) Sequences of inserted target elements. (C) Relative light units (RLU) in control or neuronal cell lines transduced at an MOI of 1.0 with lentiviral vectors containing miR-142-3pT, miR-124T, miR-125T, miR-128T, or miR-134T or in the presence of 200 nM sequence-complementary miRNA mimic. (D) miRNA expression profiling determined by TaqMan expression analysis. Fold increases indicate enrichment over miRNA expression in HeLa cells (per 5 ng small RNA). Gray asterisks, P < 0.05; black asterisks, P < 0.01.
Recombinant VSVs (rVSVs) were generated as follows. BHK cells were plated at a density of 1.5 × 106 cells/10-cm dish. Cells were infected with vaccinia virus encoding T7 polymerase (VfT7) the next day, at a multiplicity of infection (MOI) of 10 for 1 h. Virus was then removed, and cells were transfected with 2.5 μg pVSV miRT, 0.7 μg pB1-N, 1.25 μg pB1-P, and 0.5 μg pB1-L (N, P, and L plasmids were provided by Glen Barber, University of Miami), using 15 μl of Fugene 6 transfection reagent (Roche) according to the manufacturer's instructions. Cells were incubated for 3 h at 37°C, and the medium was then replaced with DMEM plus 10% FBS. After 48 h, culture medium was harvested, filtered twice through a 0.45-μm filter, and overlaid onto BHK cells in a 10-cm dish. Forty-eight hours later, if cytopathic effect (CPE) was observed, the culture medium was harvested, subjected to low-speed centrifugation, and filtered through a 0.45-μm filter. The supernatant was loaded on top of sucrose (10% [wt/vol]) and centrifuged at 70,000 × g for 2 h to pellet the particles. For virus titration, BHK cells were grown on 96-well plates and infected with serially diluted virus stock. Fifty percent tissue culture infectious dose (TCID50) values were determined by the Spearman and Karber equation.
In vitro cytotoxic activity.
The cytotoxicity of rVSVs on cell lines was measured using a standard MTT [3-(4,5-dimethylthiazolyl-2)-2,-5-diphenyltetrazolium bromide] assay. Briefly, cells were mock infected or infected with rVSV (MOI = 0.2; 2 h at 37°C), unabsorbed virus was washed, and cells were seeded into 96-well microplates at 104 cells per well in 0.1 ml medium. Plates were incubated for 24, followed by the addition of 0.01 ml of MTT to each well. The mixture was incubated for 4 h at 37°C. Formazan was extracted from the cells with 0.1 ml detergent, and the color intensity was measured with a microplate enzyme-linked immunosorbent assay reader. Experiments were performed in quadruplicate, and results were recorded as percent absorbance relative to that of untreated control cells.
miRNA mimics.
miRNA mimics were purchased from Ambion. A control miRNA mimic corresponded to a Caenorhabditis elegans miRNA with no predicted miRTs in mammalian cells, according to the manufacturer. miRNA mimics were transfected with Mirus RNA transfection reagent at 200 nM. At 4 h post-mimic transfection, cells were infected with rVSV at an MOI of 0.2, unless another time is noted. At 24 h postinfection, cells were harvested for MTT viability assay and supernatant was harvested for titration.
One-step growth curves.
BHK cells were incubated with rVSV at an MOI of 3.0 for 2 h at 37°C. Following this incubation, cells were washed and resuspended in fresh growth medium at predetermined time points (2, 4, 6, 8, 10, 24, and 48 h), and cell pellets were harvested and frozen at −80°C. At the completion of all time points, the samples were thawed and cell pellets were cleared from the samples by centrifugation, providing a cleared cell lysate fraction.
In vivo experiments.
All animal protocols were reviewed and approved by the Mayo Clinic Institutional Care and Use Committee. ICR-SCID mice were obtained from Taconic, and BALB/c mice were purchased from Jackson Laboratories. Mice were irradiated and implanted with 5 × 106 CT26 cells in the right flank. When tumors reached an average size of 0.5 by 0.5 cm, tumors were treated with rVSV. Tumor volume was measured using a hand-held caliper, and blood was collected by retro-orbital bleeds. For intracranial administration, VSV was administered in 10 μl of Opti-MEM carrier.
MicroRNA analysis.
Small RNAs were harvested from all cell lines with an Ambion miRVana microRNA isolation kit according to the manufacturer's instructions. RNA was resuspended in 50 μl nuclease-free water and quantified by spectrophotometry. For analysis of miRNA expression, an Applied Biosystems TaqMan microRNA assay system was used. For each miRNA analyzed, 5 ng of small RNA was subjected to quantitative PCR (qPCR) in triplicate on a Stratagene Mx4000 qPCR system.
Statistical analyses.
The GraphPad Prism 4.0 program (GraphPad Software, San Diego, CA) was used for data handling, analysis, and graphic representation. Survival curves were plotted according to the Kaplan-Meier method, and survival function across treatment groups was compared using log-rank test analyses.
RESULTS
Vesicular stomatitis virus causes lethal neurotoxicity in mice.
Vesicular stomatitis virus is capable of causing lethal encephalitis in rodents via almost any administration route. Direct intracranial injection of the virus, however, results in development of neuropathogenesis faster and at a lower dose than infections initiated at other sites. In order to provide the most stringent evaluation of viral restriction in the brain, we first analyzed the propensity of an engineered VSV containing a firefly luciferase reporter (VSV-Luc) to cause lethal encephalitis when the virus was administered intracerebrally. We first investigated the time course of gene expression in immunocompromised mice inoculated with 1 × 104 VSV-Luc particles and examined the luciferase expression kinetics post-viral administration. Not surprisingly, luciferase expression (a marker for viral gene expression) was visible in the brain at 24 h postadministration, and expression continued to increase until animals were euthanized or succumbed to neurotoxicity. Often, the onset of paralysis occurred at the time that luciferase was visualized in the spinal cords of infected mice (Fig. 1A). Mice inoculated with VSV-Luc intracranially exhibited classic signs of neurotoxicity, including listing, seizure, weight loss, and paralysis, and died or were euthanized prior to 15 days postinoculation. Upon histological examination of the brain, control Opti-MEM-treated mice had no histopathology outside the needle tract after administration of control carrier (Fig. 1B), while mice treated with VSV-Luc had numerous inflammatory lesions and moderate to severe meningoencephalitis, as visualized by hematoxylin and eosin (H&E) staining (Fig. 1C).
