Abstract
The central role of Raf protein kinase isoforms in human cancer demands specific anti-Raf therapeutic inhibitors. Parvoviruses are currently used in experimental cancer therapy due to their natural oncotropism and lytic life cycle. In searching for mechanisms underlying parvovirus oncolysis, we found that trimers of the major structural protein (VP) of the parvovirus minute virus of mice (MVM), which have to be imported into the nucleus for capsid assembly, undergo phosphorylation by the Raf-1 kinase. Purified Raf-1 phosphorylated the capsid subunits in vitro to the two-dimensional pattern found in natural MVM infections. VP trimers isolated from mammalian cells translocated into the nucleus of digitonin-permeabilized human cells. In contrast, VP trimers isolated from insect cells, which are devoid of Raf-1, were neither phosphorylated nor imported into the mammalian nucleus. However, the coexpression of a constitutively active Raf-1 kinase in insect cells restored VP trimer phosphorylation and nuclear transport competence. In MVM-infected normal and transformed cells, Raf-1 inhibition resulted in cytoplasmic retention of capsid proteins, preventing their nuclear assembly and progeny virus maturation. The level of Raf-1 activity in cancer cells was consistent with the extent of VP specific phosphorylation and with the permissiveness to MVM infection. Thus, Raf-1 control of nuclear translocation of MVM capsid assembly intermediates provides a novel target for viral oncolysis. MVM may reinforce specific therapies against frequent human cancers with deregulated Raf signaling.
The Raf protein isoforms (A, B, C, or Raf-1) belong to the conserved Ras/Raf/MEK/ERK mitogen-activated protein kinase (MAPK) signaling module. This signal cascade transduces mitogenic and other stimuli from the cell surface to the nucleus (reviewed in reference 41). Activation of the Raf kinases is primarily triggered by increased levels of the upstream regulator Ras-GTP protein, which directly binds and recruits cytosolic dimers of the Raf kinases to the plasma membrane (58). The membrane-associated Raf kinases are activated by phosphorylation and other events (48), assembling a MAPK signaling complex formed by the MEK and ERK kinases and scaffold proteins (8). Upon phosphorylation and dimerization (25), an active ERK kinase dissociates from the complex and translocates into the nucleus. Intranuclear ERK kinase promotes multiple protein phosphorylations and changes of gene expression that may be altered in oncogenic and deregulated signaling (50, 55).
The selectivity of lytic viruses in anticancer therapies depends on cellular targets that favor their growth. Many if not all viral infections interfere with major cellular protein kinase networks, promoting virus entry, repressing antiviral responses, or increasing metabolic activity and virus yield. The effects are, however, virus dependent: viral activation of the MAPK signaling pathway, for example, may induce cell proliferation (oncogenic DNA virus [64]), suppress transcription (hepatitis B virus [83]), reactivate translation (Ebola virus [71]), or enhance nuclear export (influenza virus [52]). The MAPK signaling cascade is essential for cellular proliferation and cancer formation (40, 67), which led to the use of lytic viruses dependent on this pathway (7) in ongoing preclinical and clinical trials (51). Molecular insights into the interactions between viral substrates and components of the MAPK pathway could thus increase specificity and therapeutic efficacy of oncolytic viruses.
The replication of virus members of the Parvoviridae relies on functions provided by proliferative cells (24). Together with diverse factors that are linked to the neoplastic growth, the underlying mechanisms allow parvoviruses to infect and lyse transformed cells preferentially (47), thus interfering with tumor formation in animal models (14, 74; reviewed in references 11 and 54). The complex molecular mechanisms supporting parvovirus intrinsic oncotropism cannot be explained by either suitable receptors responsible for virus uptake or the cytotoxicity, which is triggered by the viral nonstructural proteins (5). Parvovirus productive infection is rather mediated on different levels of the viral life cycle in a cancer-type-specific manner (65, 66). Parvoviruses may provoke death of tumor cells lacking p53 (57, 73) and show increased toxicity to transformed cells with upregulated Ras and other protein kinases (49, 69).
Progeny parvovirus production, which is required for the spreading of oncolytic effects, depends on nuclear import of de novo-synthesized structural (VP) proteins, since virion maturation is intranuclear. The parvovirus capsid is composed of 60 protein subunits sharing a common C terminus folded in an eight-strand antiparallel β-barrel motif (76). In mammalian cells infected with the parvovirus minute virus of mice (MVM), capsid assembly starts by cytoplasmic synthesis of two types of VP1/VP2 trimers in stoichiometric amounts (61). Nuclear translocation of the trimers is driven by an unconventional structured nuclear localization motif (NLM) evolutionary conserved in the parvovirus β-barrel (37). This NLM is exposed on the trimer surface but not on the assembled capsid (37, 61). Self-assembly into viruslike particles (VLPs) may occur after expression of the parvovirus major capsid protein in heterologous systems (see, for example, references 20 and 82), although little is known of the host factors regulating the nuclear targeting and assembly of parvovirus capsid subunits.
The NLM sequence and configuration differs from the best-characterized nuclear localization signals (NLS) causing protein cargo translocation across the nuclear envelope (NE). Nucleocytoplasmic transport of proteins in eukaryotic cells is a saturable and energy-dependent process occurring via the nuclear pore complex (NPC [4, 75]), a supramolecular structure embedded in the NE. The bidirectional exchange of macromolecules across the NE is mostly mediated by proteins of the importin β (karyopherin β) superfamily that comprises importins (including transportins and snurportins) and exportins. Cargo recognition is facilitated by direct or indirect interaction with specialized signals, such as an NLS or a nuclear export signal (NES) (44). In the classical nuclear import pathway, karyopherin β1/importin β binds to the adaptor importin α, which interacts with cargoes carrying NLS consisting of clusters of basic amino acids (22, 28, 62). However, many imported proteins bind karyopherin β1 directly without an adaptor, and there are diverse nonclassical NLS that have structural or linear nonhomologous domains (9, 32). A well-characterized nonclassical import pathway is mediated by karyopherin β2/transportin, which binds different substrates, such as the splicing factor hnRNP A1 (53), carrying the long and structurally disordered NLS termed PY-NLS (29, 31). Independent of the transport receptor, the directionality of the transport is driven by the concentration gradient of the small GTPase Ran in its GTP- and GDP-bound form, which triggers the dissociation of the transport complexes and the release of the cargoes (reviewed in reference 70).
The missing conclusiveness to explain increased parvovirus productive infection in cancer cells by receptors or cytotoxicity of the nonstructural proteins, led us search for underlying molecular mechanisms. To identify molecular targets of parvovirus oncolysis, we focused on the regulation of MVM assembly in normal and cancer cells. The nuclear transport competence and phosphorylation stage of isolated VP trimers was compared in insect and human cells. We sought the mammalian kinase involved and found that VP proteins phosphorylation by the Raf-1 kinase of the MAPK cascade is essential in the nuclear translocation and capsid formation of MVM assembly intermediates. The implications of these findings for MVM anticancer therapeutic potential are discussed.
MATERIALS AND METHODS
MVMp and mammalian cells.
The prototype strain of the autonomous parvovirus MVM (MVMp) (13), which infects mouse fibroblasts and human tumor cells (47, 65, 72), was used. Production of viral stocks by transfection with the pMM984 infectious plasmid (72), titration of infectivity by plaque assay, and CsCl purification of native empty or DNA-full MVMp particles were performed as previously described (43).
