Abstract
The immunomodulatory drug leflunomide is frequently used for treating polyomavirus-associated nephropathy, yet its antiviral mechanism is unclear. We characterized the effects of the active leflunomide metabolite A771726 (LEF-A) on the polyomavirus BK (BKV) life cycle in human renal tubular epithelial cells. LEF-A at 10 μg/ml reduced the extracellular BKV load by 90% (IC90) but with significant host cytostatic effects. BKV genome replication, late protein expression, and virion assembly and release were inhibited with visible disruption of the nuclear replication architecture. Both host cell and antiviral effects were largely reversed by uridine addition, implicating nonspecific pyrimidine depletion as the major anti-BKV mechanism of leflunomide.
Polyomavirus BK (BKV) is associated with two major diseases, hemorrhagic cystitis after bone-marrow transplantation and polyomavirus-associated nephropathy (PVAN) after kidney transplantation. PVAN arises in 1 to 10% of kidney transplant recipients due to uncontrolled BKV replication in the tubular epithelial cells, often resulting in graft loss (11, 22). Since there are no drugs with well-defined antipolyomavirus activity (23, 37), the main treatment is reduction of immunosuppression at the expense of an increased risk of rejection (21).
The active metabolite of the immunomodulatory drug leflunomide, A771726 (LEF-A), inhibitis mitochondrial dihydroorotate dehydrogenase (10), leading to pyrimidine depletion and cytostasis, particularly in activated lymphocytes (7). Tyrosine kinase (29), cyclooxygenase (18), and NF-κB signaling (15) may also be affected at higher concentrations. Leflunomide has demonstrated antiviral activity toward human immunodeficiency virus 1 (HIV-1) (38) and herpesviruses (25, 44) and is now also used in treatment of PVAN (2, 3, 6, 9, 12, 13, 24, 26, 30, 34, 39, 41, 47), although its clinical efficacy has not been formally tested in controlled trials. For herpesviruses, the antiviral effect is attributed to impaired nucleocapsid tegumentation (25, 44). Since BKV lacks tegument, the putative antiviral effect must be different. Two previous BKV studies performed with WI-38 and Vero cells concluded that leflunomide inhibits BKV replication (14, 24), but the detailed mechanism was not investigated. Here we report on effects of LEF-A on the BKV replication cycle in primary human renal proximal tubule epithelial cells (RPTECs).
To examine the effect of LEF-A on BKV progeny production in RPTECs, LEF-A at 2.5 to 30 μg/ml was added 2 h postinfection (h.p.i.) and extracellular BKV loads were measured by quantitative PCR (qPCR) 72 h.p.i. (5). LEF-A reduced BKV loads in a concentration-dependent manner (Fig. 1A). At 10 μg/ml (∼37 μM) and 30 μg/ml (∼111 μM), the BKV load was about 1 log (92%) and 2 logs (99%) reduced, respectively. Next, assessing cytotoxicity in BKV-infected cells, we found that LEF-A at 10 μg/ml reduced cellular DNA replication (BrdU incorporation) (5) by about 50% and mitochondrial metabolic activity (WST-1 cleavage) (5) by 40% 72 h.p.i. (Fig. 1B). LEF-A at 30 μg/ml reduced cellular DNA replication by 75% and mitochondrial metabolic activity by 47%. The overall metabolic activity (resazurin reduction) was not affected by LEF-A concentrations up to 25 μg/ml. In uninfected cells, similar results were obtained. The LEF-A 90% inhibitory concentration (IC90), 10 μg/ml, was used to determine the influence on subsequent steps in the BKV life cycle.
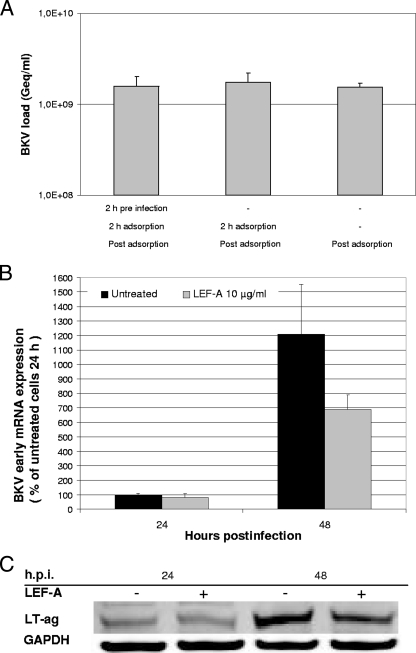
FIG. 1.