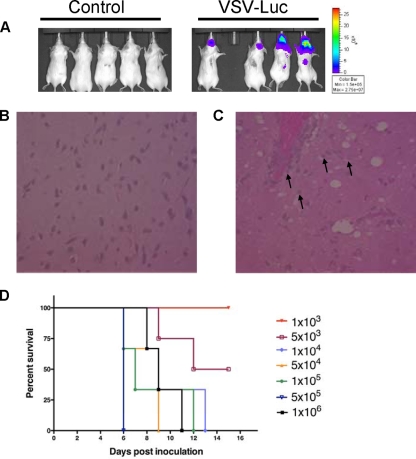
FIG. 1.
Vesicular stomatitis virus causes lethal neurotoxicity. (A) VSV-Luc localizes to and replicates in the brains of mice. Control Opti-MEM carrier or 1 × 104 VSV-Luc particles were injected intracranially into SCID mice and imaged for luminescence at 7 days postinoculation. (B) H&E-stained section of brain in Opti-MEM-injected control mouse, shown at original magnification of ×400. (C) H&E-stained section of brain of VSV-Luc-injected mouse, shown at original magnification of ×400. Black arrows represent apoptosing neurons. (D) LD50 determination with VSV-Luc administered intracranially. Kaplan-Meier survival curves of dose escalation in SCID mice are shown.
In order to determine the 50% lethal dose (LD50) of the parental VSV-Luc that would then be modified by miRNA target (miRT) insertion, we did a classic viral dose escalation study. Virus doses ranged from 1 × 102 to 1 × 107 in 10 μl of carrier. Not surprisingly, at the top doses of 1 × 104 to 1 × 107, all animals developed neurotoxicity and died or were euthanized prior to 15 days postinoculation (Fig. 1D). Animals that were administered less than 1,000 infectious units, however, were spared from lethal encephalitis, and the LD50 was determined to be 5,000 TCID50.
Neuronal microRNAs downregulate gene expression in lentiviral vectors.
Over 700 miRNAs have been identified in humans (35), many of which are known to have tissue-specific expression profiles (31). As we were interested in ameliorating viral encephalitis, we investigated those miRNAs that are known to be abundant in the CNS and which would be absent in tumors. Numerous brain-specific miRNAs have been identified (44) and are known to be expressed in different abundances, locations, and cell lineages. To identify the target elements that would most efficiently shut down gene expression in the brain, we first employed lentiviral vectors containing different brain-specific miRTs.
To select the optimal sequences for brain-specific shutdown, we utilized our previous reporter system whereby four tandem miRTs are inserted downstream of a spleen focus-forming virus (SFFV)-driven reporter gene in a lentiviral vector (Fig. 2A). We chose four of the best-characterized brain-specific target elements, as well as a hematopoiesis-specific miRT control, for further investigation. The most abundant miRNA in the brain is miR-124, a neuron-specific miRNA that has a role in neural specification in the developing brain. Vectors containing target elements corresponding to miR-124 were constructed, as well as vectors expressing miR-125T, which is expressed ubiquitously in all cell lineages of the brain; miR-128T, which has previously been shown to silence gene expression in neurons (48); and the recently identified miR-134T, which regulates spine development in addition to being heavily expressed in the hippocampus (Fig. 2B).
Vectors expressing control and brain-specific miRTs were transduced on a panel of cell lines thought to be indicative of different cell lineages that would have different miRNA expression profiles (Fig. 2D). Since neuronal cells express all four miRNAs (miR-124, miR-125, miR-128, and miR-134), we first transduced cells of different neuronal origins with our miRNA sensor vectors: Cath.A and NB41A3 are neuroblastomas, while 293T cells (once thought to be epithelial, but now reclassified) are transformed neuronal cells (45). While vectors containing target elements for all neuronal targets assayed did show significant decreases in gene expression in cells of neuronal lineage compared to HeLa and Mel-624 control cells (P < 0.05), the decrease in most cases was no greater than 30% (Fig. 2C). Even when miRT sensors were transduced into cells that contained fully complementary miRNA mimics in trans, a decrease in luciferase expression was not seen with all targets. Only when a vector containing miR-125T, miR-134T, or a combination of these targets was present was gene expression reduced by over 90% (Fig. 2C). While we (26) and others (8) have shown previously that combining more than one miRT can have additive affects in decreasing gene expression, we did not see this with neuronal targets (Fig. 2C and data not shown).
The factors affecting the efficiency with which miRNAs are able to silence sequence-complementary mRNAs are very poorly understood. While it has been shown in certain cases that more abundant miRNAs functionally silence miRTs better than do low-abundance miRNAs (8), it is clear that expression level is not the only factor that affects the regulatory capacity of miRNAs (6, 8, 19, 54).
VSV accommodates miRT insertion without slowing of growth kinetics.
Based upon our data obtained from lentiviral vectors as well as its expression profile in the brain, we determined that miR-125 provided the best candidate for attenuating viral gene expression in the brain. Vectors containing miR-125T showed a >95% decrease in luciferase expression in the presence of fully complementary miRNA mimics in vitro (Fig. 2C). In addition, all cell lineages in the brain (neurons, astrocytes, and glia cells) are thought to express miR-125 (though at different abundances). We next constructed fully replication-competent rVSVs that expressed target elements complementary to miR-125. As a control, we employed targets corresponding to miR-206, which we have shown previously to silence gene expression in muscle but to provide no protection from neurovirulence (26).
Endogenous miRTs are most often located in the 3′UTRs of cellular mRNAs. While some targets are found in coding regions of mRNAs as well as the 5′UTR, it is thought that there is a particular bias for efficacy of miRT localization in the 3′UTR (19). VSV, unlike picornaviruses, expresses each viral protein from a distinct mRNA. Unlike the design of miRNA-responsive picornaviruses, VSV provided a much greater burden for miRNA regulation, since each miRT would target only that gene in which the target was placed.