The human HeLa cell line (ATCC CCL2) and the C6 rat (ATCC CCL 107) and U-373MG (ATCC HTB17) tumor glioma cells were purchased from the American Type Culture Collection (ATCC) and maintained as previously described (65). The mouse A9 fibroblasts and the human NB324K simian virus 40-transformed newborn kidney fibroblasts are commonly used cell lines in MVM infections (72, 43). All cells of mammalian origin were maintained under a minimal number of passages in Dulbecco modified Eagle medium (DMEM) supplemented with 5% heat-inactivated fetal calf serum (FCS; Gibco-BRL) and synchronized wherever indicated by low serum growth (A9) or density arrest (NB324K and U-373MG) (to be described elsewhere). The Raf inhibitor Radicicol (Calbiochem) was stored in frozen aliquots (−70°C) and used at a 8 μM final concentration in the cultures. A9 cells stably expressing human Raf-1 with the C-terminal sequence CAAX, which constitutively activates the kinase by anchoring it to the cytoplasmic membrane (33), were obtained by cotransfection with pSV2neo and subsequent selection of clones by Geneticin G418 (Gibco).
Baculoviruses and insect cells.
Sf9 and H5 insect cells were cultured at 27°C in TC100 medium supplemented with 5% FCS. Recombinant baculoviruses of the Autographa californica (AcMNPV) species, expressing wild-type (wt) MVMp VP2 (Bac-VP2wt) or VP2-K153A (Bac-VP2K153A), were previously described (20, 59). Baculoviruses expressing wt Raf-1 and the Raf-22W constitutively active mutant form (79, 80) were obtained from T. M. Roberts (Dana-Farber Cancer Institute, Boston, MA). Baculoviruses were grown in Sf9 cells and titrated as PFU (20) or as fluorescence focus units (36). For recombinant protein expression, growing Sf9 or H5 cells were infected at a multiplicity of infection of 10 PFU/cell for each baculovirus and harvested at 72 h postinfection (hpi; unless indicated). VLPs of MVMp were purified from AcMNPV-VP2-infected insect cells according to previously described protocols (20).
Purification of MVM assembly intermediates.
NB324K human cells transfected on a large scale by electroporation (36) using the VP2-K153A expression plasmid (59) or H5 insect cells infected with baculoviruses were harvested in cold HNEM buffer (50 mM HEPES [pH 8.0], 150 mM NaCl, 2 mM MgCl2, 1 mM EDTA) supplemented with the protease inhibitor cocktail set I (Calbiochem), 100 μg of TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone) (Roche)/ml, and phosphatase inhibitors (20 mM glycerol phosphate, 5 mM NaF). Cells were disrupted in a cooled water bath sonicator, lysates were cleared by centrifugation at 15,000 × g and 4°C for 30 min in a benchtop centrifuge, and trimers from insect cells were enriched by consecutive 20 and 30% ammonium sulfate precipitations. Cellular homogenates were loaded onto 14 ml of a 5 to 20% continuous sucrose gradient in HNEM buffer prepared on ice and centrifuged at 160,000 × g in a SW40 rotor (Beckman) at 5°C and 30 h, with molecular size markers centrifuged in parallel gradients (61). Proteins in the fractions were precipitated with 10% trichloroacetic acid and 10 μg of bovine serum albumin (BSA)/ml, resuspended in Laemmli buffer, and subjected to SDS-PAGE and immunoblot analyses with anti-MVM sera. Fractions containing VP trimers (9S) were pooled, dialyzed against transport buffer (see below), concentrated by a Centricon-50 kDa (Millipore), and stored frozen in aliquots at −70°C until use. The yield was approximately 2 to 5 μg of purified VP-trimer per 107 cells.
Nuclear transport assay in permeabilized cells.
Previously described methodologies (1, 18, 56) were followed with minor modifications. HeLa cells were permeabilized with 40 μg of digitonin (Sigma)/ml in DMEM for 5 min at 37°C. Permeabilization was controlled by light microscopy before the cells were washed three times for 10 min at 4°C in transport buffer (2 mM magnesium acetate, 20 mM HEPES [pH 7.3], 110 mM potassium acetate, 1 mM EDTA, 5 mM sodium acetate, 2 mM dithiothreitol) containing 0.5% BSA and 5% goat serum (Gibco). Import reactions were performed for 15 min at 37°C in 30 μl of transport buffer containing 12 mg of rabbit reticulocyte lysate (Promega)/ml, protease inhibitors (as described above), 1 mM ATP, 5 mM creatine phosphate (Sigma), and 20 U of creatine phosphokinase (Calbiochem)/ml. Substrates were 200 ng of gradient-purified MVM capsid or VP trimers. As transport controls, BSA conjugated with the SV-40 TAg NLS (PKKKRKVED) of the α/β classical importin route, and the M9 domain (YNNQSSNFGPMK) of the transportin route (9, 22, 31), were used. Peptides were conjugated to BSA coupled with fluor agents (Alexa Fluor 488, 594, and 647; Molecular Probes) by the chemical cross-linker SMCC (Calbiochem), cleaned by chromatography in PD10 columns (Amersham), and concentrated by centrifugation through filters (3-kDa Microcon; Millipore), as described previously (18). A ratio of 10 bound peptides per BSA molecule was estimated by determining the protein molecular sizes by SDS-PAGE. For inhibition of the nuclear import, wheat germ agglutinin ([WGA] Calbiochem), a lectin that blocks glycosylated sites on the NPC that are required for interaction with the nuclear import receptors (17), was added at 100 μg/ml in the transport reaction and washing buffer. The transport reaction was terminated by placing the cells on ice, followed by three washes with transport buffer and fixation in 4% paraformaldehyde.
Immunological analyses.
Antibodies to components of the MAPK signaling pathway (Santa Cruz Biotechnology) included mouse monoclonal (mα-Raf) and rabbit polyclonal (α-Raf) antibodies recognizing the conserved C terminus of human Raf-1; rabbit polyclonal antibody (pα-ERK) recognizing the ERK1/2 proteins, and mouse monoclonal antibody (mα-P-ERK) recognizing their phosphorylated forms. A mouse monoclonal antibody (α-NPC) (MAb414; Babco, Richmond, CA) was used as marker of the nuclear envelope. Human casein kinase II was immunoprecipitated with an anti-CKIIa polyclonal antibody (Santa Cruz Biotechnology). Antibodies raised against MVM capsid antigens previously described (36, 61) included a rabbit polyclonal antiserum (polyclonal antibody α-capsid) raised against intact purified MVM empty capsids, the B7 mouse monoclonal antibody recognizing a discontinuous epitope specific of the MVM intact capsid (Mα-capsid) located at the threefold axes (23) that allows to monitor capsid formation (37, 43), and a rabbit polyclonal antiserum raised against denatured VP2 protein (α-VPs) for the general localization of VP1 and VP2 capsid proteins subunits (to be described elsewhere). Indirect double-label immunofluorescence was performed by using secondary antibodies conjugated to Texas Red or fluorescein isothiocyanate (FITC) (36), and phenotypes were visualized by epifluorescence in a Zeiss Axiovert 200 microscope equipped with a Radiance 2000 confocal laser scanning system (43). Pictures were recorded with lasers giving excitation lines at 488 nm (FITC, filter HQ 500-530), and 543 nm (TXRD, filter HQ 600 LP). For Western blot analysis, protein samples resolved in SDS-8% PAGE and electroblotted onto nitrocellulose membranes were probed with the indicated antisera as described previously (61).
Raf-1 phosphorylation of MVM capsid subunits in mammalian cells and in vitro.
