Abstract
Vaccines against the human papillomaviruses (HPVs) most frequently associated with cancer of the cervix are now available. These prophylactic vaccines, based on virus-like particles (VLPs), are extremely effective, providing protection from infection in almost 100% of cases. However, the vaccines present some limitations: they are effective primarily against the HPV type present in the vaccine, are expensive to produce, and need a cold chain. Vaccines based on the minor capsid protein L2 have been very successful in animal models and have been shown to provide a good level of protection against different papillomavirus types. The potential of L2-based vaccines to protect against many types of HPVs is discussed.
Papillomaviruses (PVs) make up a vast family that comprises hundreds of different viruses (30). PVs infect epithelia in humans and animals and cause benign hyperproliferative lesions, commonly called warts or papillomas, which can occasionally progress to squamous cell cancer or less commonly, adenocarcinoma (12). Cancer of the uterine cervix is caused by human papillomavirus (HPV), primarily types 16 and 18, but also a dozen other “high risk” HPV types that infect the genital mucosa. The presence of viral proteins, i.e., foreign antigens, in the cancers and precancers presents the opportunity for prevention or cure of the lesions via vaccination targeted against the viral proteins. The virus infectious cycle and the neoplastic progression from papilloma to carcinoma are broadly similar in humans and animals, and animal PVs and their hosts represent excellent model systems for HPVs, infection, and neoplastic progression (8, 13). Additionally, animal PVs have provided powerful models for antiviral vaccines (15). This is particularly true of the bovine papillomavirus types 1 and 4 (BPV), the cottontail rabbit papillomavirus (CRPV), and later of the canine oral papillomavirus (COPV).
In this review, we briefly describe the virus, its structure, its genomic organization, and its proteins, review the history of the development of the current prophylactic vaccines against HPV, and discuss the requirement for new broad-spectrum prophylactic vaccines. We note that a number of preclinical vaccination studies utilizing early viral antigens (not present in a virion) protect against experimental viral challenge (9, 43). Since this presumably occurs by triggering cellular immunity that clears the virus early after the initiation of infection, prior to the induction of clinically apparent disease, we classify this approach as therapeutic vaccination. Here we focus on the late proteins, L1 and L2, key structural components of the virion, and their role in prophylactic vaccination, first in animal models and then in humans.
THE VIRUS, ITS STRUCTURE, AND ITS GENOME
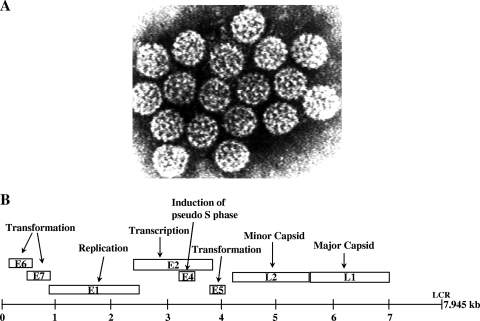
Despite their heterogeneity, PVs have a consistent virion structure and similar genetic plan. The virion (capsid) has a nonenveloped icosahedral structure of 55-nm diameter made up of 72 pentameric capsomeres (Fig. 1A). The capsid is made up of two proteins, L1, the major structural protein, and L2, the minor one (39). While the structure of the L1 part of the capsid has been defined by crystallography (6, 19), the position of L2 within the capsid is still not fully defined (11), although neutralization studies have indicated that the N terminus of L2 is accessible on the virion surface (39) (see below). The capsid contains the circular double-stranded viral DNA of approximately 8 kb associated with cellular nucleosomal proteins (39). The viral genome is divided into three parts; approximately two thirds of the genome codes for the early proteins E1 to E7, approximately one third codes for the structural proteins L1 and L2, and the remainder is mostly noncoding and contains the cis elements necessary for viral DNA replication and transcription, variously called the long control region, or LCR, or upstream regulatory region, or URR. All the genes are carried on only one strand, and therefore transcription is unidirectional (25).
FIG. 1.
(A) Electron micrograph of BPV-4. Magnification, ×80,000. (Reprinted from reference 47 with permission of the publisher.) (B) Genomic organization of a generic papillomavirus. The circular genome is represented linearly for the sake of simplicity. The viral open reading frames are represented by boxes, and the functions of their encoded viral proteins are indicated. LCR, long control region.
THE VIRUS LIFE CYCLE AND THE VIRAL PROTEINS
The life cycle of PV is totally dependent on the differentiation of the keratinocytes (31). This is an important point that has an impact on the host immune response and the design of vaccines, as will be discussed later.
The E proteins are involved in the early events of the virus life cycle. E1 is a helicase required for viral DNA replication; E2 is a transcription regulator of viral gene expression and also aids in the replicative process by helping in the recruitment of E1 to the origin of DNA replication. E4 is, properly speaking, an intermediate protein expressed during the viral DNA replicative phase. E5, E6, and E7 are the transforming proteins, all of which, to a greater or lesser extent, induce cell DNA replication and proliferation, thus helping in the replication of the viral DNA itself. Their unregulated and constitutive expression drives carcinogenesis (41). L1 and L2 are the structural proteins, major and minor, respectively, that form the capsid (Fig. 1B).