VSV contains five different viral genes, with transcriptional stop sites after each gene. Intergenic gaps function as a signal for polyadenylation and mRNA termination, and then conserved transcriptional start sites start transcription of the next gene (28). Due to transcriptase drop-off at each site, VSV genes are expressed in a gradient, with the proximal viral N gene having the most transcripts and the distal L gene having the least. This transcriptional gradient is important, as a high expression level of L is very cytotoxic and N must be expressed at sufficient levels to wrap the viral genome into a ribonucleoprotein (RNP) complex and also to provide sufficient protein to encapsidate the virus. The expression levels of M, P, and G appear to be less important, as rearrangement of these viral genes has been shown to be of little consequence to viral replication (3).
Bearing in mind that the viral L protein is expressed at the lowest levels of all viral genes, we hypothesized that it might be particularly vulnerable to miRNA-mediated inhibition. Also, while the expression level of the viral M protein is not as important in vitro, the M gene is responsible for the inhibition of host mRNA export that disables the immune response against the virus. Therefore, because the viral matrix protein inhibits an antiviral cellular signal cascade, we thought it could also be a target of potential importance. In addition to the M and L genes, we included target elements in the luciferase gene of the virus as a control. Viruses containing miRNA-targeted luciferase while potentially having less reporter should not have affected viral life cycles. Targets were therefore inserted into the 3′UTRs of the viral M, Luc, and L genes (Fig. 3A). In an effort to target the entire antigenome, we also inserted miRT sites within the viral 3′UTR, but these insertions prohibited viral rescue.
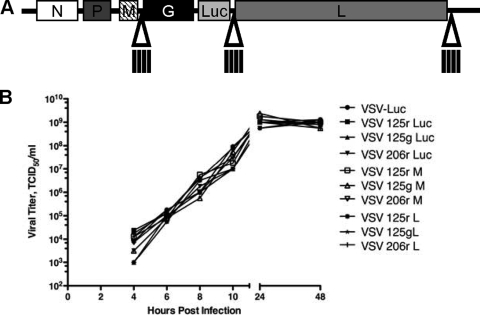
FIG. 3.
Construction and characterization of recombinant VSVs. (A) Schematic diagram of microRNA-targeted VSV. A novel NotI restriction site was created in the 3′UTR of the M, Luc, or L gene, and miRTs were cloned into this site. (B) One-step growth curves for recombinant viruses on BHK cells.
Since VSV is a minus-strand rhabdovirus, the VSV genome and mRNA are expressed in different orientations. We have shown previously that direct targeting of the viral genomes of picornaviruses provides the most efficient miRNA-mediated inhibition (Kelly et al., unpublished data). While we believed that the VSV genome would be relatively protected expressed as an RNP complex, it was nevertheless possible that miRNA recognition of the genome could occur, as the masking of target sites by secondary structure in picornaviruses did not preclude miRT recognition. We therefore oriented miRTs such that they would target either the genome (g) or mRNA (r) and inserted them in previously discussed regions of the viral genome.
Viruses were rescued and titrated in the absence of sequence-complementary miRNAs, and one-step growth curves were then performed to determine the replication kinetics of the recombinant, miRNA-targeted viruses, as determined by the TCID50 (as required by the FDA) (Fig. 3B [representative of six independent experiments]). Insertion of miRTs did not cause any slowing of virus replication on BHK cells, and all rVSVs grew to high titers in the absence of corresponding miRNAs.
miRNA-targeted VSVs are poorly responsive to sequence-complementary mimics supplemented in trans.
After constructing miRNA-targeted rVSVs, we next looked at the responsiveness of these viruses to sequence-complementary miRNAs. Viruses were analyzed to see if sequence-complementary miRNAs could provide protection from the cytolytic effects of the virus, a decrease in luciferase activity, or a decrease in viral replication.
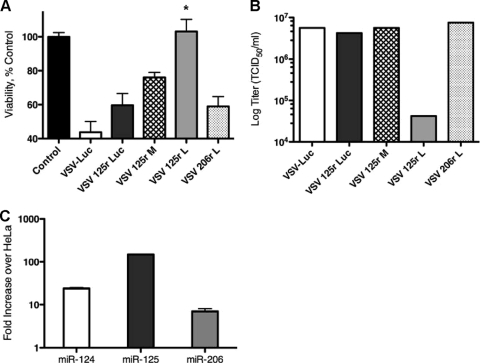
Since the matrix protein of VSV can shut down host mRNA export, including cytokines such as IFN-β that could potentially shut down viral replication, we used cell lines that are capable of inducing and responding to cellular IFN-β (52). A549 human lung epithelial cells were transfected with miRNA mimics corresponding to miR-125, miR-206, or a control miRNA thought to have no mammalian ortholog. Four hours after transfection of these miRNAs, A549 cells were infected with recombinant miRNA-targeted VSVs at a low MOI (0.2). While we have shown previously that picornaviruses assayed in this manner are fully protected from cytolytic effects of viral infection (26), VSV proved to be very poorly responsive to miRNA mimics provided in trans (Fig. 4A). Only the virus containing targets to miR-206 oriented to target the mRNA of the viral L gene had any significant increase in cell viability (P = 0.0012), and this was only ∼30%. Luciferase activity in these cells was not significantly different in the presence of sequence-complementary or control mimics (Fig. 4B).
FIG. 4.
VSVs encoding neuronal targets are largely unresponsive to sequence-complementary miRNA mimics. (A) Cell viability, as determined by MTT assay, at 24 h post-viral infection at an MOI of 0.5 with recombinant miRNA-targeted VSVs pretreated for 4 h with 200 nM miRNA mimics in A549 cells. (B) Corresponding luciferase activities of miRT VSVs in the presence of the miRNA mimics from panel A. (C) Viral titers collected at 24 h postinfection from supernatants of cells infected with miRT VSVs in the presence of miRNA mimics (representative of four repeat experiments). #, not determined; *, P = 0.0012.
In fact, the only parameter that showed a marked response was viral replication, measured by release of virus into the cell supernatant (Fig. 4C). Viral replication was decreased approximately 1.5 log in viruses containing miR-125T or miR-206T targeting the mRNA, with both inserted into the 3′UTR of L. While not on the same order of magnitude as that seen in picornaviruses (which can completely inhibit release of infectious virus in the supernatant), this reduction in viral titer could potentially attenuate the virus in the brain. A panel of other cell lines, including BHK, CT-26, and MEF (which represent cells with either productive or altered IFN responses), was utilized to study the miRNA responsiveness of VSVs, and these cells were equally (or less) responsive to miRNA-mediated viral inhibition (data not shown).