For [32P]orthophosphate in vivo labeling, MVMp-infected cells (5 PFU/cell) were starved for 4 h at 6 hpi in phosphate-free medium with 10% dialyzed FCS and labeled 10 to 24 hpi by the addition of 1 mCi of carrier-free [32P]orthophosphate (Amersham)/ml. Capsid proteins were precipitated at 4°C by using polyclonal anti-VPs antibodies bound to protein A-Sepharose in 150 mM NaCl-50 mM Tris (pH 8.0)-0.3% SDS-1% NP-40-0.75% 2-mercaptoethanol and protease and phosphatase inhibitors prior to SDS-PAGE analysis. For in vitro phosphorylation, the indicated kinases were immunoprecipitated with antibodies from human NB324K cells at 4°C in 150 mM NaCl-50 mM Tris-HCl (pH 7.5)-0.05% SDS-1% NP-40-0.5% deoxycholate in the presence of protease and phosphatase inhibitors. The Sepharose-bound precipitate was stored at −70°C. The phosphorylation reaction was performed at 37°C for 30 min in 40 μl containing immunocomplex from 106 cells, 8 μg of purified VLP in [γ-32P]ATP (10 μCi [Amersham], 3,000 Ci/mmol), 0.1 μM rATP, 25 mM HEPES (pH 7.4), 25 mM glycerophosphate, 1 mM dithiothreitol, and 10 mM MgCl2. The 32P-labeled VP2 protein resolved in gels was blotted to nitrocellulose membranes (BA85; Schleicher & Schuell) and subjected to two-dimensional tryptic phosphopeptide analysis in 20-by-20-cm thin-layer chromatography plates (Merck, Darmstadt, Germany) according to previously reported procedures (42). The plates were exposed to a radioanalytic imaging system (Fujix BAS 1000; Fuji) or to X-ray films with intensifying screens for 4 days.
Analysis of MVM capsid protein phosphorylation in insect cells.
32P labeling of VP2 protein and VLPs in Sf9 and H5 insect cells infected by baculoviruses was attempted for periods of 6 h at either 22, 38, or 48 hpi in TC100 medium without phosphate and 5% dialyzed FCS supplemented with 0.5 to 1 mCi of [32P]orthophosphate carrier-free (Amersham)/ml at 8,500 Ci/mmol. Okadaic acid (Gibco), a phosphatase inhibitor (3), was used at 350 nM during the last hour of labeling to prevent the removal of putative transient phosphate groups. VLPs were purified from the 32P-labeled cultures by CsCl gradient centrifugation to equilibrium (42). Fractions were tested for 32P counts and hemagglutination activity (20), and those corresponding to empty MVM particles were pooled and dialyzed. Detection of phospho label in the purified VP2 protein was repeatedly attempted by separating 5 μg of gradient-purified VLPs by SDS-PAGE and exposure to autoradiography with intensifying screens for 7 days. To examine the level of VP2wt and VP2K153A protein phosphorylation in the presence of baculovirus-coexpressed Raf-1 proteins, the 32P-labeled H5 cell monolayers were subjected to immunoprecipitation with the α-VPs and α-Raf-1 antibodies as described above.
RESULTS
Trimers of MVM capsid subunits assembled in insect cells are not competent for nuclear transport.
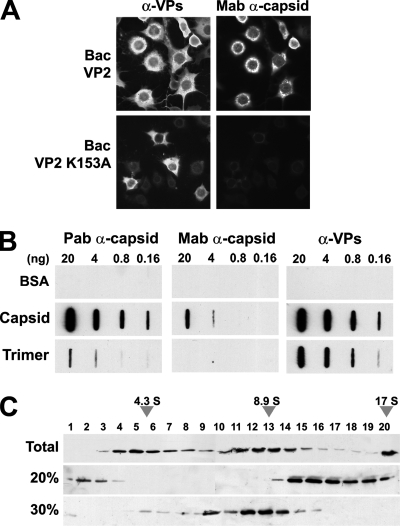
In mammalian cells susceptible to the parvovirus MVM, the capsid proteins (VPs) accumulate intranuclear (12, 37) (see also Fig. 4B below). In contrast, most baculovirus-transduced insect cells, which do not support replication of this mammalian virus, exhibited an exclusive cytoplasmic localization of the VPs (Fig. 1A, upper panels). Particle-specific antibodies characterized by slot blot analyses (see Fig. 1B) showed that, in contrast to mammalian cells, VLPs self-assembled inefficiently and primarily localized in the perinuclear cytosolic region (Fig. 1A). This finding, which confirmed previous reports on MVM (20) and canine parvovirus (82) assembly in insect cells, not only showed a cell-type-specific transport but also indicated a different assembly competence.
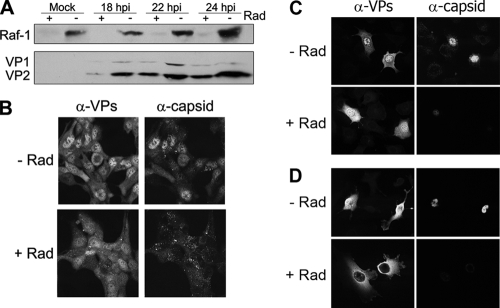
FIG. 4.
Inhibition of Raf-1 impairs MVM nuclear capsid assembly. Synchronous cultures infected with MVMp were released from the arrest and treated (+) or not (−) with Radicicol (Rad) at 14 hpi. (A) Accumulation of Raf-1 and of VP proteins along the infection of NB324K cells shown by Western blots. (B to D) Confocal analysis of the subcellular distribution of VP proteins and MVM capsid in normal and Raf-1-inhibited (+Rad) cells at 20 hpi. (B) NB324K transformed human fibroblasts; (C) A9 mouse fibroblasts; (D) U373 human glioma.
FIG. 1.
MVM capsid trimers accumulate in the cytoplasm of insect cells. (A) Subcellular distribution of MVM structural (VP2) protein (wt and K153A mutant) and capsid in H5 insect cells infected with the indicated recombinant baculoviruses. (B) Antibody specificity for MVM antigens. Capsid and VP trimers gradient-purified from human cells (see Materials and Methods) were applied to nitrocellulose membranes in phosphate saline buffer (PBS), and their immunoreactivity was analyzed by slot blot analyses. Note the lack of reactivity of VP trimers with the monoclonal α-capsid antibody, a finding in agreement with the inability of the trimers to conform in cells the capsid-specific epitope (23, 61). Capsid and VP trimers are efficiently recognized by the α-VPs polyclonal antibody. (C) Purification of VP trimers from insect cells. Extracts of Bac-VP2-K153A-infected H5 cells subjected to ammonium sulfate precipitations were sedimented in sucrose gradients and analyzed by SDS-PAGE and immunoblotting. Representative results of VP2 distribution in sucrose gradients (including monomers, trimers, and high-molecular-weight aggregates) loaded with total H5 cellular homogenate (upper) or 20% ammonium sulfate precipitate (middle) or in the supernatant of 30% ammonium sulfate precipitation (lower) are shown. The 17 S marker sedimented to the bottom of the gradients.
To avoid the possibility that capsid formation in the cytoplasm may interfere with the nuclear transport competence of VP trimers, the major assembly intermediate (61), we used the VP2-K153A mutant. This mutant forms trimers that are nuclear import competent in mammalian cells but do not assemble into capsids (61). Expression of this mutant in H5 insect cells showed a cytoplasmic phenotype with nuclear exclusion (Fig. 1A, lower panels) as for the wt VP2. Cytoplasmic capsid formation was thus not responsible for the lack of VP2 nuclear import in insect cells. Since nuclear VP2 (or VP2-K153A) localization depends on trimer formation (61), we sought to determine whether insect cells are competent for VP2 trimerization. Both wt and K153A mutant proteins expressed in insect cells sedimented with the velocity of VP2 trimers by analytical sucrose gradient ultracentrifugation (Fig. 1C) and could further be chemically cross-linked as described previously (61; data not shown). Collectively, these findings indicate that the failed VP2 nuclear translocation in insect cells was not determined by impaired trimer formation.
Active nuclear import of VP trimers isolated from human cells.