Effect of LEF-A titration on BKV load and RPTEC cytotoxicity. RPTECs (Lonza) (passage 4) were seeded in 24- or 96-well plates and supernatant infected with BKV-Dunlop at 50% confluency from Vero cells (multiplicity of infection [MOI] of 1) or left uninfected. At 2 h.p.i., virus or supernatant was removed, cells were washed, and medium with increasing LEF-A concentrations (A771726; Calbiochem) or without LEF-A was added. (A) Supernatants were harvested 72 h.p.i., and extracellular BKV loads were measured by qPCR with primers and probe targeting the LT-ag gene (5). Data are presented as Geq/ml (Geq = genome equivalents). (B) The cytotoxicity of LEF-A was monitored 72 h.p.i. by measuring cellular DNA replication by cell proliferation enzyme-linked immunosorbent assay (ELISA), BrdU (Roche Applied Science) (5), mitochondrial metabolic activity with cell proliferation reagent WST-1 (Roche Applied Science) (5), and total cellular metabolic activity (monotoring mithochondrial, microsomal, and cytosolic enzymes) with the resazurin-based assay TOX-8 (Sigma-Aldrich). For all three assays, colorimetric measurements were performed as described by the manufacturer. Absorbance for untreated cells was set as 100%.
To study the effect on viral entry, LEF-A was added: (i) 2 h before, (ii) together with, or (iii) 2 h after BKV infection. Comparing extracellular BKV loads 72 h.p.i. (Fig. 2A) or large T-antigen (LT-ag) mRNA expression 24 h.p.i. by reverse transcription (RT)-qPCR (5) (data not shown), no significant differences were found, suggesting that BKV entry is unaffected by LEF-A. To investigate the effect of LEF-A on early gene expression, first LT-ag transcripts were measured. At 24 h.p.i., the levels were barely affected, whereas at 48 h.p.i., a reduction of 43% was found (Fig. 2B). Similarly, LT-ag protein levels (5) seemed unaffected at 24 h.p.i. but 46% reduced at 48 h.p.i. (Fig. 2C). Immunofluorescence staining of LT-ag (5) 24 h.p.i. showed about the same proportion of LT-ag-stained cells in untreated and LEF-A-treated cells (Fig. 2D). At 48 h.p.i., LEF-A-treated cells were fewer and weakly stained (Fig. 2D). Nuclear DNA staining also revealed fewer cells, suggesting reduced cell proliferation. Thus, LT-ag expression was not significantly affected before the onset of BKV DNA replication.
FIG. 2.
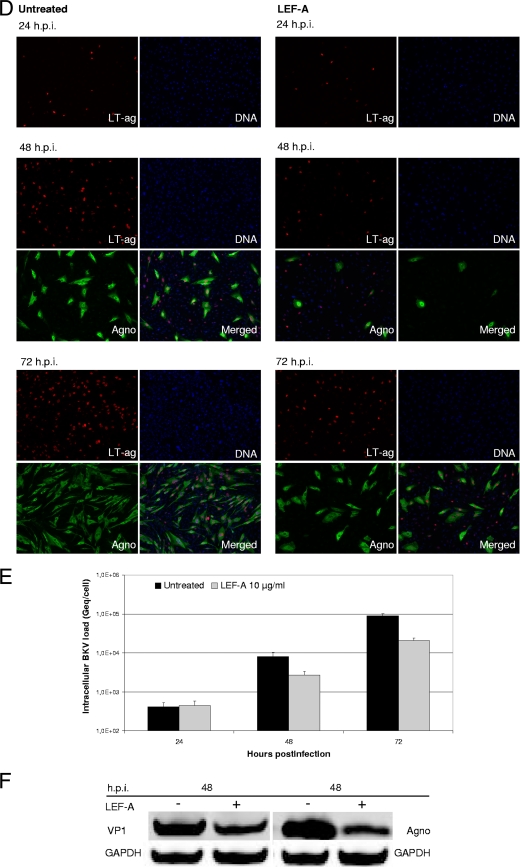
Influence of LEF-A on different steps in the BKV life cycle. RPTECs were seeded and infected as described earlier. (A) The influence of LEF-A on BKV adsorption and entry was monitored by comparing LEF-A addition 2 h before, together with, or 2 h after BKV infection. Supernatants were harvested at 72 h.p.i., and extracellular BKV loads were measured by qPCR as described. (B) LT-ag transcription was measured 24 and 48 h.p.i. Total RNA was extracted using the mirVana Paris kit (Ambion, Applied Biosystems) and treated with DNase turbo (Ambion, Applied Biosystems) before cDNA was generated from 225 ng RNA per sample using the High Capacity cDNA kit (Applied Biosystems). LT-ag transcripts were quantified by RT-qPCR and normalized to the levels of endogenous human hypoxanthine phosphoribosyltransferase (huHPRT) transcripts by the 2−ΔΔC(T) method (5, 28). Results are presented as the changes in LT-ag transcript levels, with the level in the untreated sample 24 h.p.i. arbitrarily set to 100%. (C) LT-ag protein levels at 24 and 48 h.p.i. were examined by Western blotting. RPTECs were lysed in cell disruption buffer (mirVana Paris kit; Ambion), and Western blotting was performed as