Neuronal target insertion protects primary brain cells from cytopathic effects in vitro.
While the expression of miRNA mimics in trans served to effectively shut down the expression of lentiviral vectors containing miR-125T, by >95%, they had very little effect on luciferase expression in rVSVs, nor did they serve to protect cells from cytolytic effects of the virus (Fig. 3). While we have shown previously that miRNA mimics are able to functionally suppress picornavirus infection in the presence of fully complementary targets (26), we have also seen that expression of miRNAs in trans cannot serve to functionally silence targets as efficiently as that of abundant endogenous miRNAs (Kelly et al., unpublished data). We therefore employed primary cells that express large amounts of endogenous miR-125 to see if endogenous miRNAs could indeed affect VSV replication.
VSV is capable of infecting neurons, astrocytes, and glia cells in the brain. While it is likely that neuropathogenesis is a result of cytotoxic effects on neuronal cells, cytokines such as interleukin-2 (IL-2) and nitric oxide synthase (NOS), produced by astrocytes and microglia, can have protective effects on surrounding cells (29). To determine if the presence of miR-125 in primary astrocytes could protect these cells from infection with rVSVs bearing miR-125T in different regions of the virus, we infected primary astrocytes with miRT VSVs and looked at cytotoxicity and viral replication when cells were infected at a low MOI.
Twenty-four hours after infection with VSV (even at an MOI as low as 0.2), severe cytopathic effects were seen in primary astrocytes. Analysis by MTT assay showed that ∼50% of cells were killed by rVSVs expressing control miR-206T. Insertion of miR-125T in luciferase provided no protection from cytopathic effects in primary astrocytes. However, the presence of miR-125T in the M protein rescued cell viability by roughly 15%, while miR-125r in L completely restored cell viability, such that it was not significantly different from that of mock-infected cells (P = 0.515) (Fig. 5A). In addition, miR-125r in L caused a >2-log reduction in viral titers released into the supernatants of infected astrocytes (Fig. 5B).
FIG. 5.
Viral replication of miRT VSVs is restricted in primary brain cells. (A) Cell viability, as determined by MTT assay, at 24 h post-viral infection at an MOI of 0.5 with recombinant miRNA-targeted VSVs in primary astrocytes. (B) Viral titers collected at 24 h postinfection from supernatants of cells infected with miRT VSVs in primary astrocytes (representative of three repeat experiments). (C) miRNA abundance in primary astrocytes, as determined by TaqMan miRNA expression analysis. Fold increases in miR-124, miR-125, or miR-206 over corresponding miRNAs in HeLa cells (per 5 ng small RNA) are shown. *, P < 0.001.
To validate that primary astrocytes did indeed contain miR-125, we performed TaqMan miRNA expression analysis of these cells to confirm that this brain-specific miRNA was upregulated compared to that in cells of other lineages (Fig. 5C). While miRNA mimics added in trans could not significantly protect cells from VSV toxicity (Fig. 4), here we show that higher levels of endogenous miRNAs expressed in astrocytes enabled VSV to become responsive to cellular miRNAs. While this result is modest compared to the effects seen in picornavirus-infected cells (5) or adenovirus-infected cells (55), it is nevertheless statistically significant (P < 0.001). Decreases of viral titers in vivo by >2 log could certainly provide protection from viral toxicities.
miRT insertion protects against neuropathogenesis when VSV is administered intracerebrally.
Having determined that rVSVs did respond to cellular miRNAs in vitro, we next proceeded with in vivo evaluation of the toxicity profiles of these viruses. While neuropathology develops from VSV infection with almost any administration route in immunocompromised rodent models, intracranial inoculation clearly provides the largest burdens for safety evaluation.
To assay for VSV attenuation in the brain, we therefore delivered 2× LD50 of VSV-Luc. This was also the LD100 for the virus (Fig. 1D). Immunocompromised mice were injected with 1 × 104 rVSV particles and imaged for bioluminescence 2, 5, 7, 10, 12, 14, 28, 35, and 42 days after inoculation with the virus. After virus administration, animals were monitored closely for signs of neurotoxicity, and no complications were seen in animals injected intracerebrally with control carrier.
Based upon in vitro analysis of our recombinant viruses (as well as small pilot experiments), we chose three recombinant, miRNA-targeted viruses for further evaluation (as well as the parental VSV-Luc). Viruses with miR-125T targeting the mRNA (125r) inserted in either the M or L gene were analyzed, as was a virus used as a control for insertional attenuation, containing miR-206r in L. Viral replication visualized by luciferase expression was seen at 24 h postinoculation in animals treated with VSV-Luc and the control VSV 206r L. In addition, the insertion of miR-125T in M did not attenuate virus replication in the brain 2 days after inoculation. However, only 1 of 10 animals administered 1 × 104 VSV 125r L particles had any detectable luciferase signal at the same time point (data not shown).
One week after delivery of rVSV, severe neurotoxicity had developed in groups treated with VSV-Luc, VSV 125r M, and VSV 206r L. Intense luciferase expression (>1 × 107 photons/s/brain) was seen in the majority of these animals 7 days after virus administration (Fig. 6A to D). However, 9 of 10 animals treated with VSV 125r L had no luciferase expression in the brain and no symptoms of neurotoxicity. These animals remained healthy and negative for luciferase expression up to 35 days postinoculation. At this time, all animals were euthanized and brains were harvested for tissue overlay to look for viral recovery or were sent for histological examination.
FIG. 6.