Although the nuclear transport process is evolutionarily well conserved, there are some differences between distantly related organisms, since some proteins are imported into the nucleus in a species-dependent manner (10). To exclude that such phenomena cause the lack of import of the VP trimers in insect cells, we purified VP2-K153A trimers from NB324K human and H5 insect cells and tested them with digitonin-permeabilized human HeLa cells. Transport was facilitated by import receptors that are present in rabbit reticulocyte lysate, which supports nuclear entry in various permeabilized mammalian cell lines. A control experiment was performed using native empty capsids purified from MVM-infected cells. Consistent with the localization of the NLM in the internal capsid surface (37), no capsids were detectable within the nucleus of the HeLa cells (Fig. 2A, upper panels). However, human cell line-derived VP2 trimers translocated into the nucleus. In agreement with our previous report (61), this result provided further direct evidence of the important role that the VP trimeric intermediate plays in MVM capsid assembly. Transport was temperature dependent, indicating active nuclear import (Fig. 2A middle panels). Active receptor-mediated transport was confirmed by using the nuclear import inhibitor WGA (Fig. 2A, lower panels). In contrast, H5 insect cell-expressed trimers failed to accumulate in HeLa nuclei even under transport supporting conditions (Fig. 2B).
FIG. 2.
VP trimers isolated from mammalian and insect cells differ in their nuclear transport competence. (A) Confocal analysis of the nuclear transport of mammalian VP trimers in permeabilized cells. Equal amounts of MVM capsid (upper) and VP trimer (lower) purified from NB324K human cells were incubated with permeabilized HeLa cells and stained with the α-VPs antibody. (B) Transport assay with VP-trimers isolated from human NB324K (mammal) or H5 (insect) cells in the presence of fluorescent BSA-M9 of the transportin pathway.
Site-specific VP2 phosphorylation by Raf-1 in vitro.
In searching for the mechanism underlying the different nuclear transport competence of mammalian and insect VP trimers, we investigated the role of phosphorylation, known to be a major regulator of cargo-receptor interactions in nuclear import (60). We observed that wt VLPs and VP2-K153A trimers purified from H5 and Sf9 insect cells showed no detectable phospho label, irrespective of the conditions of metabolic labeling (see Materials and Methods). In contrast, the VP capsid subunits from MVM-infected human and mouse cells are phosphorylated (42, 43). Phosphorylation occurred mainly at Ser-2, -6, and -10 of the VP2 N-terminal domain (2Nt) (42), overlapping with within two contiguous S/TXXXS/T canonical activation loop motifs of the MAPK signaling pathway (40, 78). To explore whether differences in the MAPK signaling pathway between insect and mammalian cells can account for the different VP2 phosphorylation, we subjected insect cell-derived VP2 to in vitro phosphorylation assays with human Ser/Thr protein kinases after precipitation by different antibodies. We focused on the Raf family (A-Raf, B-Raf, and C-Raf [or Raf-1]), which are involved in the development of a high proportion of glioma, melanoma, and other human malignancies (15, 16, 38, 78, 77), by promoting the nuclear translocation of phosphorylated downstream effectors (16, 41). As a control, CKIIα was used since it affects the nuclear transport capacity of some NLS-containing proteins (60).
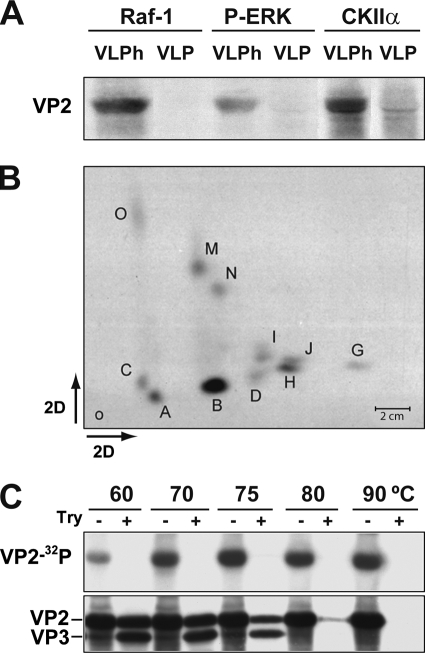
Intact VLP could not be phosphorylated, irrespective of the anti-protein kinase antibody used for pulldown. In contrast, heat-disassembled capsid underwent substantial phosphorylation by Raf-1 and by CKIIα and, to lower extent, by the P-ERK kinase (Fig. 3A). The two-dimensional phosphopeptide pattern obtained with Raf-1 (Fig. 3B) was remarkably identical to the pattern of VP2 with native empty capsids purified from MVM-infected cells (42, 43). In contrast, P-ERK and CKIIα phosphorylations analyzed by two-dimensional chromatography lacked the characteristic prominence of phosphopeptide B (data not shown). These results indicate that Raf-1 can directly phosphorylate VP2 at the sites and to a similar extent found in MVM infections, but on unassembled protein subunits only.
FIG. 3.
Raf-1 phosphorylation of MVM assembly intermediates in vitro. (A) Phosphorylation of VP2 subunits of native (VLP) or heated-denatured (VLPh) VLPs by immunoprecipitated kinases. Autoradiography after SDS-PAGE is shown. (B) Two-dimensional phosphopeptide map of the VP2 protein phosphorylated in vitro by pulldown Raf-1. Phosphopeptides are named according to a previous nomenclature (42). (C) Raf-1 phosphorylation of MVM capsid disassembly intermediates. Purified wtVLP particles heated at the indicated temperatures were subjected to Raf-1 phosphorylation (see Materials and Methods) and, wherever indicated (+), to subsequent trypsin digestion at 37°C as described previously (20, 42). Samples were resolved by SDS-8% PAGE and electroblotted, and filters were exposed to autoradiography (upper) and subsequently developed with the anti-VPs antibody (lower).
Raf-1 phosphorylation of capsid assembly intermediates is required for MVM productive infection.
The MVM capsid surface lacked Raf-1 phosphorylation sites but these sites became exposed on heat-treated capsid (Fig. 3A and B). The exposure of Raf-1 phosphorylation sites on MVM capsid disassembly intermediates was further analyzed in vitro by using purified intact VLPs subjected to graded heat-induced conformational rearrangements. MVM native capsid and VLPs undergo conformational changes along a 60 to 90°C temperature range that can be monitored by fluorescence (6) and are associated with the externalization out of the coat of the VP2 2Nt that becomes accessible to trypsin digestion (20). As shown in Fig. 3C, the VP2 phosphorylation by Raf-1 gradually increased when the samples had been heated to temperatures that cause partial or total VLP disassembly. It is noteworthy that Raf-1 only phosphorylated residues heat externalized out of the coat in VP2 domains fully accessible to the protease. At the lower temperatures of 60 to 75°C, these phosphorylated residues were localized in the 2Nt domain, since they were removed from the capsid as the VP2 protein was cleaved by trypsin to produce VP3. This experiment confirmed that 2Nt, the major phosphorylated domain of MVM capsid in infected cells (42), is also a main target of Raf-1 phosphorylation in vitro.
The assembly-dependent exposure of Raf-1 phosphorylation sites in vitro suggested that VP phosphorylation in cells must occur prior to capsid assembly, which follows VP nuclear import (61). To confirm the latter hypothesis in vivo, the effect of Raf-1 activity on VP transport and assembly was investigated. We used Radicicol, which selectively depletes Raf kinase (68). Inhibitor treatment instead of a genetic silencing approach was chosen since permanent Raf-1 inhibition affects cell proliferation, which is mandatory for parvovirus replication (24). The inhibitor was added on synchronous cultures of three MVMp permissive cells with different origins and tumorigenic potentials, at postinfection times and in a concentration that allowed both effective Raf-1 depletion and accumulation of capsid proteins to high levels (Fig. 4A). The Radicicol treatment caused a significant cytosolic retention of the VP proteins that was partial in human NB324K and mouse A9 fibroblasts (Fig. 4B and C) and absolute in U373 glioma cells (Fig. 4D). The different degrees of VP transport inhibition among the cell lines occurred consistently, for reasons that were not further investigated. However, a severely reduced capsid formation was consistently observed in all three cell cultures (Fig. 4B to D, α-capsid/+Rad panels), corresponding to an inhibition in infectious virus yields by ∼1,000-fold within a single replication cycle (data not shown). Collectively, these results indicated that inhibition of Raf-1 signaling cascade interferes with the nuclear accumulation of VP proteins, which is a prerequisite for capsid assembly (61). Consistently, a severe impairment of MVM capsid formation and progeny virus production was observed.