described previously (5) using polyclonal rabbit anti-LT-ag serum (20) and a monoclonal antibody directed against the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam). The secondary antibodies used were IRDye800CW goat anti-rabbit IgG (Rockland) and Alexa Fluor 680 goat anti-mouse IgG (Invitrogen). (D) Early and late protein expression was investigated by indirect immunofluorescence staining. RPTECs were methanol fixed 24, 48, and 72 h.p.i., blocked with 3% goat serum in phosphate-buffered saline (PBS) for 30 min, and then treated as described earlier (27). The primary antibodies, SV40 LT-ag monoclonal (Pab416; Calbiochem) (red) and polyclonal rabbit anti-agnoprotein serum (20) (green), and the secondary antibodies, Alexa fluor 568 goat antimouse (Invitrogen) and Alexa fluor 488 goat antirabbit (Invitrogen), were used. Cell nuclei (blue) were stained with DRAQ5 (Biostatus). Images were collected using a Nikon TE2000 microscope equipped and processed with the NIS Elements Basic Research software program, version 2.2 (Nikon Corporation). (E) Intracellular BKV DNA loads were quantified from RPTECs harvested at 24, 48, and 72 h.p.i. After DNA extraction, BKV DNA loads were measured by qPCR and normalized for cellular DNA using the aspartoacylase (ACY) qPCR (5, 35, 36). Data are presented as Geq/cell. (F) Late protein expression was detected by Western blotting 48 h.p.i. as described above (5), using as primary antibodies polyclonal rabbit anti-VP1 serum (17), polyclonal rabbit anti-agnoprotein serum (20), and the monoclonal antibody directed against GAPDH (Abcam).
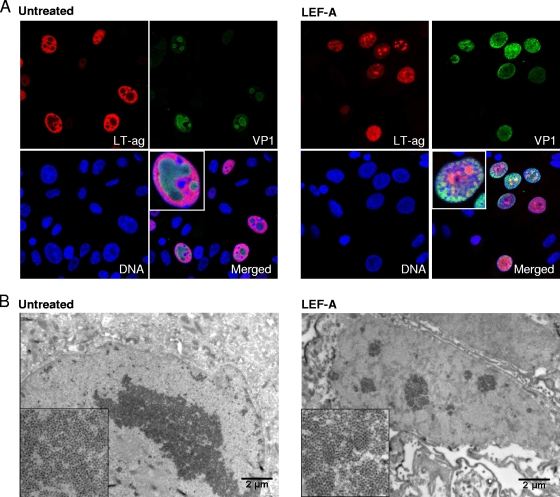
To investigate whether BKV DNA replication was affected by LEF-A, intracellular BKV loads at 24, 48, and 72 h.p.i. were measured by qPCR and normalized to the cell number (5). In untreated cells, BKV loads steadily increased from 24 h.p.i. In LEF-A-treated cells, this increase was curtailed by 67% (2.6 × 103 copies/cell) at 48 h.p.i. and by 77% (2 × 104 copies/cell) at 72 h.p.i. (Fig. 2E). Thus, BKV genome replication is significantly but not completely inhibited by the LEF-A IC90. To investigate viral late expression, we compared VP1 and agnoprotein expression at 48 h.p.i. and found a 60% and 70% reduction, respectively (Fig. 2F). Immunofluorescence staining indicated a similar reduction of agnoprotein-stained (Fig. 2D) and VP1-stained (data not shown) cells. Moreover, VP1 nuclear distribution and signal intensity were different in LEF-A-treated cells. Confocal microscopy of untreated cells revealed large nuclear inclusions with strong VP1 signals surrounded by LT-ag staining (Fig. 3A). This characteristic nuclear architecture of polyomavirus replication was absent in the majority of LEF-A-treated cells. Instead, they showed strong but dispersed VP1 staining mixed with LT-ag. When immunoelectron microscopy (IEM) with gold-labeled VP1 antibodies was performed, large nuclear clusters of viral particles were observed in untreated RPTECs while two morphologies were seen in LEF-A-treated cells, either small virion clusters (Fig. 3B) or scattered VP1 labeling without assembled viral particles (data not shown). These results suggest that BKV assembly is affected by LEF-A. To investigate virion release, we compared infectious intracellular and extracellular BKV titers at 48 h.p.i. by seeding cell lysates and supernatants on RPTECs. We found a larger fraction of the total viral load intracellularly in LEF-A-treated (84%) than in untreated (73%) cells, suggesting that virion release also is affected.