VSVs encoding neuron-specific miR-125T have reduced neurotoxicity. Mice were inoculated intracranially with 1 × 104 rVSV particles and were imaged at the indicated times, using an IVIS200 system (Xenogen Corp., Alameda, CA), and monitored for symptoms of neurotoxicity. (A) VSV-Luc at 7 days postinfection (p.i.). (B) VSV 125r M at 7 days p.i. (C) VSV 125r L at 7 days p.i. (D) VSV 206r L at 7 days p.i. (E) Kaplan-Meier survival graphs for mice inoculated intracranially with 1 × 104 rVSV particles. (F) Quantification of the amount of bioluminescence output per brain, plotted over time, as an indication of viral gene expression for mice inoculated with rVSV (values are averages for 10 animals per group ± standard deviations). (G) A mouse administered 1 × 104 VSV 125 L particles (negative until imaged on day 28) developed encephalitis, as seen at the labeled times postinfection. *, P = 0.0025.
After intracranial virus administration, 90% of animals treated with 1 × 104 VSV 125r L particles survived to the terminal point of the study (35 days) (Fig. 6E). This survival rate was highly significantly different from those with parental VSV-Luc (P < 0.001) and the control for insertional attenuation (P = 0.0025). While animals treated with VSV-125r M did trend toward increased survival, with 30% surviving to the terminal point in the study, this survival rate was not significantly increased over that with control miRT. Examination of luciferase expression in the brain also revealed that VSV 125r L was attenuated in the brain, with only one animal treated with the virus ever becoming positive for luciferase expression (and quickly succumbing to lethal encephalitis) (Fig. 6F). The observation that 10% of mice manifested with neurotoxicity even when viral expression was targeted by sequence-complementary miRNAs in the brain provides further evidence that this targeting paradigm, while effective for negative-strand viruses, is not as efficient as that with positive-strand picornaviruses and lentiviral vectors.
While the inclusion of target elements for miR-125 targeting the mRNA of the L gene of VSV did significantly attenuate the virus in the brain and increase the therapeutic index of VSV, mutation of these target elements is always of potential concern. Because only ∼100 nt of sequence insert provided the entire basis of the reduced toxicity for this virus, we looked at the potential of this virus to mutate the sequence insert. In vitro selective pressure did not provide any change in the sequence insert of the virus, nor did the animal treated with VSV 125r L that succumbed to neurotoxicity (Fig. 6E) have any sequence alterations in the insert sequence. However, in a pilot study in which 1 × 104 VSV 125r L particles were administered to immunocompromised mice, we did see one animal become positive for luciferase expression 4 weeks after administration. The signal gained in intensity over a period of 2 weeks, finally manifesting in the spinal cord, in addition to expression in the brain (Fig. 6G). Forty-two days after inoculation, this animal was sacrificed, and its brain was harvested to recover virus. This virus was then amplified on BHK cells in vitro, and the miRT insert and flanking region were cloned into TOPO and sequenced, but no sequence alterations in the miRT insert were found. Therefore, the delayed development of VSV encephalitis could be a result of second-site mutations, or perhaps saturation of endogenous miR-125 as a result of VSV infection.
In vivo evaluation of recombinant miRNA-targeted VSVs showed that insertion of target elements for miR-125T targeting the L gene of the virus significantly ameliorated neurotoxicity. However, as with all viral engineering, selective pressure to mutate this sequence does occur and can hamper the efficacy of this targeting paradigm, even in negative-strand viruses.
miRNA-targeted VSVs retain oncolytic efficacy in vivo.
Our previous experiment showing that VSV incorporating miRNA targets within the viral genome can actually attenuate the virus in the brain is of great import. However, since these viruses are to be used therapeutically as anticancer agents, we next wanted to validate that they did indeed retain their oncolytic efficacy in models of oncolysis in vivo.
miRNAs are known not only to have tissue-specific signatures but to have cancer-specific expression profiles as well (12). While there are miRNAs that are indicated as oncosuppressive and are found to be downregulated significantly in tumors, so too are there miRNAs that are oncogenic and found to be highly enriched in tumors (23). miR-125, in fact, has actually been indicated to have a role in breast cancer. While miR-125 is most highly enriched in the brain, it is also expressed (though at much lower abundance) in normal breast tissue (24). However, in many breast cancers, miR-125 is found to be downregulated significantly or even altogether absent.
While downregulation of miRNAs in tumors should pose no barriers for effective oncolysis by viruses that express these miRTs, increased expression of miRNAs in tumors could hamper their efficacy. While there have been no published works suggesting that any brain-specific miRNA is upregulated in cancerous cells of any origin, we nevertheless decided to test that miRNA-regulated viruses did in fact retain oncolytic efficacy.
Many groups have published that mouse colorectal cancer is particularly susceptible to VSV oncolysis (21, 49). Since CT-26 cells are colorectal carcinoma cells derived from BALB/c mice, they provided a good model system, since they could be implanted as subcutaneous grafts and studied in fully immunocompetent mice. Therefore, 5 × 106 CT-26 cells were injected subcutaneously into the right flanks of immunocompetent BALB/c mice. When tumors reached approximately 250 mm3, they were treated with one intratumoral dose of 1 × 109 rVSV on day 0 and one intravenous dose of 1 × 109 rVSV on day 3. Tumors were monitored daily, and bioluminescence imaging was performed on days 2, 5, 7, and 10 after first virus administration.
Control tumors grew quickly and were unencumbered by control carrier treatment, and all animals were euthanized by day 18 after treatment (when tumors reached >2,000 mm3 or ulcerated). Animals treated with parental VSV-Luc or VSVs containing miRT inserts showed prolonged tumor progression (Fig. 7A) and survival (Fig. 7B), but tumors in all groups eventually grew such that they were larger than 10% of body weight (>2,000 mm3) or ulcerated, necessitating euthanasia of all animals due to tumor size.
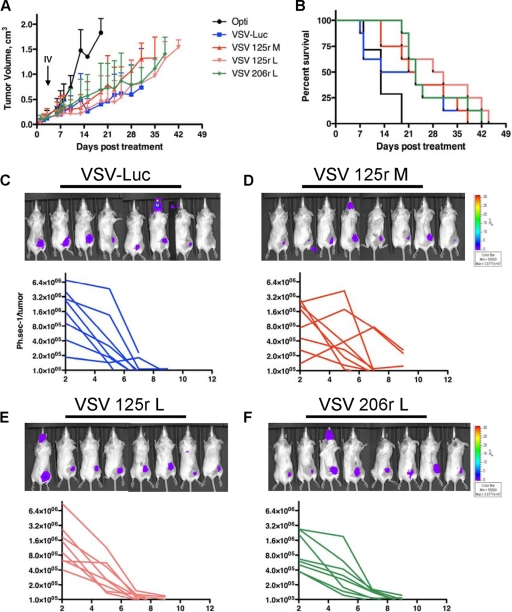
FIG. 7.