The virus infects the basal cells and delivers the genome to the nucleus. The episomal viral genome starts expressing the early proteins that maintain the keratinocyte in a proliferative state. As the keratinocyte continues to divide, it also differentiates, and the viral genome, typically maintained at a low copy number, undergoes many rounds of replication, aided by E4 (26). The late proteins are expressed in the granular layer, where they start to encapsidate the viral genome, and mature infectious virus is released with the keratin squames (31). Occasionally the virus life cycle is not completed, and cell transformation without accompanying differentiation leads to neoplasia (81). For a fuller description of the function of the viral proteins during the virus life cycle and neoplastic progression, see references 14, 44, 63, 67, and 76.
The fact that the entire virus cycle takes place above the basal layer and without directly triggering cell lysis limits the interaction of viral antigens with systemic immune cells that patrol for pathogens underneath the basal membrane and are thus unaware of the infection that takes place in the epithelium, particularly of the expression of the late proteins in the upper layers. Furthermore, papillomaviruses cause the depletion of intraepithelial Langerhans cells, which are crucial for T-cell priming (58, 61), downregulate surface major histocompatibility complex (MHC) class I, the major presenter of antigenic peptides to T cells (3, 4), and interfere with type I interferon (IFN) signaling (2, 35). Together these events help explain the general lack of local inflammation, the poor immune response to the virus, and the long persistence of the papillomas, even in immunocompetent individuals. It is still not clear what triggers clearance of infection and rejection of papillomas. Often in cattle, mechanical abrasion of the papilloma brings about an influx of immune cells, with subsequent tumor regression (57). Treatments of warts in patients often employ destruction of local tissue, like cryotherapy, or nonspecific immunostimulatory agents, including a Toll-like receptor (TLR) agonist delivered topically. Furthermore, warts are typically recalcitrant in HIV-positive or organ transplant patients, further suggesting the importance of cellular immune responses in clearing HPV infections and the potential of therapeutic HPV vaccination (45). Despite poor recognition of the viral proteins by the host immune cells, the viral proteins are immunogenic, especially the late ones, as shown by their experimental inoculation in cattle and rabbits (16, 48, 59). These observations have been pivotal in the development of prophylactic vaccines, first in animals and later in humans. We address the BPV/cattle system in greater detail, with reference also to the CRPV/rabbit and COPV/dog systems.
VIRUS VACCINES
The first anti-PV vaccines were based on virus preparations (50, 64). Vaccinated animals were protected from further challenges, and protection was due to the production of virus-neutralizing antibodies (21, 49). There are two major classes of neutralizing antibodies, both binding to L1, which prevent infection by two distinct mechanisms: inhibition of virus binding to the cell surface in vitro and prevention of virion uptake and/or uncoating without inhibiting virus binding to cell monolayers (7). Recent studies have identified HPV binding to laminin 5 in the extracellular matrix (ECM) (24) and to heparan sulfate proteoglycans in the basement membrane in vivo (51), with the ECM and basement membrane appearing to act as depots for virions prior to their attachment to keratinocytes. Distinct classes of L1-specific neutralizing antibodies that block ECM binding or direct cell surface binding have been described (29).
The original virus vaccines were more effective than natural infection in triggering an immune response because the vaccine was delivered intramuscularly and was therefore made available to antigen presenting cells (APC), while in a natural infection, the virus infects the epithelium and is in poor contact with APC (see above). Importantly, however, protection conferred by virus vaccines was virus type restricted; for example, cattle vaccinated with BPV type 4 (BPV-4) were not protected against challenge with BPV-2, and vice versa (Table 1) (49). This clearly showed that individual virus types had distinct neutralizing determinants on the surfaces of their virions and that vaccinating against only one type would not protect against infection by other types unless they were closely related.
TABLE 1.
Summary of protection studies with cattle using inactivated BPV virion preparations for immunization and different BPV genotypes for challenge
| Vaccine | Protection induced by challenge with indicated virusa |
||||
|---|---|---|---|---|---|
| BPV-1 | BPV-2 | BPV-4 | BPV-5 | BPV-6 | |
| BPV-1 | + | +b | − | ND | ND |
| BPV-2 | +/−c | + | − | ND | ND |
| BPV-4 | − | − | + | − | ND |
| BPV-5 | ND | − | − | + | ND |
| BPV-6 | − | − | − | ND | + |
+, protected from infection; −, not protected; ND, not done.
In the study by Shafti-Keramat et al. (73), antisera raised against BPV-1 or BPV-2 VLPs could cross-neutralize the homologous as well as the heterologous type in in vitro experiments.
L1 VACCINES
Subsequent vaccines consisted of the BPV or CRPV L1 protein, often expressed in bacteria in fusion form with either beta-galactosidase (β-Gal), glutathione-S-transferase (GST), or trpE and purified under denaturing conditions (16, 48, 59). These L1 vaccines were partially protective and generated the production of low titers of virus-neutralizing antibodies (48). These experiments suggested that some neutralizing epitopes encoded by L1 are linear or that the antigen may partially refold. Recently it has been shown that GST-L1 fusions of COPV made in Escherichia coli can assemble into pentameric structures known as capsomeres, which display conformational epitopes, induce high titers of neutralizing antibodies, and effectively protect dogs against COPV challenge (78).