FIG. 3.
Influence of LEF-A on nuclear BKV replication architecture. (A) For confocal microscopy, RPTECs were seeded in fibronectin-coated chamber slides, infected, and treated with LEF-A (10 μg/ml) as described earlier. At 72 h.p.i., cells were fixed in 4% paraformaldehyde, followed by methanol permabilization and blocking with 3% goat serum in PBS for 30 min. Then, indirect immunofluorescence was performed as described earlier (27) using as primary antibodies an SV40 LT-ag monoclonal (Pab416; Calbiochem) (red) and polyclonal rabbit anti-VP1 serum (17) (green) and the secondary antibodies Alexa fluor 568 goat anti-mouse IgG (Invitrogen) and Alexa fluor 488 goat anti-rabbit IgG (Invitrogen). Cell nuclei (blue) were stained with DRAQ5 (Biostatus). Confocal microscopy analysis was performed using a microscope (Axiovert 200; Carl Zeis, Inc.) equipped with an LSM510 confocal module and processed using the LSM5 software program, version 3.2 (Carl Zeiss, Inc.). (B) For IEM analysis, RPTECs were grown in 6 wells, infected, and treated with LEF-A (10 μg/ml) as earlier described. At 48 h.p.i., cells were fixed with 4% formaldehyde, washed in 0.12% glycin, scraped off, and pelleted in 12% gelatin. The pellet was placed in 2.3 M sucrose overnight, cut in cubes, mounted on cryo-pins, frozen by immersion in liquid nitrogen, and sectioned using a Leica EM UC6 ultramicrotome. The sections were submerged in 1% cold-water fish skin gelatin overnight and incubated with polyclonal rabbit anti-VP1 serum (17) and then with protein A-gold (10 nm). The specimens were contrasted with a mixture of uranyl acetate and methylcellulose and examined by using a Jeol 1010 transmission electron microscope.
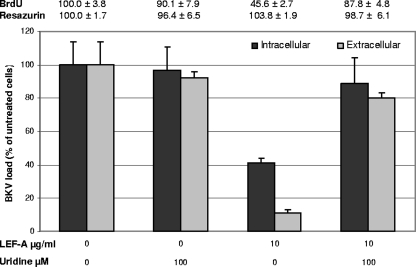
Finally, we investigated the role of pyrimidine depletion; uridine at 100 μM was added 2 h.p.i. to BKV-infected cells with or without LEF-A treatment. Addition of uridine almost completely restored LEF-A (10 μg/ml)-mediated inhibition of viral and cellular DNA replication and the extracellular BKV load (Fig. 4). Uridine also reversed LEF-A-mediated reduction of late viral protein expression and restored the nuclear replication architecture of VP1 and LT-ag (data not shown). With LEF-A at 30 μg/ml, however, uridine at 100 μM could neither restore cellular DNA replication nor extracellular BKV loads, and microscopy revealed significant cytotoxic effects independently of uridine addition (data not shown). Thus, the IC90 antiviral effect of LEF-A is caused mainly by pyrimidine depletion and is, as such, closely linked to the cytostatic effect of LEF-A caused by pyrimidine depletion.
FIG. 4.