Raf-1 phosphorylation confers nuclear transport competence to the VP trimer.
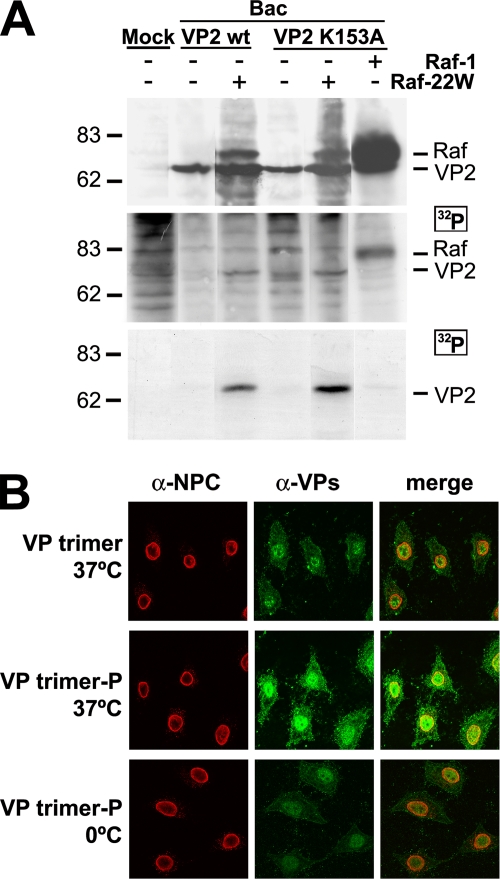
To directly relate Raf-1 phosphorylation to the nuclear transport of MVM capsid proteins, we used insect cell-expressed VP2 trimers coexpressed with the wt or with a constitutively active form of the Raf-1 kinase (Raf-22W). Expression of Raf-22W was performed since—in contrast to mammalian cells—insect cells require the coexpression of p21ras, pp60v-src, or JAK2 oncoproteins to obtain full wt Raf-1 activation (79, 81). Phosphorylation of the VP2 protein in the insect cells was evaluated by in vivo labeling with [32P]orthophosphate. VP2 phosphorylation was almost undetectable in baculovirus infections even under a very high level of accumulation of phosphorylated wt Raf-1 protein (Fig. 5A). However, wt and K153A mutant VP2 proteins were phosphorylated to similarly high levels in insect cells coexpressing the constitutively active Raf-22W protein (Fig. 5A, middle and lower panels). We next purified such phosphorylated VP2 trimer and its nonphosphorylated equivalent and analyzed their transport competence. In agreement with the results shown in Fig. 2, the nonphosphorylated VP2 trimer did not enter the nuclei of digitonin-permeabilized HeLa cells (Fig. 5B, upper panels). In contrast, VP2 trimers isolated from Raf-22W-expressing insect cells were efficiently and actively imported (Fig. 5B, lower panels). We thus concluded that Raf-1 phosphorylation confers nuclear transport competence to the VP2 trimer in mammalian cells.
FIG. 5.
Raf-1 phosphorylation suffices VP2 trimer for nuclear import. (A) VP2 phosphorylation by Raf-1 in insect cells. H5 cells infected with the indicated baculoviruses were labeled at 22 to 28 hpi with [32P]orthophosphate. The upper section blot shows VP2 and Raf-1 expression in the [32P]orthophosphate-labeled cultures. For the middle panel, autoradiography of the same filter was performed. The lower panel shows phosphorylation of VP2wt and VP2-K153A proteins in Raf-22W-expressing insect cells demonstrated by immunoprecipitation with the α-VPs antibody. Approximately 105 labeled cells were subjected to each analysis. (B) Confocal laser scanning microscopy of the nuclear transport of phosphorylated VP2 trimer in mammalian cells. Gradient-purified VP2 trimers from insect cells infected by the Bac-VP2wt (VP trimer) or by the Bac-VP2wt and the Bac-Raf1-22W baculoviruses (VP trimer-P) were subjected to transport analysis in permeabilized HeLa cells. The lower panels show the results of a control experiment with permeabilized cells kept on ice showing no significant nuclear localization caused by diffusion.
Raf-1 activity in mouse and human transformed cells increases permissiveness to MVM infection.
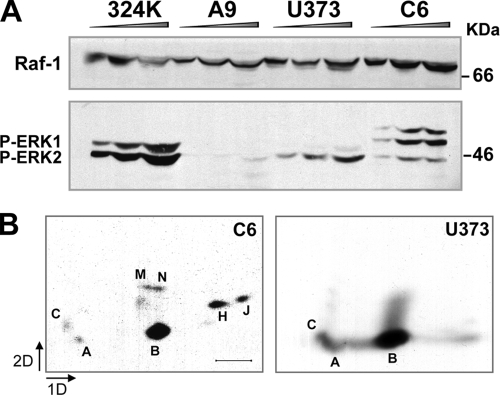
We finally investigated the relationship between Raf-1 activity and the susceptibility to MVM infection and oncolysis. The simian virus 40-transformed human NB324K and the mouse A9 fibroblasts are two common MVM cell hosts (72). Virus production was significantly higher in the NB324K cells (43, 72), which corresponded to an increased degree of VP2-specific phosphorylation (43). We thus analyzed whether Raf-1 activation correlated with these parameters. Figure 6A demonstrates that the amount of Raf-1 was similar in both fibroblast cell lines. Activation of the MAPK signaling pathway was, however, higher in NB324K cells. To exclude a cell-type-specific activation that is independent to permissiveness, we further analyzed the human U373 and the rat C6 glioma cell lines, which are both highly permissive to MVM cytotoxicity and productive infection (65). Both glioma cells exhibited MAPK signaling pathway activation (Fig. 6A) that corresponded to the characteristic VP2 phosphorylation pattern found in MVM infections (Fig. 6B). Of note, phosphopeptide B, representing the VP2 2Nt (42), was particularly prominent in the human U3T3 glioma cells. Conclusively, the levels of Raf-1 activation corresponded with increased virus production and VP2-specific phosphorylation in MVM permissive transformed fibroblasts and glioma cells.
FIG. 6.
Activation of the MAPK signaling cascade in MVM permissive cells. (A) Levels of Raf-1, and of phosphorylated ERK protein isoforms, accumulated in the indicated cell lines, as determined by SDS-PAGE and immunoblotting. Cells were starved 24 h with low serum and stimulated 6 h with 10% FCS before harvest. Equivalent graded amounts of total protein extracts were loaded per slot. (B) Pattern of VP2 phosphorylation in glioma cells. The figure shows two-dimensional phosphopeptide maps of VP2, immunoprecipitated from the indicated MVM-infected glioma cells labeled with [32P]orthophosphate. Scale bar, 2 cm.
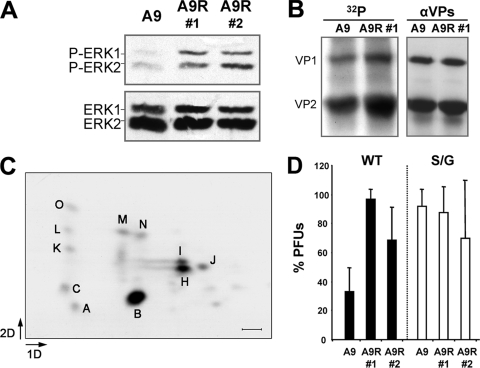
To gain direct evidence that an activated Raf-1 does increase cell permissiveness to MVM, mouse A9 cells were stably transfected with the constitutively active RafCAAX mutant (33). Two clones transfected by the RafCAAX expression vector harboring an increased Raf-1 activity with respect to the parental A9 cells were selected (Fig. 7A). Upon MVM infection, these clones showed a moderately enhanced VP phosphorylation (Fig. 7B). Moreover, the two-dimensional phosphopeptide map of the expressed VP2 (Fig. 7C) remained identical to that found after infection of the parental A9 cells (43).