VIRUS-LIKE PARTICLE VACCINES
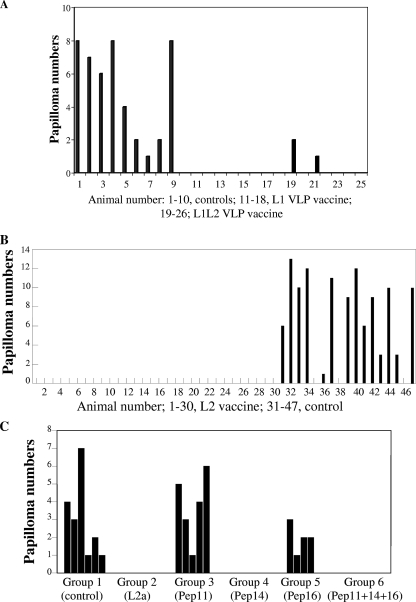
Virus-like particles (VLPs) are empty PV particles, without the viral genome, that are formed by expression of L1 alone or of L1 and L2 (53, 55). They are assembled in insect or yeast cells, display remarkable structural and antigenic similarity to virions, and can induce high titers of antibodies in vaccinated individuals (20, 42, 54, 79, 80). VLP vaccination was tested and validated against mucosal BPV-4 and cutaneous CRPV challenge, with protection approaching 100% independently of the presence of the L2 protein in the VLP (Fig. 2A) (23, 54). That naïve animals could be protected from experimental viral challenge simply by passive transfer of immune IgG suggests the importance of neutralizing antibodies in mediating protection (10). Consistent data were also obtained with the mucosal COPV challenge model (75). These observations opened the way for the use of VLPs in vaccines against HPVs.
FIG. 2.
Prophylactic vaccination of cattle with BPV-4 VLPs (A), BPV-4 L2 protein (B), and BPV-4 L2 N terminus peptides (C). (A) Animals were vaccinated with either L1 or L1L2 VLPs. Each calf was inoculated with two doses 4 weeks apart of either 150 μg L1 VLP (each dose) or 200 μg L1L2 VLP (each dose) in Alhydrogel. Challenge virus was delivered 2 weeks after the second dose, and calves were examined for tumors at 21 weeks postchallenge. Two calves in the L1L2 VLP group developed tumors 21 weeks after challenge, but the tumors were smaller that the tumors in the control animals. (B) L2 was in a GST fusion form. Each calf was inoculated with two doses of 1 mg GST-L2 in Freund's incomplete adjuvant delivered 4 weeks apart. Challenge virus was delivered 2 weeks after the second inoculation. Calves were examined for tumors 8 weeks after challenge. (C) L2a is the N terminus of L2, comprising residues 11 to 200, in a GST fusion form; peptide 11 (Pep11) corresponds to amino acids (aa) 101 to 120; Pep14 to aa 131 to 151, and Pep16 to aa 151 to 170 of BPV-4 L2. Pep11 + Pep14 + Pep16 is an equimolar mixture of the three peptides. All peptides were conjugated to keyhole limpet hemocyanin (KLH). Each calf was inoculated with two doses of 50 μg of peptide in Alhydrogel 4 weeks apart. In the case of the Pep11 + Pep14 + Pep16 vaccine, each peptide was present at 50 μg. Challenge virus was delivered 2 weeks after the second inoculation. Calves were examined for tumors 10 weeks after challenge. In all panels, the controls are unvaccinated calves. See text for details. (Reprinted from references 16 and 17 and 54 with permission of the publishers.)
HUMAN VLP VACCINES
Two versions of L1-only HPV VLP vaccines are now available in a number of countries through the various health service systems or privately. One version, Gardasil, developed and marketed by Merck Sharp & Dohme, comprises VLPs of the four major mucosal HPVs: HPV type 16 (HPV-16) and HPV-18 (the primary causes of cervical cancer) and HPV-6 and HPV-11 (the causes of genital warts). The other version, Cervarix, developed and marketed by GlaxoSmithKline (GSK), comprises VLPs of HPV-16 and HPV-18. Both vaccines have proven nearly 100% effective in protecting against the HPV types targeted by each vaccine in clinical trials that included hundreds of thousands of women and were fully licensed in 2006 (72). The vaccines are targeted to adolescent girls, with catch-up programs for older girls. The precise ages of the targeted girls vary from country to country; for instance, the ages are 12 and 13 years with catch up to 18 years in Great Britain (http://www.fightcervicalcancer.org.uk/cervical-cancer/index.aspx) and 11 and 12 years with a recommendation for vaccination from ages 13 to 26 years in the United States (http://www.cancer.gov/cancertopics/types/cervical/). Despite these differences, it is clear that vaccination should ideally be performed before the onset of sexual activity, as neither vaccine has demonstrated therapeutic activity for preexisting infections.
The vaccine is not only efficacious but also safe; infrequent serious adverse events and deaths have been reported after vaccination, but data to date have not causally linked these to the vaccine, although surveillance continues (66) (http://www.nhs.uk/Conditions/HPV-vaccination/Pages/Sideeffects.aspx and http://www.cdc.gov/vaccinesafety/updates/hpv_faqs.htm).