MicroRNA-targeted VSVs have equivalent antitumor activities in CT-26 model. (A) CT-26 cells (5 × 106) were injected subcutaneously into the right flank of BALB/c mice, and mice were administered one intratumoral dose of virus (1 × 109) on day 0 and one intravenous dose (1 × 109) on day 3 and monitored for tumor progression. (B) Kaplan-Meier survival curves for the mice in panel A. Images show CT-26 tumor-bearing mice treated with rVSV on day 2, and graphs show the quantification of bioluminescence output per flank, plotted over time, as an indication of viral gene expression for mice treated with VSV-Luc (C), VSV 125r M (D), VSV 125r L (E), and VSV 206r L (F).
To visualize viral replication in tumors (as well as the potential for neurotoxicity), we imaged all mice at days 2, 5, 7, and 10 after the first virus administration. At 2 days posttreatment, virus was present at similar abundances in mice treated with VSV-Luc or miRT-targeted viruses, but luciferase activity quickly decreased, such that no viral replication was detected in subcutaneous tumors or other tissues by 10 days posttreatment (Fig. 7C to F). While we did observe a luciferase signal in the head for 1/8 mice in each group (Fig. 7E), it never appeared to be a result of neurotoxicity. These animals had no symptomatic manifestation of neuropathogenesis, and upon sacrifice and harvest of the brain, no virus was detected. We therefore hypothesize that this may actually be a signal in the mucosa of the mouth and/or nose. There was no significant difference in antitumor efficacies of miRT recombinants (P = 0.2353), nor was there a significant difference in viral replication in tumors, as analyzed by luminescence activity on the right flank (P = 0.9294).
DISCUSSION
MicroRNA targeting clearly provides an effective means to restrict viral replication in specific tissues, based on different miRNA profiles of different cell lineages. Unlike other targeting paradigms that are very specific to viral class, miRNA-mediated restriction should be able to regulate any virus or vector and has already proven efficacious in vivo for retroviruses, double-stranded DNA (dsDNA) viruses, positive-strand RNA viruses, (27), and now negative-strand RNA viruses. Unlike inclusion of tissue-specific promoters and display of nonnative receptors on viruses, miRNA targeting restricts rather than expands host range, increasing safety rather than enabling the potential for greater viral pathogenicity. Perhaps importantly for small, replication-competent viruses, the typical insert (∼100 bp) is highly economical in terms of sequence size and should not be prohibitive for any virus or vector.
VSV has been shown to have potent activity against a variety of tumor models, including colorectal cancer (49), hepatocellular cancer (1), glioma (38), myeloma (18), and bladder cancer (20). In addition, it may act in synergy with the host immune system to provide even greater antitumor activity (30). While VSV therefore seems an ideal virus for translation into the clinic as an anticancer therapeutic, lingering doubts about the neurotropism of the virus, among other issues, have as yet hindered clinical development of the virus.
Here we have engineered a VSV encoding firefly luciferase to carry target elements for tissue-specific miRNAs in an effort to curb lethal encephalitis caused by viral infection. While we used miRNA targets in orientations such that they targeted either the genome or mRNA, only viruses containing miRTs targeting the mRNA were at all responsive to sequence-complementary miRNAs. Because all engineered VSVs were much less responsive in vitro than picornaviruses or adenoviruses, we conducted small pilot in vivo experiments where genome-targeted miRTs were administered intracranially. These provided no reduction in either luciferase expression or protection from neuropathology (data not shown), with all animals succumbing to neurotoxicity or showing symptoms necessitating euthanasia at times that were not significantly different from those for control mice.
In addition to looking at the orientation of miRTs, we also looked at the impact of site selection in VSV. Unlike picornaviruses, which encode one mRNA (which also serves as its genome), VSV contains five distinct mRNAs (one for each viral gene). Viruses that contained miRTs in the 3′UTRs of the M, luciferase, and L genes were therefore rescued. Both in vitro and in vivo, miRT insertion in L proved more efficacious than the same sequence inserted in M, while (as predicted) luciferase insertions did not have any effect on viral replication whatsoever. While it is possible that a decrease (without complete ablation) of L protein could be more crippling to VSV than would a decrease in M protein, due to the transcriptional gradient causing L to already be expressed at the lowest level of all viral genes, little is known about the actual levels of protein required for viral replication. While viral rescue occurs in vitro only with a specific in trans N:P:L ratio, shuffling of the internal M, P, and G genes in the virus has been shown to have a negligible impact on viral replication (3). Therefore, our actual empirical determination that miRT insertion in L is superior to miRT insertion in M is of particular import. However, it is likely that combination viruses whereby one or more miRTs are inserted in multiple genes will be most effective.
We also investigated the efficacy of different brain-specific miRTs in the silencing of gene expression. In lentiviral vectors used as reporter constructs, all brain-specific miRNAs proved to be less efficient in silencing sequence-complementary mRNA expression than our previously described muscle, hematopoietic, and tumor suppressor miRNAs (26; Kelly et al., unpublished data). Though the miRNA expression level has been implicated in affecting the regulatory efficiency of a specific miRNA, other unknown factors clearly contribute to miRNA silencing. However, despite the superior functioning of other tissue-specific miRNAs in vitro, target elements for miR-125 do downregulate gene expression >90% in the presence of supersaturating miRNA mimics supplemented in trans, and endogenous miR-125 expression in the brain functions to protect mice from the development of lethal encephalitis in vivo. While miR-125 downregulated gene expression in vitro better than did miR-124, miR-128, or miR-134, we do not dismiss the potential of these miRNAs for exploitation in the future. In fact, pilot studies where miR-124T was inserted into the 3′UTR of M are suggestive that this abundant miRNA could indeed act to prevent viral replication in the brain (data not shown).