FIG. 7.
Raf-1 activity increases cell permissiveness to MVM infection. (A) Activity of the MAPK signaling pathway in untransfected and RafCAAX transfected A9 mouse fibroblasts, as measured by blot analysis of the basal and phosphorylated ERK1/2 isoforms. (B) Level of VP phosphorylation in MVMp-infected A9 and A9-RafCAAX cells. VP proteins immunoprecipitated at 20 hpi from 32P-labeled cells were separated by SDS-PAGE and blotted. Filters were exposed to autoradiography (left) and then probed with the α-VPs antibody (right). (C) Two-dimensional phosphopeptide map of the VP2 protein in MVM-infected A9-RafCAAX cells. Major phosphopeptides are marked according to an earlier study (42). Scale bar, 2 cm. (D) Comparative plaque-forming capacity of wt MVM and of the S/G mutant in normal A9 cells and in two A9-RafCAAX-transfected clones. Cell monolayers were inoculated in parallel with wtMVM and S/G viral samples normalized for PFU titers, and plaques were scored in triplicate. Means with the standard errors from four assays are shown.
Raf-1 activation thus enhanced VP2 specific phosphorylation in vivo. To correlate the extent of Raf-1 phosphorylation with the level of productive infection, we analyzed MVM plaque-forming capacity in the parental and Raf-1-transfected A9 cells. As shown in Fig. 7D, the higher extent of VP2 phosphorylation in RafCAAX-expressing cells correlated well with a three- to fourfold increase in the number of MVMwt plaques formed in the transfected clones. For further confirmation, we infected these cells with an MVMp mutant harboring the three serine residues targeted by Raf-1 at the 2Nt domain mutated to glycine (42). This mutant (S/G) lacking 2Nt phosphorylation grows poorly but can be purified from NB324K cells (42), although it can form clear plaques in A9 fibroblasts (43). In contrast to the wt, this mutant showed no significant alteration in its plaque phenotype (regarding both number and size of the plaques) comparing A9 and A9-RafCAAX cells (Fig. 7D, right). Thus, a constitutively active Raf-1 does not enhance the plaque-forming capacity of a MVM mutant lacking the target Ser residues at 2Nt.
DISCUSSION
Lack of MVM capsid protein phosphorylation in insect cells.
Most vertebrate proteins expressed by baculoviruses in insect cells are correctly phosphorylated. However, quantitatively altered and nonphysiological phosphorylation sites were described for a number of mammalian virus proteins (21, 34). The lack of MVM capsid protein phosphorylation in insect cells described in the present study is thus uncommon. It was unexpected given the overall MAPK signaling pathway conservation in evolution, including the Raf homolog (Draf) found in Drosophila melanogaster (46). Although Draf contains all three conserved Raf domains, its sequence similarity to Raf-1 is lower across their N-terminal regulatory region (35), suggesting that insect Raf proteins may be not homologous enough to interact with and phosphorylate VP2. Our results are consistent with the requirement of coexpressed oncoproteins of this pathway in insect cells for mammalian Raf-1 activation (79-81).
Raf-1 phosphorylation regulates the MVM life cycle.
The Raf-1 kinase plays an essential role in MVM cycle, since Raf-1 activity increased MVM infection (Fig. 6 and 7). The site-specific in vitro phosphorylation of fully and partly disassembled VLP protein subunits by Raf-1 (Fig. 3) strongly suggests that newly synthesized VP proteins in MVM-infected mammalian cells are phosphorylated by the normally cytoplasmic Raf-1 kinase (33). Raf-1 phosphorylation was required (Fig. 2) and sufficient (Fig. 5) to confer competence for nuclear translocation to the VP subunits. Phosphorylation was thus needed for the interaction of VP trimers with nuclear transport factors. The mechanism by which Raf-1 phosphorylation enhances NE translocation of MVM assembly intermediates is in agreement with the largely recognized association of parvovirus infections with mitogenic and proliferative cell processes (2, 24).
In insect cells devoid of Raf-1 activity, VP2 proteins lacking any phospho label (Fig. 3A and 5A and data not shown) self-assembled into cytoplasmic VLPs but rather inefficiently (Fig. 1A), as was previously shown for the canine parvovirus (82). We further show here that the VP2 proteins accumulate as trimers and large aggregates in the insect cytoplasm (Fig. 1C). Therefore, VP phosphorylation may be also important in the orchestrated parvovirus morphogenesis, in particular at the stage of assembly of the trimeric intermediate into the T=1 capsid. Interestingly, the capsid subunits must indeed undergo a conformational change along this stage of the assembly process, since trimers expressed in cells (61) (Fig. 1A) or isolated as protein complex in vitro (Fig. 1B) failed to configure the epitope recognized by the B7 monoclonal antibody at the threefold axis of the capsid (23). VP phosphorylation may facilitate this conformational change or the recognition of the trimers by a morphogenetic cellular factor. However, VLPs were shown to exhibit the same three-dimensional crystal structure as native phosphorylated MVM capsid (20, 27, 42), indicating that phosphorylation does not shape the final configuration of the amino acid residues in the formed capsid.
Raf-1 targeting in MVM anticancer potential.
The Raf kinase isoforms are frequently overexpressed, activated, or mutated in glioma, melanoma, and pancreatic carcinomas (15, 16, 38, 77, 78). The findings that Raf-1 phosphorylation (i) plays a key role in the nuclear entry of MVM capsid assembly intermediates, (ii) is required for virus maturation, and (iii) supports propagation of the lytic infection in glioma and other human transformed cells is thus supporting current searches for inhibitors of the Raf signaling cascade for cancer treatment (19, 45, 63). Disruption of the Raf-1-Rb interaction by RRD-251 suppresses tumor growth (26), and Sorafenib or Nexavar (Bayer 43-9006), designed to counter Raf kinases, is currently in phase III clinical trials for melanoma treatment and is approved for metastatic renal cancer (30). However, concerns about the specificity and efficacy of these inhibitors in the clinic were raised, since therapeutic benefit may be caused by side inhibition of V-EGFR needed for angiogenesis (reviewed in reference 39) and Raf-1 may be activated as consequence of B-Raf inhibition (77). Parvoviruses, which show antineoplastic activity in animal models (11, 54), may thus become alternative and more specific agents in combined therapies against certain cancer types. Our results support MVM, a mouse parvovirus that is apathogenic for humans, as a cytotoxic and replicative inhibitor targeting common cancers with a deregulated Raf signaling cascade.
Acknowledgments
We gratefully acknowledge C. J. Marshall (CRC, Institute of Cancer Research, London, United Kingdom) for the plasmid expressing the RafCAAX active form of Raf-1 and T. M. Roberts (Dana-Farber Cancer Institute, Boston, MA) for the Bac-Raf-1 and Bac-Raf22W constructs.
This study was supported by grants from the Spanish Ministerio de Ciencia e Innovación (SAF2008-03238) and Comunidad de Madrid (S-SAL/0185/2006) to the laboratory of J.M.A. and by an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa.” L.R. was supported in part by a short-term EMBO fellowship.
Footnotes
Published ahead of print on 25 November 2009.