Despite their undeniable success, there are drawbacks to the present VLP vaccines. As shown for the various types of BPVs (49) (Table 1), VLP vaccines are highly effective against the virus types from which the L1 was derived, but their efficacy against other types is variable, depending in part upon phylogenetic similarity (68). Thus, the current vaccinees will be protected against the two most common oncogenic types found in cervical cancer, HPV-16 and HPV-18, but not against all high-risk mucosal HPVs. It is clear that a multivalent vaccine that protects against a multitude of HPVs is desirable, and efforts to develop a nine-type L1 VLP combination vaccine are ongoing. Another drawback is that the production of VLPs occurs in eukaryotic cells, and therefore the vaccine is very expensive. Production of L1 pentamers in bacteria (78) may provide a cheaper alternative to VLPs. Furthermore, vaccine distribution requires a cold chain. These last two points make the vaccine unlikely to be adopted widely in the near term by developing countries, where it is most needed because of their lack of cytologic screening programs.
L2 VACCINE
An alternative, or addition, to VLP vaccines may be immunization against the minor capsid protein L2. Vaccination of naïve cattle with BPV-4 L2 in a GST fusion form was highly protective against challenge with the virus (Fig. 2B) (16). Protection was long lived (at least 1 year when an alum adjuvant was used), and L2 vaccination elicited the production of neutralizing antibodies and the generation of memory cells capable of responding to challenge years after the original vaccination (40, 62). Similar results were observed in the rabbit model (22, 32, 59).
Neutralizing determinants of BPV-4 L2 were mapped to 30 amino acids within the N terminus of L2 (Fig. 2C) (17, 18, 56), and the antibodies elicited by this peptide are capable of recognizing the equivalent peptides of HPV-16 and HPV-6 L2 (17). Indeed, antibodies raised against bacterially expressed L2 of HPV-6, -16 or -18 neutralize not only the homologous virus type but also heterologous types (69). The cross-neutralizing epitope(s) resides in the N terminus of L2 (65), as is the case for BPV-1 L2, and cross-neutralization extends to many other genital HPV types, to cutaneous HPV types (38), and to BPV and CRPV (65). Cross-neutralization is due to the extensive homology of the N terminus of L2, and in particular of the neutralizing epitope, across many PV types from different species (38). Taken together, the protection studies in cows and rabbits described above and in vitro neutralization assays strongly suggest that, unlike VLP-based vaccines, L2 vaccines might provide broad spectrum protection.
Such a suggestion is strengthened by two important in vivo observations. Rabbits vaccinated with the N terminus of HPV-16 L2 were immune to infection by CRPV or rabbit oral papillomavirus (ROPV) (37), an impressive finding, as these three viruses are evolutionarily distant. Second, human volunteers vaccinated with the candidate prophylactic/therapeutic vaccine HPV-16 L2E6E7 fusion protein (33, 52, 77) induced L2-specific antibodies that neutralized a divergent type of HPV (36). Another potential benefit of a single cross-protective antigen generated in bacteria is cheaper production compared to that of highly multivalent VLP vaccines produced in yeast or insect cells.
Given that in infected unvaccinated animals, the levels of L2 antibody titers are below detection (16) and that many L2 epitopes are not on the virus surface (60), the question arises of how antibodies against the N terminus of L2 can neutralize virus. The question was addressed by the elegant work of Day and colleagues (27). The L2-neutralizing epitope defined by monoclonal antibody RG-1 is internal to the capsid; however, it becomes exposed when the virus binds to the cell surface and changes its conformation. Infection requires the removal of an L2 N-terminal sequence by the cellular protease furin, and this process is associated with binding and entry of keratinocytes (28). Furin cleavage renders L2 accessible on the capsid surface and displays the RG-1 epitope. The binding of the anti-L2 antibodies to the exposed L2 epitope(s) blocks virus transfer from the extracellular matrix to the cell surface (27) and hence prevents infection (28).
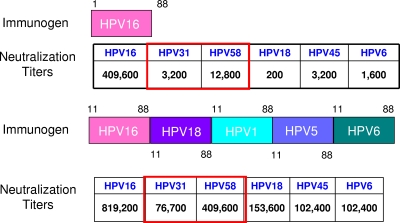
Despite the great promise of L2 as a broadly neutralizing prophylactic vaccine, the titers of neutralizing antibody produced against L2 are rather low compared to those produced against L1 VLPs (69). How is it possible to boost the L2-specific titers, and in what form should an L2 vaccine be delivered? One option is to insert the L2-neutralizing epitope on the surfaces of VLPs, thus increasing the titers of neutralizing antibodies approximately 10-fold (74). However, this solution does not solve the problem of the expensive production of VLPs. Possible alternatives could be a synthetic L2 lipopeptide in which the cross-neutralizing L2 peptide is linked to both a T-helper epitope and a ligand for TLR2 (1), tandem repeats of the same peptide displayed on bacterial thioredoxin (71), or concatenated L2 peptides from different papillomavirus types (Fig. 3) (46). In all cases, the neutralizing antibody titers are noticeably increased, while the cross-neutralization properties are maintained or enhanced.