Impact of uridine on BKV replication and cellular viability in LEF-A-treated RPTECs. RPTECs were seeded and infected as earlier described. At 2 h.p.i., virus was removed and replaced with LEF-A-containing medium with or without exogenous uridine at 100 μM. For intracellular DNA measurements, cells were harvested 48 h.p.i., and for extracellular BKV load, supernatants were harvested 72 h.p.i. BKV and cellular DNA loads were measured by qPCR as described earlier, and BKV loads of untreated cells were set as 100%. In addition, RPTEC cellular DNA replication was measured by cell proliferation ELISA, BrdU, and total cellular metabolic activity determined using TOX-8 at 48 h.p.i. as described earlier. Absorbance for untreated cells was set as 100%.
In summary, our study shows that LEF-A inhibits BKV DNA replication and changes nuclear VP1 and LT-ag distribution and suggests that viral assembly and release are inhibited. At the same time, host cell proliferation is affected. Since LEF-A-mediated inhibition at 10 μg/ml (IC90) can be overcome with uridine, it seems to depend upon pyrimidine depletion. Potential effects of higher LEF-A concentrations are difficult to study in RPTECs since host cell viability is severely affected.
We have previously shown that the nucleoside analogue cidofovir acts almost exclusively at the level of cellular and BKV DNA replication and that the IC90 also inhibits BKV DNA replication by at least 90% (5). Similarly, LEF-A inhibited BKV DNA replication as the first step, but the LEF-A IC90 gave only a 67% reduction, suggesting inhibition of additional steps downstream of DNA replication. LEF-A reduced late protein levels by 60 to 70%, most likely resulting from fewer available DNA templates for late gene transcription (8). However, our data implicate inhibition of virion assembly and release, with altered nuclear distribution of VP1 and LT-ag. This could be caused by modified signal transduction pathways or posttranslational modifications of VP1, i.e., phosphorylations, acetylation, and methylation (32). Furthermore LEF-A may inhibit Ca2+ mobilization (46), which plays an important role in viral assembly (33). Finally, the changed nuclear architecture may result from lower BKV DNA levels, since DNA may act as a scaffold, increasing the local VP1 concentration or changing the tertiary structure of VP1 as earlier reported for simian virus 40 (SV40) (42).
LEF-A inhibition of BKV replication coincided with a 50% reduction in cellular DNA replication, and microscopic evaluation of the RPTECs revealed a lower cell number. The cells maintained integrity, and the overall metabolic activity was not significantly affected even though mitochondrial metabolic activity was decreased by 40%. In agreement with our results, uridine has been reported to restore cellular DNA replication in other leflunomide-treated cells (4, 31). Pyrimidines are precursors for RNA and DNA synthesis, membrane lipid biosynthesis, protein glycosylation, energy metabolism, intracellular messengers, and enzyme cofactors and therefore essential for proliferation (19). Since kidney tubule regeneration seems to rely on division of differentiated, normally slowly cycling cells (43), we may assume that the cytostatic effects of LEF-A are negligible in a healthy kidney. However, a PVAN-afflicted kidney probably requires higher cell cycling and faster pyrimidine synthesis to regenerate the integrity of the tubular epithelium (16), which may be jeopardized by leflunomide. In fact, a delayed recovery was recently described for 7 patients on leflunomide therapy (1).
How do our observations compare with those of other studies? The IC90 of LEF-A found for BKV-infected RPTECs, 10 μg/ml, could yield only a 50% inhibitory concentration (IC50) in Vero cells (24) and in lung fibroblast lines (14). Interestingly, uridine addition was not found to affect the LEF-A anti-BKV activity in Vero cells (24), while HIV inhibition in peripheral blood mononuclear cells was completely abolished (38). Blood LEF-A levels of >40 μg/ml have been suggested to be required to significantly decrease BKV loads in kidney transplant patients (45), but the concentration inside the renal tubule and its cells is unknown (40). A confounder of the clinical effect is the concomitant reduction in immunosuppression, since typically mycophenolate mofetil is replaced by leflunomide.
We conclude that LEF-A inhibits the BKV life cycle in primary human RPTECs at the level of DNA replication and, to a lesser extent, at the level of virion assembly and release. These effects are closely linked to the cytostatic properties of LEF-A, which at 10 μg/ml seem mainly to involve pyrimidine depletion. While inhibition of viral DNA replication was somewhat greater than that of cellular DNA replication, the marginal difference may be either weakened or strengthened in vivo depending on the proliferative status of the tubular epithelial cells.