REFERENCES
- 1.Adam, S. A., R. S. Marr, and L. Gerace. 1990. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns, K., and C. R. Parrish. 2007. Parvoviridae, p. 2437-2477. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 3.Bialojan, C., and A. Takai. 1988. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases: specificity and kinetics. Biochem. J. 256:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehmer, T., S. Jeudy, Ian C. Berke, and T. U. Schwartz. 2008. Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex. Mol. Cell 30:721-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillet-Fauquet, P., M. Perros, A. Brandenburge, P. Spegelaere, and J. Rommelaere. 1990. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding nonstructural proteins. EMBO J. 9:2989-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreira, A., M. Menéndez, J. Reguera, J. M. Almendral, and M. G. Mateu. 2004. In vitro disassembly of a parvovirus capsid and effect on capsid stability of heterologous peptide insertions in surface loops. J. Biol. Chem. 279:6517-6525. [DOI] [PubMed] [Google Scholar]
- 7.Chiocca, E. A. 2002. Oncolytic viruses. Nat. Rev. Cancer 2:938-950. [DOI] [PubMed] [Google Scholar]
- 8.Chong, H., H. G. Vikis, and K. L. Guan. 2003. Mechanisms of regulating the Raf kinase family. Cell Signal. 15:463-469. [DOI] [PubMed] [Google Scholar]
- 9.Cingolani, G., J. Bednenko, M. T. Gillespie, and L. Gerace. 2002. Molecular basis for the recognition of a nonclassical nuclear localization signal by importin beta. Mol. Cell 10:1345-1353. [DOI] [PubMed] [Google Scholar]
- 10.Citovsky, V., D. Warnick, and P. Zambryski. 1994. Nuclear import of Agrobacterium Vird2 and VirE2 proteins in maize and tobacco. Proc. Natl. Acad. Sci. U. S. A. 91:3210-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis, J. J., L. Deleu, U. Koch, and R. Rommelaere. 2006. Parvovirus oncosuppression, p. 365-378. In J. R. Kerr, S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 12.Cotmore, S. F., and P. Tattersall. 2007. Parvoviral host range and cell entry mechanisms. Adv. Virus Res. 70:183-232. [DOI] [PubMed] [Google Scholar]
- 13.Crawford, L. V. 1966. A minute virus of mice. Virology 29:605-612. [DOI] [PubMed] [Google Scholar]
- 14.Cukor, G., N. R. Blacklow, S. Kibrick, and I. C. Swan. 1975. Effect of adeno-associated virus on cancer expression by herpesvirus-transformed hamster cells. J. Natl. Cancer Inst. 44:957-959. [DOI] [PubMed] [Google Scholar]
- 15.Davies, H., G. R. Bignell, C. Cox, P. Stephens, S. Edkins, S. Clegg, J. Teague, H. Woffendin, M. J. Garnett, W. Bottomley, N. Davis, E. Dicks, R. Ewing, Y. Floyd, K. Gray, S. Hall, R. Hawes, J. Hughes, V. Kosmidou, A. Menzies, C. Mould, A. Parker, C. Stevens, S. Watt, S. Hooper, R. Wilson, H. Jayatilake, B. A. Gusterson, C. Cooper, J. Shipley, D. Hargrave, K. Pritchard-Jones, N. Maitland, G. Chenevix-Trench, G. J. Riggins, D. D. Bigner, G. Palmieri, A. Cossu, A. Flanagan, A. Nicholson, J. W. Ho, S. Y. Leung, S. T. Yuen, B. L. Weber, H. F. Seigler, T. L. Darrow, H. Paterson, R. Marais, C. J. Marshall, R. Wooster, M. R. Stratton, and P. A. Futreal. 2002. Mutations of the BRAF in human cancer. Nature 417:949-954. [DOI] [PubMed] [Google Scholar]
- 16.Dhomen, N., J. S. Reis-Filho, S. da Rocha Dias, R. Hayward, K. Savage, V. Delmas, L. Larue, C. Pritchard, and R. Marais. 2009. Oncogenic Braf induces melanocyte senescente and melanoma in mice. Cancer Cell 15:294-303. [DOI] [PubMed] [Google Scholar]
- 17.Finlay, D. R., D. D. Newmeywe, T. M. Price, and D. J. Forbes. 1987. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J. Cell Biol. 104:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorlich, D., S. Prehn, R. A. Laskey, and E. Hartmann. 1994. Isolation of a protein that is essential for the first step of nuclear protein import. Cell 79:767-778. [DOI] [PubMed] [Google Scholar]
- 19.Gray-Schopfer, V., C. Wellbrock, and R. Marais. 2007. Melanoma biology and new targeted therapy. Nature 445:851-857. [DOI] [PubMed] [Google Scholar]
- 20.Hernando, E., A. L. Llamas-Saiz, C. Foces-Foces, R. McKenna, L. Portman, M. Agbandje-McKenna, and J. M. Almendral. 2000. Biochemical and physical characterization of parvovirus minute virus of mice virus-like particles. Virology 267:299-309. [DOI] [PubMed] [Google Scholar]
- 21.Hoss, A., I. Moarefi, K. H. Scheidtmann, L. J. Cisek, J. L. Corden, I. Dornreiter, A. K. Arthur, and E. Fanning. 1990. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J. Virol. 64:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalderon, D., W. D. Richardson, A. F. Markham, and A. E. Smith. 1984. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 311:33-38. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann, B., A. López-Bueno, M. G. Mateu, P. R. Chipman, C. D. S. Nelson, C. R. Parrish, J. M. Almendral, and M. G. Rossmann. 2007. Minute virus of mice, a parvovirus, in complex with the Fab fragment of a neutralizing monoclonal antibody. J. Virol. 81:9851-9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr, J. K., S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish (ed.). 2006. Parvoviruses. Hodder Arnold, London, United Kingdom.