FIG. 3.
Diagram depicting the polymeric L2 vaccine concept. (Courtesy of D. Lowy, reproduced with permission.). In vitro neutralization data for hyperimmune sera from rabbits vaccinated with each immunogen in Freund's adjuvant as described in Jagu et al. (46) are presented. Vaccination with the N terminus of L2 is protective against diverse papillomavirus types in association with the induction of serum antibodies that neutralize diverse papillomavirus types at low titers and the homologous type (i.e., HPV-16) at higher titers (top panel). The polymeric immunogen (bottom panel) is formed by concatenation of the N termini of L2 polypeptides derived from multiple diverse papillomavirus types. This concatenation of L2 of diverse types results in the repetitive display of epitopes with sequences conserved among types but not the type-specific epitopes. Repetitive display of B-cell epitopes is associated with enhanced antibody production. Indeed, this polymeric L2 approach results in antisera that neutralize at higher titers not only the types included in the multimeric immunogen but also other types (i.e., HPV-31 and HPV-58, boxed in red) and is associated with both a more robust and more broadly protective immune response than a monomeric L2 antigen.
In conclusion, the L1 VLP vaccines are very effective in preventing new infections by the two most common oncogenic HPV types and, if widely and properly used, will dramatically reduce the rates of HPV-associated cancer over time. However, there is a perceived need for new broad-spectrum vaccines capable of protecting against the many other HPV types involved in cervical cancer and of being produced more economically. The discovery of a causal role for oncogenic HPV in a subset of head and neck cancers suggests that HPV vaccines could potentially have an impact beyond the prevention of genital warts and cancers. We believe that L2 peptides, properly delivered, are excellent candidates for a low-cost broad spectrum protective vaccine.
There are also a number of other candidate low-cost approaches for the delivery of L1 antigen, such as L1 capsomer vaccines (70, 78), and even needle-free approaches such as live L1-recombinant Salmonella enterica serovar Typhimurium or Typhi (5, 34), although multivalent formulations would still be required for broad protection. Local production in emerging economies may also provide an additional mechanism to drive down the cost of manufacture so that those most in need of HPV vaccines have the opportunity to receive them. While L1- and L2-based vaccines do elicit cellular immune responses, there is no clear evidence to date that they have an impact on the clinical course of preexisting HPV disease (72). This highlights the potential and continued need for development of therapeutic HPV vaccines for those currently afflicted with HPV-associated precancer and cancer.
Acknowledgments
The authors gratefully acknowledge the support of grants to M.S.C. from Cancer Research UK, of which she is a Fellow, and to R.B.S.R. from the National Institutes of Health (P50 CA098252, RO1 CA118790, and RO1 CA133749) and the American Cancer Society (RSG-08-116-01-CCE). M.S.C. is an inventor of patents for the use of papillomavirus L2 protein and L2 peptides. The patents are managed by Cancer Research Technology. R.B.S.R. has served as a paid consultant of Merck & Co., and R.B.S.R.'s student has received unrestricted educational grant funding from GSK. R.B.S.R. is an inventor of L2 patents licensed to Shantha Biotechnics, PaxVax, Inc., and Acambis, Inc. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
We thank Doug Lowy for his artistic contribution and intellectual input.
Footnotes
Published ahead of print on 11 November 2009.
REFERENCES
- 1.Alphs, H. H., R. Gambhira, B. Karanam, J. N. Roberts, S. Jagu, J. T. Schiller, W. Zeng, D. C. Jackson, and R. B. S. Roden. 2008. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc. Natl. Acad. Sci. U.S.A. 105:5850-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonsson, A., E. Payne, K. Hengst, and N. A. McMillan. 2006. The human papillomavirus type 16 E7 protein binds human interferon regulatory factor-9 via a novel PEST domain required for transformation. J. Interferon Cytokine Res. 26:455-461. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafi, G. H., D. R. Brown, K. H. Fife, and M. S. Campo. 2006. Down-regulation of MHC class I is a property common to papillomavirus E5 proteins Virus Res. 120:208-211. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafi, G. H., H. Mohammad, B. Marchetti, and M. S. Campo. 2006. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 119:2105-2112. [DOI] [PubMed] [Google Scholar]
- 5.Baud, D., F. Ponci, M. Bobst, P. De Grandi, and D. Nardelli-Haefliger. 2004. Improved efficiency of a Salmonella-based vaccine against human papillomavirus type 16 virus-like particles achieved by using a codon-optimized version of L1. J. Virol. 78:12901-12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop, B., J. Dasgupta, M. Klein, R. L. Garcea, N. D. Christensen, R. Zhao, and X. S. Chen. 2007. Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies. J. Biol. Chem. 282:31803-31811. [DOI] [PubMed] [Google Scholar]
- 7.Booy, F. P., R. B. Roden, H. L. Greenstone, J. T. Schiller, and B. L. Trus. 1998. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 Å resolution. J. Mol. Biol. 281:95-106. [DOI] [PubMed] [Google Scholar]
- 8.Borzacchiello, G., F. Roperto, L. Nasir, and M. S. Campo. 2009. Human papillomavirus research: do we still need animal models? Int. J. Cancer 125:739-740. [DOI] [PubMed] [Google Scholar]
- 9.Brandsma, J. L., M. Shlyankevich, Y. Su, D. Zelterman, J. K. Rose, and L. Buonocore. 14 July 2009, posting date. Reversal of papilloma growth in rabbits therapeutically vaccinated against E6 with naked DNA and/or vesicular stomatitis virus vectors. Vaccine [Epub ahead of print.] doi: 10.1016/j.vaccine.2009.04.082. [DOI] [PMC free article] [PubMed]
- 10.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck, C. B., N. Cheng, C. D. Thompson, D. R. Lowy, A. C. Steven, J. T. Schiller, and B. L. Trus. 2008. Arrangement of L2 within the papillomavirus capsid. J. Virol. 82:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campo, M. 2003. Papillomavirus and disease in humans and animals. Vet. Comp. Oncol. 1:3-14. [DOI] [PubMed] [Google Scholar]
- 13.Campo, M. S. 2002. Animal models of papillomavirus pathogenesis. Virus Res. 89:249-261. [DOI] [PubMed] [Google Scholar]
- 14.Campo, M. S. 2006. Papillomavirus research: from natural history to vaccine and beyond. Caister Academic Press, Norfolk, United Kingdom.