Acknowledgments
We thank Kenneth Bowitz Larsen at the Bioimaging Core Facility and Helga Marie Bye at the Department of Electron Microscopy, both at the University of Tromsø, and Bettina Aasnæs and Kjersti Sandvik at the Department of Microbiology and Infection Control at the University Hospital of North Norway for excellent technical assistance.
This work was supported by EXTRA funds from the Norwegian Foundation for Health and Rehabilitation to E.B. and Swiss National Fund Grant 3200B0-110040/1 to H.H.H.
Footnotes
Published ahead of print on 2 December 2009.
REFERENCES
- 1.Awdishu, L., E. Sonbol, A. Feist, F. Lin, O. Alemi, R. Huang, and R. Pharmacy. 2009. Leflunomide delays recovery in kidney transplant recipients with BK viremia. American Transplant Congress 2009, abstr. L 503.
- 2.Bansal, S., M. S. Lucia, and A. Wiseman. 2008. A case of polyomavirus-associated nephropathy presenting late after transplantation. Nat. Clin. Pract. Nephrol. 4:283-287. [DOI] [PubMed] [Google Scholar]
- 3.Basse, G., C. Mengelle, N. Kamar, J. Guitard, D. Ribes, L. Esposito, and L. Rostaing. 2007. Prospective evaluation of BK virus DNAemia in renal transplant patients and their transplant outcome. Transplant. Proc. 39:84-87. [DOI] [PubMed] [Google Scholar]
- 4.Baumann, P., S. Mandl-Weber, A. Volkl, C. Adam, I. Bumeder, F. Oduncu, and R. Schmidmaier. 2009. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol. Cancer Ther. 8:366-375. [DOI] [PubMed] [Google Scholar]
- 5.Bernhoff, E., T. J. Gutteberg, K. Sandvik, H. H. Hirsch, and C. H. Rinaldo. 2008. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am. J. Transplant. 8:1413-1422. [DOI] [PubMed] [Google Scholar]
- 6.Chang, C. Y., A. Gangji, K. Chorneyko, and A. Kapoor. 2005. Urological manifestations of BK polyomavirus in renal transplant recipients. Can. J. Urol. 12:2829-2836. [PubMed] [Google Scholar]
- 7.Cherwinski, H. M., D. McCarley, R. Schatzman, B. Devens, and J. T. Ransom. 1995. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J. Pharmacol. Exp. Ther. 272:460-468. [PubMed] [Google Scholar]
- 8.Cole, C. N. 1996. Polyomavirinae: the viruses and their replication, p. 1997-2043. In B. N. Fields, D. M. Knipe, and P. H. Howley (ed.), Fields virology, vol. 2. Lippincott-Raven, New York, NY.
- 9.Crew, R. J., G. Markowitz, and J. Radhakrishnan. 2006. Therapeutic options in BK virus-associated interstitial nephritis. Kidney Int. 70:399-402. [DOI] [PubMed] [Google Scholar]
- 10.Davis, J. P., G. A. Cain, W. J. Pitts, R. L. Magolda, and R. A. Copeland. 1996. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry 35:1270-1273. [DOI] [PubMed] [Google Scholar]
- 11.Drachenberg, C. B., J. C. Papadimitriou, R. Wali, C. L. Cubitt, and E. Ramos. 2003. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am. J. Transplant. 3:1383-1392. [DOI] [PubMed] [Google Scholar]
- 12.Duclos, A. J., V. Krishnamurthi, M. Lard, E. Poggio, M. Kleeman, C. Winans, R. Fatica, and S. Nurko. 2006. Prevalence and clinical course of BK virus nephropathy in pancreas after kidney transplant patients. Transplant. Proc. 38:3666-3672. [DOI] [PubMed] [Google Scholar]
- 13.Faguer, S., H. H. Hirsch, N. Kamar, C. Guilbeau-Frugier, D. Ribes, J. Guitard, L. Esposito, O. Cointault, A. Modesto, M. Lavit, C. Mengelle, and L. Rostaing. 2007. Leflunomide treatment for polyomavirus BK-associated nephropathy after kidney transplantation. Transpl. Int. 20:962-969. [DOI] [PubMed] [Google Scholar]
- 14.Farasati, N. A., R. Shapiro, A. Vats, and P. Randhawa. 2005. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation 79:116-118. [DOI] [PubMed] [Google Scholar]
