Abstract
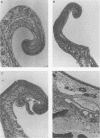
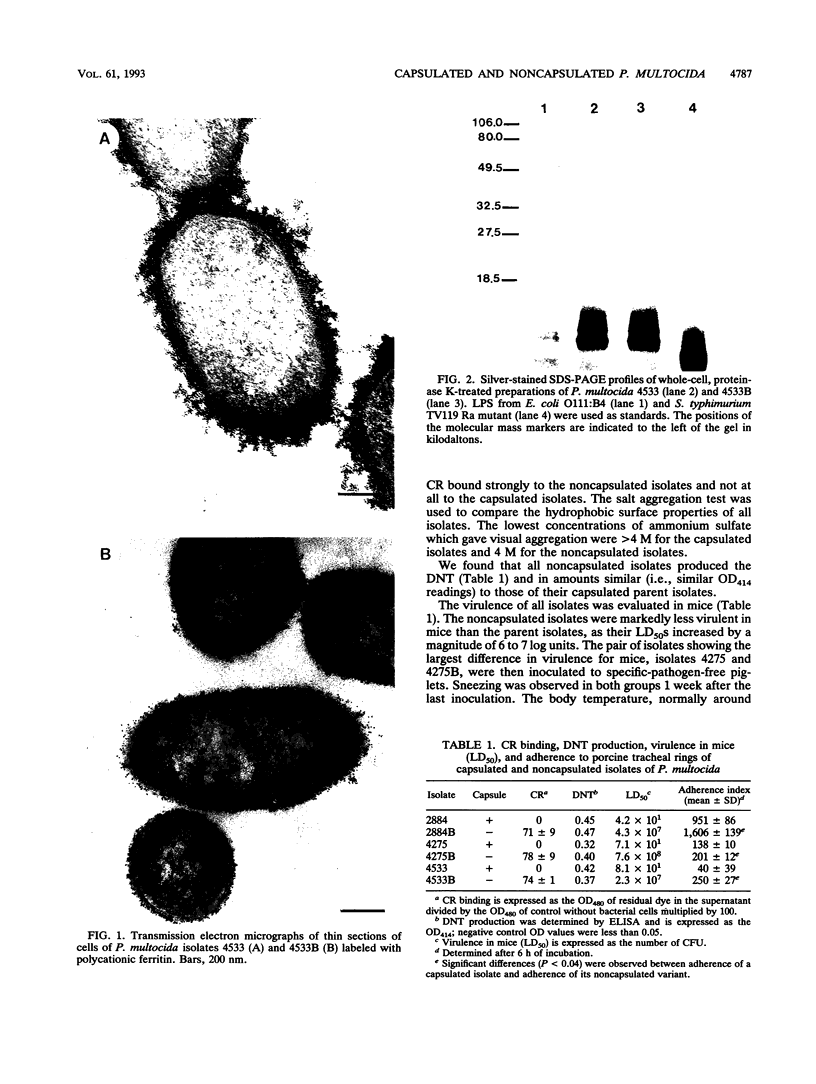
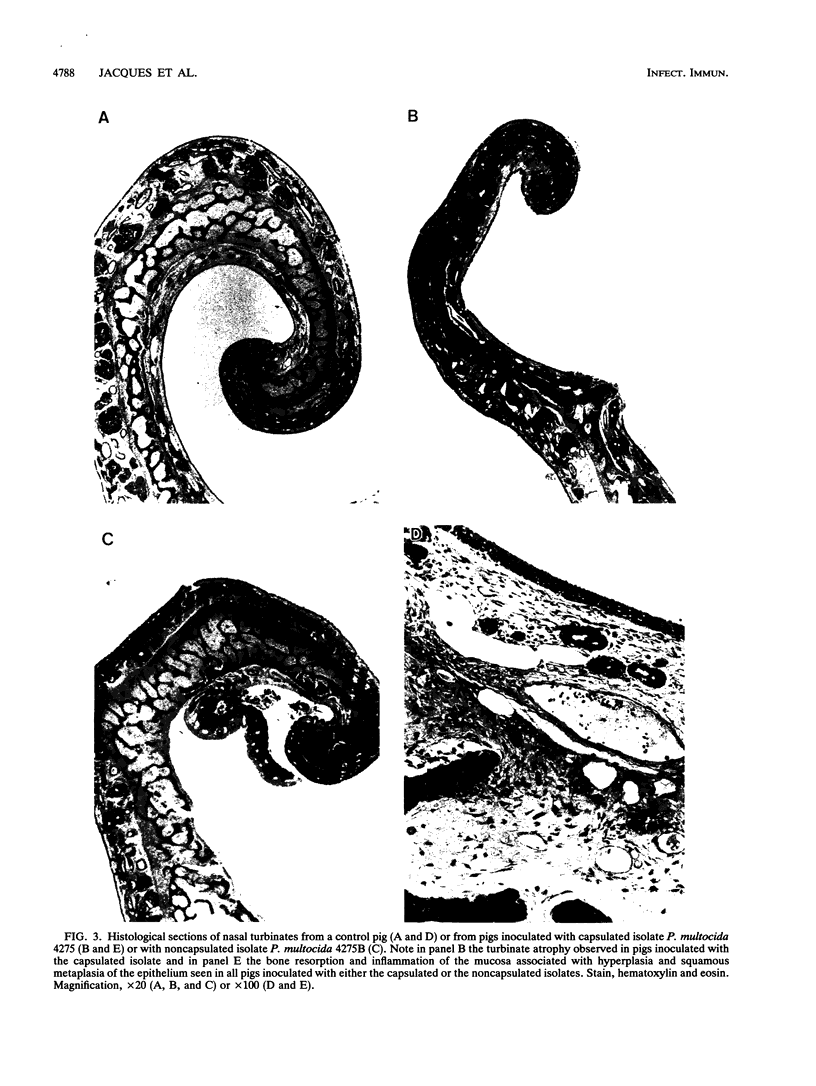
The virulence and the adherence to porcine respiratory tract cells and mucus of three toxigenic, capsular type D Pasteurella multocida isolates and their noncapsulated variants were evaluated in the present study. Loss of capsule by P. multocida, verified by transmission electron microscopy after polycationic ferritin labeling, was associated with a massive reduction in virulence of the organisms in mice. Specific-pathogen-free piglets inoculated intranasally with one of the capsulated isolates or its noncapsulated variant developed turbinate lesions characterized by bone resorption and by an inflammation of the mucosa associated with hyperplasia and squamous metaplasia of the epithelium. Infection with the capsulated isolate led to more severe lesions and atrophy of turbinates. The interactions of these P. multocida isolates with porcine respiratory tract cells and mucus were studied in vitro. The presence of capsule resulted in a decrease in binding of respiratory tract mucus were studied in vitro. The presence of capsule resulted in a decrease in binding of respiratory tract mucus to P. multocida isolates as determined by a dot blot assay. The presence of capsule also resulted in a significant decrease in adherence to porcine tracheal rings maintained in culture. The capsule seemed to mask outer membrane components which are involved in adherence. One of these components might be lipopolysaccharide since purified lipopolysaccharide bound respiratory tract mucus and blocked adherence of this microorganism to porcine tracheal rings. Our data indicate that capsular material does not seem to be involved in adherence of P. multocida to respiratory tract cells and mucus, but capsulated isolates are more virulent in mice and also in piglets.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard B., Vena M. M., Cavalier A., Le Lannic J., Gouranton J., Kobisch M. Electron microscopic observation of the respiratory tract of SPF piglets inoculated with Mycoplasma hyopneumoniae. Vet Microbiol. 1992 Mar;30(4):329–341. doi: 10.1016/0378-1135(92)90020-t. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Rioux S., Foiry B., Jacques M. Affinity for porcine respiratory tract mucus is found in some isolates of Actinobacillus pleuropneumoniae. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):119–125. doi: 10.1111/j.1574-6968.1992.tb05450.x. [DOI] [PubMed] [Google Scholar]
- Bötcher L., Lübke A., Hellmann E. In vitro binding of Pasteurella multocida cell wall preparations to tracheal mucus of cattle and swine and to a tracheal epithel cell wall preparation of cattle. Zentralbl Veterinarmed B. 1991 Dec;38(10):721–730. doi: 10.1111/j.1439-0450.1991.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Chanter N. Molecular aspects of the virulence of Pasteurella multocida. Can J Vet Res. 1990 Apr;54 (Suppl):S45–S47. [PubMed] [Google Scholar]