- 15.Campo, M. S. 1997. Vaccination against papillomavirus in cattle. Clin. Dermatol. 15:275-283. [DOI] [PubMed] [Google Scholar]
- 16.Campo, M. S., G. J. Grindlay, B. W. O'Neil, L. M. Chandrachud, G. M. McGarvie, and W. F. Jarrett. 1993. Prophylactic and therapeutic vaccination against a mucosal papillomavirus J. Gen. Virol. 74:945-953. [DOI] [PubMed] [Google Scholar]
- 17.Campo, M. S., B. W. O'Neil, G. J. Grindlay, F. Curtis, G. Knowles, and L. Chandrachud. 1997. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology 234:261-266. [DOI] [PubMed] [Google Scholar]
- 18.Chandrachud, L. M., G. J. Grindlay, G. M. McGarvie, B. W. O'Neil, E. R. Wagner, W. F. Jarrett, and M. S. Campo. 1995. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 211:204-208. [DOI] [PubMed] [Google Scholar]
- 19.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 20.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 21.Christensen, N. D., and J. W. Kreider. 1990. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J. Virol. 64:3151-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen, N. D., J. W. Kreider, N. C. Kan, and S. L. DiAngelo. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181:572-579. [DOI] [PubMed] [Google Scholar]
- 23.Christensen, N. D., C. A. Reed, N. M. Cladel, R. Han, and J. W. Kreider. 1996. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 70:960-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Culp, T. D., L. R. Budgeon, M. P. Marinkovich, G. Meneguzzi, and N. D. Christensen. 2006. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruses by binding virions and transferring them to adjacent cells. J. Virol. 80:8940-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danos, O., L. W. Engel, E. Y. Chen, M. Yaniv, and P. M. Howley. 1983. Comparative analysis of the human type 1a and bovine type 1 papillomavirus genomes. J. Virol. 46:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davy, C., and J. Doorbar. 2007. G2/M cell cycle arrest in the life cycle of viruses. Virology 368:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day, P. M., R. Gambhira, R. B. S. Roden, D. R. Lowy, and J. T. Schiller. 2008. Mechanisms of human papillomavirus type 16 neutralization by L2 cross-neutralizing and L1 type-specific antibodies. J. Virol. 82:4638-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day, P. M., D. R. Lowy, and J. T. Schiller. 2008. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. J. Virol. 82:12565-12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day, P. M., C. D. Thompson, C. B. Buck, Y. Y. Pang, D. R. Lowy, and J. T. Schiller. 2007. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J. Virol. 81:8784-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Villiers, E.-M., C. Fauquet, T. R. Broker, H.-U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 324:17-27. [DOI] [PubMed] [Google Scholar]
- 31.Doorbar, J. 2005. The papillomavirus life cycle. J. Clin. Virol. 32(Suppl.):S7-S15. [DOI] [PubMed] [Google Scholar]
- 32.Embers, M. E., L. R. Budgeon, M. Pickel, and N. D. Christensen. 2002. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J. Virol. 76:9798-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiander, A. N., A. J. Tristram, E. J. Davidson, A. E. Tomlinson, S. Man, P. J. Baldwin, J. C. Sterling, and H. C. Kitchener. 2006. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int. J. Gynecol. Cancer 16:1075-1081. [DOI] [PubMed] [Google Scholar]
- 34.Fraillery, D., D. Baud, S. Y. Pang, J. Schiller, M. Bobst, N. Zosso, F. Ponci, and D. Nardelli-Haefliger. 2007. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin. Vaccine Immunol. 14:1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frazer, I. H., R. Thomas, J. Zhou, G. R. Leggatt, L. Dunn, N. McMillan, R. W. Tindle, L. Filgueira, P. Manders, P. Barnard, and M. Sharkey. 1999. Potential strategies utilised by papillomavirus to evade host immunity. Immunol. Rev. 168:131-142. [DOI] [PubMed] [Google Scholar]
- 36.Gambhira, R., P. E. Gravitt, I. Bossis, P. L. Stern, R. P. Viscidi, and R. B. S. Roden. 2006. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 66:11120-11124. [DOI] [PubMed] [Google Scholar]
- 37.Gambhira, R., S. Jagu, B. Karanam, P. E. Gravitt, T. D. Culp, N. D. Christensen, and R. B. S. Roden. 2007. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J. Virol. 81:11585-11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gambhira, R., B. Karanam, S. Jagu, J. N. Roberts, C. B. Buck, I. Bossis, H. Alphs, T. Culp, N. D. Christensen, and R. B. S. Roden. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 81:13927-13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcea, R. L., and X. Chen. 2007. Papillomavirus structure and assembly, p. 69-88. In R. L. Garcea and D. DiMaio (ed.) The papillomaviruses. Springer, New York, NY.