- 15.Feng, H., X. Y. Li, J. R. Zheng, J. W. Gao, L. F. Xu, and M. Y. Tang. 2005. Inhibition of the nuclear factor-kappaB signaling pathway by leflunomide or triptolide also inhibits the anthralin-induced inflammatory response but does not affect keratinocyte growth inhibition. Biol. Pharm. Bull. 28:1597-1602. [DOI] [PubMed] [Google Scholar]
- 16.Funk, G. A., R. Gosert, P. Comoli, F. Ginevri, and H. H. Hirsch. 2008. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am. J. Transplant. 8:2368-2377. [DOI] [PubMed] [Google Scholar]
- 17.Grinde, B., M. Gayorfar, and C. H. Rinaldo. 2007. Impact of a polyomavirus (BKV) infection on mRNA expression in human endothelial cells. Virus Res. 123:86-94. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton, L. C., I. Vojnovic, and T. D. Warner. 1999. A771726, the active metabolite of leflunomide, directly inhibits the activity of cyclo-oxygenase-2 in vitro and in vivo in a substrate-sensitive manner. Br. J. Pharmacol. 127:1589-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann, M. L., R. Schleyerbach, and B. J. Kirschbaum. 2000. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 47:273-289. [DOI] [PubMed] [Google Scholar]
- 20.Hey, A. W., J. I. Johnsen, B. Johansen, and T. Traavik. 1994. A two fusion partner system for raising antibodies against small immunogens expressed in bacteria. J. Immunol. Methods 173:149-156. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79:1277-1286. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3:611-623. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, M., J. R. Abend, S. F. Johnson, and M. J. Imperiale. 2009. The role of polyomaviruses in human disease. Virology 384:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josephson, M. A., D. Gillen, B. Javaid, P. Kadambi, S. Meehan, P. Foster, R. Harland, R. J. Thistlethwaite, M. Garfinkel, W. Atwood, J. Jordan, M. Sadhu, M. J. Millis, and J. Williams. 2006. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation 81:704-710. [DOI] [PubMed] [Google Scholar]
- 25.Knight, D. A., A. Q. Hejmanowski, J. E. Dierksheide, J. W. Williams, A. S. Chong, and W. J. Waldman. 2001. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplantation 71:170-174. [DOI] [PubMed] [Google Scholar]
- 26.Leca, N., K. A. Muczynski, J. A. Jefferson, I. H. de Boer, J. Kowalewska, E. A. Kendrick, R. Pichler, and C. L. Davis. 2008. Higher levels of leflunomide are associated with hemolysis and are not superior to lower levels for BK virus clearance in renal transplant patients. Clin. J. Am. Soc. Nephrol. 3:829-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuenberger, D., P. A. Andresen, R. Gosert, S. Binggeli, E. H. Strom, S. Bodaghi, C. H. Rinaldo, and H. H. Hirsch. 2007. Human polyomavirus type 1 (BK virus) agnoprotein is abundantly expressed, but immunologically ignored. Clin. Vaccine Immunol. 14:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 29.Mattar, T., K. Kochhar, R. Bartlett, E. G. Bremer, and A. Finnegan. 1993. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett. 334:161-164. [DOI] [PubMed] [Google Scholar]
- 30.Mazzucco, G., C. Costa, M. Bergallo, G. P. Segoloni, and G. Monga. 2008. Severe crescentic BK virus nephropathy with favourable outcome in a transplanted patient treated with Leflunomide. Clin. Nephrol. 70:163-167. [DOI] [PubMed] [Google Scholar]
- 31.Nair, R. V., W. Cao, and R. E. Morris. 1995. Inhibition of smooth muscle cell proliferation in vitro by leflunomide, a new immunosuppressant, is antagonized by uridine. Immunol. Lett. 48:77-80. [DOI] [PubMed] [Google Scholar]