- Choi-Kim K., Maheswaran S. K., Felice L. J., Molitor T. W. Relationship between the iron regulated outer membrane proteins and the outer membrane proteins of in vivo grown Pasteurella multocida. Vet Microbiol. 1991 Jun;28(1):75–92. doi: 10.1016/0378-1135(91)90100-t. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugal F., Bélanger M., Jacques M. Enhanced adherence of Pasteurella multocida to porcine tracheal rings preinfected with Bordetella bronchiseptica. Can J Vet Res. 1992 Jul;56(3):260–264. [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W. Virulence attributes of the liposaccharides of the HAP group organisms. Can J Vet Res. 1990 Apr;54 (Suppl):S28–S32. [PubMed] [Google Scholar]
- Foged N. T., Nielsen J. P., Pedersen K. B. Differentiation of toxigenic from nontoxigenic isolates of Pasteurella multocida by enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Jul;26(7):1419–1420. doi: 10.1128/jcm.26.7.1419-1420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M., Jacques M. Hemagglutination by Pasteurella multocida of porcine origin. J Clin Microbiol. 1987 May;25(5):938–939. doi: 10.1128/jcm.25.5.938-939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frymus T., Wittenbrink M. M., Petzoldt K. Failure to demonstrate adherence of Pasteurella multocida involved in atrophic rhinitis to swine nasal epithelial cells. Zentralbl Veterinarmed B. 1986 Mar;33(2):140–144. doi: 10.1111/j.1439-0450.1986.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Harmon B. G., Glisson J. R., Latimer K. S., Steffens W. L., Nunnally J. C. Resistance of Pasteurella multocida A:3,4 to phagocytosis by turkey macrophages and heterophils. Am J Vet Res. 1991 Sep;52(9):1507–1511. [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Bélanger M., Roy G., Foiry B. Adherence of Actinobacillus pleuropneumoniae to porcine tracheal epithelial cells and frozen lung sections. Vet Microbiol. 1991 Apr;27(2):133–143. doi: 10.1016/0378-1135(91)90004-y. [DOI] [PubMed] [Google Scholar]
- Jacques M., Foiry B. Electron microscopic visualization of capsular material of Pasteurella multocida types A and D labeled with polycationic ferritin. J Bacteriol. 1987 Aug;169(8):3470–3472. doi: 10.1128/jb.169.8.3470-3472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Parent N., Foiry B. Adherence of Bordetella bronchiseptica and Pasteurella multocida to porcine nasal and tracheal epithelial cells. Can J Vet Res. 1988 Apr;52(2):283–285. [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Trust T. J. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida. J Bacteriol. 1985 Dec;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letellier A., Dubreuil D., Roy G., Fairbrother J. M., Jacques M. Determination of affinity of Pasteurella multocida isolates for porcine respiratory tract mucus, and partial characterization of the receptors. Am J Vet Res. 1991 Jan;52(1):34–39. [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Morioka H., Tachibana M., Machino M., Suganuma A. Polymyxin B binding sites in Escherichia coli as revealed by polymyxin B-gold labeling. J Histochem Cytochem. 1987 Feb;35(2):229–231. doi: 10.1177/35.2.3025293. [DOI] [PubMed] [Google Scholar]