- 40.Gaukroger, J. M., L. M. Chandrachud, B. W. O'Neil, G. J. Grindlay, G. Knowles, and M. S. Campo. 1996. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J. Gen. Virol. 77:1577-1583. [DOI] [PubMed] [Google Scholar]
- 41.Hä fner, N., C. Driesch, M. Gajda, L. Jansen, R. Kirchmayr, I. B. Runnebaum, and M. Durst. 2007. Integration of the HPV16 genome does not invariably result in high levels of viral oncogene transcripts. Oncogene 27:1610-1617. [DOI] [PubMed] [Google Scholar]
- 42.Hagensee, M. E., N. H. Olson, T. S. Baker, and D. A. Galloway. 1994. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J. Virol. 68:4503-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han, R., N. M. Cladel, C. A. Reed, X. Peng, and N. D. Christensen. 1999. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J. Virol. 73:7039-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howie, H. L., R. A. Katzenellenbogen, and D. A. Galloway. 2009. Papillomavirus E6 proteins. Virology 384:324-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung, C. F., T. C. Wu, A. Monie, and R. Roden. 2008. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol. Rev. 222:43-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagu, S., B. Karanam, R. Gambhira, S. V. Chivukula, R. J. Chaganti, D. R. Lowy, J. T. Schiller, and R. B. S. Roden. 2009. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J. Natl. Cancer Inst. 101:782-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarrett, W. F., J. Murphy, B. W. O'Neil, and H. M. Laird. 1978. Virus-induced papillomas of the alimentary tract of cattle. Int. J. Cancer 22:323-328. [DOI] [PubMed] [Google Scholar]
- 48.Jarrett, W. F., K. T. Smith, B. W. O'Neil, J. M. Gaukroger, L. M. Chandrachud, G. J. Grindlay, G. M. McGarvie, and M. S. Campo. 1991. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology 184:33-42. [DOI] [PubMed] [Google Scholar]
- 49.Jarrett, W. F. H., B. W. O'Neil, J. M. Gaukroger, K. T. Smith, H. M. Laird, and M. S. Campo. 1990. Studies on vaccination against papillomaviruses: the immunity after infection and vaccination with bovine papillomaviruses of different types. Vet. Rec. 126:473-475. [PubMed] [Google Scholar]
- 50.Jarrett, W. F. H., B. W. O'Neil, J. M. Gaukroger, H. M. Laird, K. T. Smith, and M. S. Campo. 1990. Studies on vaccination against papillomaviruses: a comparison of purified virus, tumour extract and transformed cells in prophylactic vaccination. Vet. Rec. 126:449-452. [PubMed] [Google Scholar]
- 51.Johnson, K. M., R. C. Kines, J. N. Roberts, D. R. Lowy, J. T. Schiller, and P. M. Day. 2009. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J. Virol. 83:2067-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karanam, B., R. Gambhira, S. Peng, S. Jagu, D.-J. Kim, G. W. Ketner, P. L. Stern, R. J. Adams, and R. B. S. Roden. 2009. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine 27:1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. U. S. A. 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirnbauer, R., L. M. Chandrachud, B. W. O'Neil, E. R. Wagner, G. J. Grindlay, A. Armstrong, G. M. McGarvie, J. T. Schiller, D. R. Lowy, and M. S. Campo. 1996. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 219:37-44. [DOI] [PubMed] [Google Scholar]
- 55.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knowles, G., G. J. Grindlay, M. S. Campo, L. M. Chandrachud, and B. W. O'Neil. 1997. Linear B-cell epitopes in the N-terminus of L2 of bovine papillomavirus type 4. Res. Vet. Sci. 62:289-291. [DOI] [PubMed] [Google Scholar]
- 57.Knowles, G., B. W. O'Neil, and M. S. Campo. 1996. Phenotypical characterization of lymphocytes infiltrating regressing papillomas. J. Virol. 70:8451-8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leong, C. M., J. Doorbar, I. Nindl, H.-S. Yoon, and M. H. Hibma. 17 September 2009. Loss of epidermal Langerhans cells occurs in human papillomavirus α, γ, and μ but not β genus infections. J. Investig. Dermatol. [Epub ahead of print.] doi: 10.1038/jid.2009.266. [DOI] [PubMed]
- 59.Lin, Y. L., L. A. Borenstein, R. Selvakumar, R. Ahmed, and F. O. Wettstein. 1992. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 187:612-619. [DOI] [PubMed] [Google Scholar]
- 60.Liu, W. J., L. Gissmann, X. Y. Sun, A. Kanjanahaluethai, M. Muller, J. Doorbar, and J. Zhou. 1997. Sequence close to the N-terminus of L2 protein is displayed on the surface of bovine papillomavirus type 1 virions. Virology 227:474-483. [DOI] [PubMed] [Google Scholar]
- 61.Matthews, K., C. M. Leong, L. Baxter, E. Inglis, K. Yun, B. T. Backstrom, J. Doorbar, and M. Hibma. 2003. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down Regulation of E-cadherin. J. Virol. 77:8378-8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGarvie, G. M., L. M. Chandrachud, J. Gaukroger, G. J. Grindlay, B. W. O'Neil, J. W. Baird, E. R. Wagner, W. F. H. Jarrett, and M. S. Campo. 1994. Vaccination of cattle with L2 protein prevents BPV-4 infection, p. 283-290. In M. A. Stanley (ed.), Papillomavirus immunology. Plenum Publishing Corporation, New York, NY.