While miRNA targeting has great potential, mutation and selection of viruses containing altered miRNA target sequences could be a potential pitfall, with mutations in the miRT sequence reducing the efficiency with which target recognition can take place. Therefore, combination viruses in which miRT inserts are included in multiple genes could provide advantages in terms of both perturbing viral replication and providing buffers to mutation of miRT inserts. However, miRNA saturation, where multiple target sites act as sponges to soak up endogenous miRNAs (16), could then also come into play. One might envisage the use of different miRNA targets in different genes to circumvent both of these issues in designing viruses for future use.
Overall, our data provide important proof of principle that miRNA targeting can curb the development of VSV encephalitis in mice, even when the virus is administered intracranially. In addition, we looked at the regulatory efficiencies of different miRTs, the impact of miRT orientation in a negative-strand virus, and the regulatory impact of the viral insertion site. Most importantly, however, we have engineered VSV to have an increased therapeutic index by increasing the safety of the virus without perturbing the potent oncolytic activity of this virus.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Altomonte, J., R. Braren, S. Schulz, S. Marozin, E. J. Rummeny, R. M. Schmid, and O. Ebert. 2008. Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology 48:1864-1873. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51-65. [DOI] [PubMed] [Google Scholar]
- 3.Ball, L. A., C. R. Pringle, B. Flanagan, V. P. Perepelitsa, and G. W. Wertz. 1999. Phenotypic consequences of rearranging the P, M, and G genes of vesicular stomatitis virus. J. Virol. 73:4705-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber, G. N. 2005. VSV-tumor selective replication and protein translation. Oncogene 24:7710-7719. [DOI] [PubMed] [Google Scholar]
- 5.Barnes, D., M. Kunitomi, M. Vignuzzi, K. Saksela, and R. Andino. 2008. Harnessing endogenous miRNAs to control virus tissue tropism as a strategy for developing attenuated virus vaccines. Cell Host Microbe 4:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281-297. [DOI] [PubMed] [Google Scholar]
- 7.Bi, Z., M. Barna, T. Komatsu, and C. S. Reiss. 1995. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J. Virol. 69:6466-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, B. D., B. Gentner, A. Cantore, S. Colleoni, M. Amendola, A. Zingale, A. Baccarini, G. Lazzari, C. Galli, and L. Naldini. 2007. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat. Biotechnol. 25:1457-1467. [DOI] [PubMed] [Google Scholar]
- 9.Brown, B. D., M. A. Venneri, A. Zingale, L. Sergi Sergi, and L. Naldini. 2006. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 12:585-591. [DOI] [PubMed] [Google Scholar]
- 10.Carneiro, F. A., M. L. Bianconi, G. Weissmuller, F. Stauffer, and A. T. Da Poian. 2002. Membrane recognition by vesicular stomatitis virus involves enthalpy-driven protein-lipid interactions. J. Virol. 76:3756-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawood, R., H. H. Chen, F. Carroll, M. Bazan-Peregrino, N. van Rooijen, and L. W. Seymour. 2009. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 5:e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C. Z. 2005. MicroRNAs as oncogenes and tumor suppressors. N. Engl. J. Med. 353:1768-1771. [DOI] [PubMed] [Google Scholar]
- 13.Dal Canto, M. C., S. G. Rabinowitz, and T. C. Johnson. 1976. Status spongiousus resulting from intracerebral infection of mice with temperature-sensitive mutants of vesicular stomatitis virus. Br. J. Exp. Pathol. 57:321-330. [PMC free article] [PubMed] [Google Scholar]
- 14.Edge, R. E., T. J. Falls, C. W. Brown, B. D. Lichty, H. Atkins, and J. C. Bell. 2008. A let-7 microRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol. Ther. 16:1437-1443. [DOI] [PubMed] [Google Scholar]
- 15.Fu, H., Y. Tie, C. Xu, Z. Zhang, J. Zhu, Y. Shi, H. Jiang, Z. Sun, and X. Zheng. 2005. Identification of human fetal liver miRNAs by a novel method. FEBS Lett. 579:3849-3854. [DOI] [PubMed] [Google Scholar]
- 16.Gentner, B., G. Schira, A. Giustacchini, M. Amendola, B. D. Brown, M. Ponzoni, and L. Naldini. 2009. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat. Methods 6:63-66. [DOI] [PubMed] [Google Scholar]
- 17.Godlewski, J., H. B. Newton, E. A. Chiocca, and S. E. Lawler. 2009. MicroRNAs and glioblastoma; the stem cell connection. Cell Death Differ. [Epub ahead of print.] doi: 10.1038/cdd.2009.71. [DOI] [PubMed]
- 18.Goel, A., S. K. Carlson, K. L. Classic, S. Greiner, S. Naik, A. T. Power, J. C. Bell, and S. J. Russell. 2007. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood 110:2342-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, S., L. Jin, F. Zhang, P. Sarnow, and M. A. Kay. 2009. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 16:144-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadaschik, B. A., K. Zhang, A. I. So, L. Fazli, W. Jia, J. C. Bell, M. E. Gleave, and P. S. Rennie. 2008. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 68:4506-4510. [DOI] [PubMed] [Google Scholar]
- 21.Huang, T. G., O. Ebert, K. Shinozaki, A. Garcia-Sastre, and S. L. Woo. 2003. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol. Ther. 8:434-440. [DOI] [PubMed] [Google Scholar]
- 22.Huneycutt, B. S., I. V. Plakhov, Z. Shusterman, S. M. Bartido, A. Huang, C. S. Reiss, and C. Aoki. 1994. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain Res. 635:81-95. [DOI] [PubMed] [Google Scholar]
- 23.Iorio, M. V., P. Casalini, E. Tagliabue, S. Menard, and C. M. Croce. 2008. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur. J. Cancer 44:2753-2759. [DOI] [PubMed] [Google Scholar]