- Nakai T., Kume K., Yoshikawa H., Oyamada T., Yoshikawa T. Adherence of Pasteurella multocida or Bordetella bronchiseptica to the swine nasal epithelial cell in vitro. Infect Immun. 1988 Jan;56(1):234–240. doi: 10.1128/iai.56.1.234-240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijoan C., Trigo F. Bacterial adhesion to mucosal surfaces with special reference to Pasteurella multocida isolates from atrophic rhinitis. Can J Vet Res. 1990 Apr;54 (Suppl):S16–S21. [PubMed] [Google Scholar]
- Rimler R. B., Brogden K. A. Pasteurella multocida isolated from rabbits and swine: serologic types and toxin production. Am J Vet Res. 1986 Apr;47(4):730–737. [PubMed] [Google Scholar]
- Rimler R. B. Comparisons of Pasteurella multocida lipopolysaccharides by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to determine relationship between group B and E hemorrhagic septicemia strains and serologically related group A strains. J Clin Microbiol. 1990 Apr;28(4):654–659. doi: 10.1128/jcm.28.4.654-659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W. Capsule reduces adherence of enterotoxigenic Escherichia coli to isolated intestinal epithelial cells of pigs. Infect Immun. 1984 Sep;45(3):737–740. doi: 10.1128/iai.45.3.737-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush H. G. Resistance of some capsular serotype D strains of Pasteurella multocida to rabbit polymorphonuclear neutrophil phagocytosis. Vet Microbiol. 1989 May;20(1):79–87. doi: 10.1016/0378-1135(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Rutter J. M. Atrophic rhinitis in swine. Adv Vet Sci Comp Med. 1985;29:239–279. [PubMed] [Google Scholar]
- Snipes K. P., Hansen L. M., Hirsh D. C. Plasma- and iron-regulated expression of high molecular weight outer membrane proteins by Pasteurella multocida. Am J Vet Res. 1988 Aug;49(8):1336–1338. [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect Immun. 1991 Apr;59(4):1325–1333. doi: 10.1128/iai.59.4.1325-1333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo E., Pijoan C. Presence of pili in Pasteurella multocida strains associated with atrophic rhinitis. Vet Rec. 1988 Jan 2;122(1):19–19. doi: 10.1136/vr.122.1.19. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vena M. M., Blanchard B., Thomas D., Kobisch M. Adherence of Pasteurella multocida isolated from pigs and relationship with capsular type and dermonecrotic toxin production. Ann Rech Vet. 1991;22(2):211–218. [PubMed] [Google Scholar]
- Virji M., Kayhty H., Ferguson D. J., Alexandrescu C., Moxon E. R. Interactions of Haemophilus influenzae with cultured human endothelial cells. Microb Pathog. 1991 Mar;10(3):231–245. doi: 10.1016/0882-4010(91)90057-h. [DOI] [PubMed] [Google Scholar]
- Whiteley L. O., Maheswaran S. K., Weiss D. J., Ames T. R. Immunohistochemical localization of Pasteurella haemolytica A1-derived endotoxin, leukotoxin, and capsular polysaccharide in experimental bovine Pasteurella pneumonia. Vet Pathol. 1990 May;27(3):150–161. doi: 10.1177/030098589002700302. [DOI] [PubMed] [Google Scholar]
- Whiteley L. O., Maheswaran S. K., Weiss D. J., Ames T. R., Kannan M. S. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J Vet Intern Med. 1992 Jan-Feb;6(1):11–22. doi: 10.1111/j.1939-1676.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]