- 63.McLaughlin-Drubin, M. E., and K. Münger. 2009. The human papillomavirus E7 oncoprotein. Virology 384:335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson, C., D. Segre, and L. V. Skidmore. 1959. Immunity to bovine cutaneous papillomatosis produced by vaccine homologous to the challenge agent. J. Am. Vet. Med. Assoc. 135:499-502. [PubMed] [Google Scholar]
- 65.Pastrana, D. V., R. Gambhira, C. B. Buck, Y.-Y. S. Pang, C. D. Thompson, T. D. Culp, N. D. Christensen, D. R. Lowy, J. T. Schiller, and R. B. S. Roden. 2005. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 337:365-372. [DOI] [PubMed] [Google Scholar]
- 66.Reiter, P. L., N. T. Brewer, S. L. Gottlieb, A. L. McRee, and J. S. Smith. 16 September 2009, posting date. How much will it hurt? HPV vaccine side effects and influence on completion of the three-dose regimen. Vaccine 27:6840-6844. [Epub ahead of print.] doi: 10.1016/j.vaccine.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts, S., S. R. Kingsbury, K. Stoeber, G. L. Knight, P. H. Gallimore, and G. H. Williams. 2008. Identification of an arginine-rich motif in human papillomavirus type 1 E1Ê4 protein necessary for E4-mediated inhibition of cellular DNA synthesis in vitro and in cells. J. Virol. 82:9056-9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roden, R., and T. C. Wu. 2006. How will HPV vaccines affect cervical cancer? Nat. Rev. Cancer 6:753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roden, R. B. S., W. H. Yutzy, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270:254-257. [DOI] [PubMed] [Google Scholar]
- 70.Rose, R. C., W. I. White, M. Li, J. A. Suzich, C. Lane, and R. L. Garcea. 1998. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J. Virol. 72:6151-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubio, I., A. Bolchi, N. Moretto, E. Canali, L. Gissmann, M. Tommasino, M. Müller, and S. Ottonello. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20-38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949-1956. [DOI] [PubMed] [Google Scholar]
- 72.Schiller, J. T., X. Castellsagué, L. L. Villa, and A. Hildesheim. 2008. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 26(Suppl. 10):K53-K61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shafti-Keramat, S., C. Schellenbacher, A. Handisurya, N. Christensen, B. Reininger, S. Brandt, and R. Kirnbauer. 3 September 2009, posting date. Bovine papillomavirus type 1 (BPV1) and BPV2 are closely related serotypes. Virology 393:1-6. [Epub ahead of print.] doi: 10.1016/j.virol.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slupetzky, K., R. Gambhira, T. D. Culp, S. Shafti-Keramat, C. Schellenbacher, N. D. Christensen, R. B. S. Roden, and R. Kirnbauer. 2007. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine 25:2001-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. U. S. A. 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Talbert-Slagle, K., and D. DiMaio. 2009. The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango. Virology 384:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Burg, S. H., K. M. C. Kwappenberg, T. O'Neill, R. M. P. Brandt, C. J. M. Melief, J. K. Hickling, and R. Offringa. 2001. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine 19:3652-3660. [DOI] [PubMed] [Google Scholar]
- 78.Yuan, H., P. A. Estes, Y. Chen, J. Newsome, V. A. Olcese, R. L. Garcea, and R. Schlegel. 2001. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J. Virol. 75:7848-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou, J., D. J. Stenzel, X.-Y. Sun, and I. H. Frazer. 1993. Synthesis and assembly of infectious bovine papillomavirus particles in vitro. J. Gen. Virol. 74:763-768. [DOI] [PubMed] [Google Scholar]
- 80.Zhou, J., X. Y. Sun, D. J. Stenzel, and I. H. Frazer. 1991. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 185:251-257. [DOI] [PubMed] [Google Scholar]
- 81.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nature Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]