- 24.Iorio, M. V., M. Ferracin, C. G. Liu, A. Veronese, R. Spizzo, S. Sabbioni, E. Magri, M. Pedriali, M. Fabbri, M. Campiglio, S. Menard, J. P. Palazzo, A. Rosenberg, P. Musiani, S. Volinia, I. Nenci, G. A. Calin, P. Querzoli, M. Negrini, and C. M. Croce. 2005. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65:7065-7070. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. E., F. Nasar, J. W. Coleman, R. E. Price, A. Javadian, K. Draper, M. Lee, P. A. Reilly, D. K. Clarke, R. M. Hendry, and S. A. Udem. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360:36-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly, E. J., E. M. Hadac, S. Greiner, and S. J. Russell. 2008. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat. Med. 14:1278-1283. [DOI] [PubMed] [Google Scholar]
- 27.Kelly, E. J., and S. J. Russell. 2009. MicroRNAs and the regulation of vector tropism. Mol. Ther. 17:409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, and M. A. Martin (ed.). 2006. Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Komatsu, T., D. D. Ireland, N. Chen, and C. S. Reiss. 1999. Neuronal expression of NOS-1 is required for host recovery from viral encephalitis. Virology 258:389-395. [DOI] [PubMed] [Google Scholar]
- 30.Kottke, T., R. M. Diaz, K. Kaluza, J. Pulido, F. Galivo, P. Wongthida, J. Thompson, C. Willmon, G. N. Barber, J. Chester, P. Selby, S. Strome, K. Harrington, A. Melcher, and R. G. Vile. 2008. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol. Ther. 16:1910-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagos-Quintana, M., R. Rauhut, A. Yalcin, J. Meyer, W. Lendeckel, and T. Tuschl. 2002. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12:735-739. [DOI] [PubMed] [Google Scholar]
- 32.Lang, K. S., A. A. Navarini, M. Recher, P. A. Lang, M. Heikenwalder, B. Stecher, A. Bergthaler, B. Odermatt, S. Akira, K. Honda, H. Hengartner, and R. M. Zinkernagel. 2007. MyD88 protects from lethal encephalitis during infection with vesicular stomatitis virus. Eur. J. Immunol. 37:2434-2440. [DOI] [PubMed] [Google Scholar]
- 33.Lee, C. Y., P. S. Rennie, and W. W. Jia. 2009. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin. Cancer Res. 15:5126-5135. [DOI] [PubMed] [Google Scholar]
- 34.Letchworth, G. J., L. L. Rodriguez, and J. Del Cbarrera. 1999. Vesicular stomatitis. Vet. J. 157:239-260. [DOI] [PubMed] [Google Scholar]
- 35.Lewis, B. P., C. B. Burge, and D. P. Bartel. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15-20. [DOI] [PubMed] [Google Scholar]
- 36.Obuchi, M., M. Fernandez, and G. N. Barber. 2003. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77:8843-8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otsuka, M., Q. Jing, P. Georgel, L. New, J. Chen, J. Mols, Y. J. Kang, Z. Jiang, X. Du, R. Cook, S. C. Das, A. K. Pattnaik, B. Beutler, and J. Han. 2007. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 27:123-134. [DOI] [PubMed] [Google Scholar]
- 38.Ozduman, K., G. Wollmann, J. M. Piepmeier, and A. N. van den Pol. 2008. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J. Neurosci. 28:1882-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez, J. T., A. M. Pham, M. H. Lorini, M. A. Chua, J. Steel, and B. R. tenOever. 2009. MicroRNA-mediated species-specific attenuation of influenza A virus. Nat. Biotechnol. 27:572-576. [DOI] [PubMed] [Google Scholar]
- 40.Rao, P. K., R. M. Kumar, M. Farkhondeh, S. Baskerville, and H. F. Lodish. 2006. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. USA 103:8721-8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiss, C. S., I. V. Plakhov, and T. Komatsu. 1998. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann. N. Y. Acad. Sci. 855:751-761. [DOI] [PubMed] [Google Scholar]
- 42.Schellekens, H., E. Smiers-de Vreede, A. de Reus, and R. Dijkema. 1984. Antiviral activity of interferon in rats and the effect of immune suppression. J. Gen. Virol. 65:391-396. [DOI] [PubMed] [Google Scholar]
- 43.Schratt, G. M., F. Tuebing, E. A. Nigh, C. G. Kane, M. E. Sabatini, M. Kiebler, and M. E. Greenberg. 2006. A brain-specific microRNA regulates dendritic spine development. Nature 439:283-289. [DOI] [PubMed] [Google Scholar]
- 44.Sempere, L. F., S. Freemantle, I. Pitha-Rowe, E. Moss, E. Dmitrovsky, and V. Ambros. 2004. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 5:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw, G., S. Morse, M. Ararat, and F. L. Graham. 2002. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 16:869-871. [DOI] [PubMed] [Google Scholar]
- 46.Shingara, J., K. Keiger, J. Shelton, W. Laosinchai-Wolf, P. Powers, R. Conrad, D. Brown, and E. Labourier. 2005. An optimized isolation and labeling platform for accurate microRNA expression profiling. RNA 11:1461-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinozaki, K., O. Ebert, A. Suriawinata, S. N. Thung, and S. L. Woo. 2005. Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J. Virol. 79:13705-13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smirnova, L., A. Grafe, A. Seiler, S. Schumacher, R. Nitsch, and F. G. Wulczyn. 2005. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 21:1469-1477. [DOI] [PubMed] [Google Scholar]
- 49.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan, C. S., A. T. Grundhoff, S. Tevethia, J. M. Pipas, and D. Ganem. 2005. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature 435:682-686. [DOI] [PubMed] [Google Scholar]
- 51.Sun, Y., S. Koo, N. White, E. Peralta, C. Esau, N. M. Dean, and R. J. Perera. 2004. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 32:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umareddy, I., K. F. Tang, S. G. Vasudevan, S. Devi, M. L. Hibberd, and F. Gu. 2008. Dengue virus regulates type I interferon signalling in a strain-dependent manner in human cell lines. J. Gen. Virol. 89:3052-3062. [DOI] [PubMed] [Google Scholar]
- 53.van den Pol, A. N., K. P. Dalton, and J. K. Rose. 2002. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 76:1309-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westerhout, E. M., M. Ooms, M. Vink, A. T. Das, and B. Berkhout. 2005. HIV-1 can escape from RNA interference by evolving an alternative structure in its RNA genome. Nucleic Acids Res. 33:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ylosmaki, E., T. Hakkarainen, A. Hemminki, T. Visakorpi, R. Andino, and K. Saksela. 2008. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific microRNA. J. Virol. 82:11009-11015. [DOI] [PMC free article] [PubMed] [Google Scholar]