- 32.Ng, J., O. Koechlin, M. Ramalho, D. Raman, and N. Krauzewicz. 2007. Extracellular self-assembly of virus-like particles from secreted recombinant polyoma virus major coat protein. Protein Eng. Des. Sel. 20:591-598. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson, J., N. Miyazaki, L. Xing, B. Wu, L. Hammar, T. C. Li, N. Takeda, T. Miyamura, and R. H. Cheng. 2005. Structure and assembly of a T=1 virus-like particle in BK polyomavirus. J. Virol. 79:5337-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott, U., T. Steiner, M. Busch, J. Gerth, and G. Wolf. 2008. A single-center experience with BK virus nephropathy. Clin. Nephrol. 69:244-250. [DOI] [PubMed] [Google Scholar]
- 35.Randhawa, P., R. Shapiro, and A. Vats. 2005. Quantitation of DNA of polyomaviruses BK and JC in human kidneys. J. Infect. Dis. 192:504-509. [DOI] [PubMed] [Google Scholar]
- 36.Randhawa, P. S., A. Vats, D. Zygmunt, P. Swalsky, V. Scantlebury, R. Shapiro, and S. Finkelstein. 2002. Quantitation of viral DNA in renal allograft tissue from patients with BK virus nephropathy. Transplantation 74:485-488. [DOI] [PubMed] [Google Scholar]
- 37.Rinaldo, C. H., and H. H. Hirsch. 2007. Antivirals for the treatment of polyomavirus BK replication. Expert Rev. Anti Infect. Ther. 5:105-115. [DOI] [PubMed] [Google Scholar]
- 38.Schlapfer, E., M. Fischer, P. Ott, and R. F. Speck. 2003. Anti-HIV-1 activity of leflunomide: a comparison with mycophenolic acid and hydroxyurea. AIDS 17:1613-1620. [DOI] [PubMed] [Google Scholar]
- 39.Sessa, A., A. Esposito, A. Giliberti, M. Bergallo, C. Costa, R. Rossano, E. Lettieri, and M. Capuano. 2008. BKV reactivation in renal transplant recipients: diagnostic and therapeutic strategy—case reports. Transplant. Proc. 40:2055-2058. [DOI] [PubMed] [Google Scholar]
- 40.Teschner, S., P. Gerke, M. Geyer, J. Wilpert, B. Krumme, and G. Walz. 2008. Leflunomide therapy for polyomavirus-induced allograft nephropathy. American Transplant Congress 2008, abstr. L 1063. [DOI] [PubMed]
- 41.Teschner, S., M. Geyer, J. Wilpert, E. Schwertfeger, T. Schenk, G. Walz, and J. Donauer. 2006. Remission of polyomavirus-induced graft nephropathy treated with low-dose leflunomide. Nephrol. Dial. Transplant. 21:2039-2040. [DOI] [PubMed] [Google Scholar]
- 42.Tsukamoto, H., M. A. Kawano, T. Inoue, T. Enomoto, R. U. Takahashi, N. Yokoyama, N. Yamamoto, T. Imai, K. Kataoka, Y. Yamaguchi, and H. Handa. 2007. Evidence that SV40 VP1-DNA interactions contribute to the assembly of 40-nm spherical viral particles. Genes Cells 12:1267-1279. [DOI] [PubMed] [Google Scholar]
- 43.Vogetseder, A., T. Palan, D. Bacic, B. Kaissling, and M. Le Hir. 2007. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am. J. Physiol. Cell Physiol. 292:C807-C813. [DOI] [PubMed] [Google Scholar]
- 44.Waldman, W. J., D. A. Knight, L. Blinder, J. Shen, N. S. Lurain, D. M. Miller, D. D. Sedmak, J. W. Williams, and A. S. Chong. 1999. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology 42:412-418. [DOI] [PubMed] [Google Scholar]
- 45.Williams, J. W., B. Javaid, P. V. Kadambi, D. Gillen, R. Harland, J. R. Thistlewaite, M. Garfinkel, P. Foster, W. Atwood, J. M. Millis, S. M. Meehan, and M. A. Josephson. 2005. Leflunomide for polyomavirus type BK nephropathy. N. Engl. J. Med. 352:1157-1158. [DOI] [PubMed] [Google Scholar]
- 46.Xu, X., J. W. Williams, E. G. Bremer, A. Finnegan, and A. S. Chong. 1995. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J. Biol. Chem. 270:12398-12403. [DOI] [PubMed] [Google Scholar]
- 47.Zavos, G., M. Gazouli, E. Psimenou, I. Papaconstantinou, J. Bokos, J. Boletis, A. Zografidis, and A. Kostakis. 2004. Polyomavirus BK infection in Greek renal transplant recipients. Transplant. Proc. 36:1413-1414. [DOI] [PubMed] [Google Scholar]