- 25.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605-615. [DOI] [PubMed] [Google Scholar]
- 26.Kinkade, R., P. Dasgupta, A. Carie, D. Pernazza, M. Carless, S. Pillai, N. Lawrence, S. M. Sebti, and S. Chellappan. 2008. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Canc. Res. 68:3810-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontou, M., L. Govindasamy, H. J. Nam, N. Bryant, A. L. Llamas-Saiz, C. Foces-Foces, E. Hernando, M. P. Rubio, R. McKenna, J. M. Almendral, and M. Agbandje-McKenna. 2005. Structural determinants of tissue tropism and in vivo pathogenicity for the parvovirus minute virus of mice. J. Virol. 79:10931-10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosugi, S., M. Hasebe, N. Matsumura, H. Takashima, E. Miyamoto-Sato, M. Tomita, and H. Yanagawa. 2009. Six classes of nuclear localization signals specific to different binding grooves of importin α. J. Biol. Chem. 284:478-485. [DOI] [PubMed] [Google Scholar]
- 29.Lange, A., R. E. Mills, S. E. Devine, and A. H. Corbett. 2008. A PY-NLS nuclear targeting signal is required for nuclear localization and function of the Saccharomyces cerevisiae mRNA-binding protein Hrp1. J. Biol. Chem. 283:12926-12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larkin, J. M., S. Chowdhury, and M. E. Gore. 2007. Drug insight: advances in renal cell carcinoma and the role of targeted therapies. Nat. Clin. Pract. Oncol. 4:470-479. [DOI] [PubMed] [Google Scholar]
- 31.Lee, B., A. E. Cansizoglu, K. E. Suel, T. H. Louis, Z. Zhang, and Y. M. Chook. 2006. Rules for nuclear localization sequence recognition by karyopherin β2. Cell 126:543-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, S. J., T. Sekimoto, E. Yamashita, E. Nagoshi, A. Nakagawa, N. Imamoto, M. Yoshimura, H. Sakai, K. T. Chong, T. Tsukihara, and Y. Yoneda. 2003. The structure of importin-β bound to SREBP-2: nuclear import of a transcription factor. Science 302:1571-1575. [DOI] [PubMed] [Google Scholar]
- 33.Leevers, S. J., H. F. Paterson, and C. J. Marshall. 1994. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369:411-414. [DOI] [PubMed] [Google Scholar]
- 34.Li, M., S. E. Delos, L. Montross, and R. L. Garcea. 1995. Polyomavirus VP1 phosphorylation: coexpression with the VP2 capsid protein modulates VP1 phosphorylation in Sf9 insect cells. Proc. Natl. Acad. Sci. U. S. A. 92:5992-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, W., M. Melnick, and N. Perrimon. 1998. Dual function of Ras in Raf activation. Development 125:4999-5008. [DOI] [PubMed] [Google Scholar]
- 36.Lombardo, E., J. C. Ramírez, J. García, and J. M. Almendral. 2002. Complementary roles of multiple nuclear targeting signals in the capsid proteins of the parvovirus minute virus of mice during assembly and onset of infection. J. Virol. 76:7049-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lombardo, E., J. C. Ramírez, M. Agbandje-Mckenna, and J. M. Almendral. 2000. A β-stranded motif drives capsid proteins oligomers of the parvovirus minute virus of mice into the nucleus for viral assembly. J. Virol. 74:3804-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyustikman, Y., H. Momota, W. Pao, and E. C. Holland. 2008. Constitutive activation of Raf-1 induces glioma formation in mice. Neoplasia 10:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madhunapantula, S. V., and G. P. Robertson. 2008. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 68:5-8. [DOI] [PubMed] [Google Scholar]
- 40.Mansour, S. J., W. T. Matten, A. S. Hermann, J. M. Candia, S. Rong, K. Fukasawa, G. F. Vande Woude, and N. G. Ahn. 1994. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265:966-970. [DOI] [PubMed] [Google Scholar]
- 41.Marais, R., and C. J. Marshall. 1996. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 27:101-125. [PubMed] [Google Scholar]
- 42.Maroto, B., J. C. Ramírez, and J. M. Almendral. 2000. Phosphorylation status of the parvovirus minute virus of mice particle: mapping and biological relevance of the major phosphorylation sites. J. Virol. 74:10892-10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maroto, B., N. Valle, R. Saffrich, and J. M. Almendral. 2004. Nuclear export of the non-enveloped parvovirus virion is directed by an unordered protein signal exposed on the capsid surface. J. Virol. 78:10685-10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattaj, I. W., and L. Englmeier. 1998. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67:265-306. [DOI] [PubMed] [Google Scholar]
- 45.McCubrey, J. A., L. S. Steelman, W. H. Chappell, S. L. Abrams, E. W. T. Wong, F. Chang, B. Lehmann, D. M. Terrian, M. Milella, A. Tafuri, F. Stivala, M. Libra, J. Basecke, C. Evangelisti, A. M. Martelli, and R. A. Franklin. 2007. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation, and drug resistance. Biochem. Biophys. Acta 1773:1263-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mogila, V., F. Xia, and W. X. Li. 2006. An intrinsic cell cycle checkpoint pathway mediated by MEK and ERK in Drosophila. Dev. Cell 11:575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mousset, S., and J. Rommelaere. 1982. Minute virus of mice inhibits cell transformation by simian virus 40. Nature 300:537-539. [DOI] [PubMed] [Google Scholar]
- 48.Noble, C., K. Mercer, J. Hussain, L. Carragher, S. Giblett, R. Hayward, C. Patterson, R. Marais, and C. A. Pritchard. 2008. CRAF autophosphorylation of serine 621 is required to prevent its proteasome-mediated degradation. Mol. Cell 31:862-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuesch, J. P. F., and J. Rommelaere. 2007. A viral adaptor protein modulating casein kinase II activity induces cytopathic effects in permissive cells. Proc. Natl. Acad. Sci. U. S. A. 104:12482-12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Old, W. M., J. B. Shabb, S. Houel, H. Wang, K. L. Couts, C. Y. Yen, E. S. Litman, C. H. Croy, K. Meyer-Arendt, J. G. Miranda, R. A. Brown, E. S. Witze, R. E. Schweppe, K. A. Resing, and N. G. Ahn. 2009. Functional proteomics identifies targets of phosphorylation by B-Raf signalling in melanoma. Mol. Cell 34:115-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parato, K. A., D. Senger, P. A. J. Forsyth, and J. C. Bell. 2005. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 5:965-976. [DOI] [PubMed] [Google Scholar]
- 52.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 53.Pollard, V. W., W. M. Michael, S. Nakielny, M. C. Siomi, F. Wang, and G. Dreyfuss. 1996. A novel receptor-mediated nuclear protein import pathway. Cell 86:985-994. [DOI] [PubMed] [Google Scholar]
- 54.Ponnazhagan, S. 2008. Adeno-associated virus, p. 55-68. In K. J. Harrington, R. G. Vile, and H. S. Pandha (ed.), Viral therapy of cancer. John Wiley & Sons, Ltd., West Sussex, United Kingdom.
- 55.Pratilas, C. A., B. S. Taylor, Q. Ye, A. Viale, C. Sander, D. B. Solit, and N. Rosen. 2009. v600EBRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc. Natl. Acad. Sci. U. S. A. 106:4519-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabe, B., A. Vlachou, N. Pante, A. Helenius, and M. Kann. 2003. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc. Natl. Acad. Sci. U. S. A. 100:9849-9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raj, K., P. Ogston, and P. Beard. 2001. Virus-mediated killing of cells that lack p53 activity. Nature 412:914-918. [DOI] [PubMed] [Google Scholar]
- 58.Raman, M., W. Chen, and M. H. Cobb. 2007. Differential regulation and properties of MAPKs. Oncogene 26:3100-3112. [DOI] [PubMed] [Google Scholar]
- 59.Reguera, J., A. Carreira, L. Riolobos, J. M. Almendral, and M. G. Mateu. 2004. Role of interfacial amino acid residues in assembly, stability, and conformation of a spherical virus capsid. Proc. Natl. Acad. Sci. U. S. A. 101:2724-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rihs, H.-P., D. A. Jans, H. Fan, and R. Peters. 1991. The rate of nuclear cytoplasmic protein transport is determined by the casein kinase II site flanking the nuclear localization sequence of the SV40 T-antigen. EMBO J. 10:633-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riolobos, L., J. Reguera, M. G. Mateu, and J. M. Almendral. 2006. Nuclear transport of trimeric assembly intermediates exerts a morphogenetic control on the icosahedral parvovirus capsid. J. Mol. Biol. 357:1026-1038. [DOI] [PubMed] [Google Scholar]
- 62.Robbins, J., S. M. Dilworth, R. A. Laskey, and C. Dingwall. 1991. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64:651-653. [DOI] [PubMed] [Google Scholar]
- 63.Roberts, P. J., and C. J. Der. 2007. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291-3310. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Viciana, P., C. Collins, and M. Fried. 2006. Polyoma and SV40 proteins differentially regulate PP2A to activate distinct cellular signaling pathways involved in growth control. Proc. Natl. Acad. Sci. U. S. A. 103:19290-19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubio, M. P., S. Guerra, and J. M. Almendral. 2001. Genome replication and postencapsidation functions mapping to the nonstructural gene restrict the host range of a murine parvovirus in human cells. J. Virol. 75:11573-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salomé, N., B. van Hille, N. Duponchel, G. Meneguzzi, F. Cuzin, J. Rommelaere, and J. J. Cornelis. 1990. Sensitization of transformed rat cells to parvovirus MVMp is restricted to specific oncogenes. Oncogene 5:123-130. [PubMed] [Google Scholar]
- 67.Schubbert, S., K. Shannon, and G. Bollag. 2007. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7:295-308. [DOI] [PubMed] [Google Scholar]
- 68.Soga, S., T. Kozawa, H. Narumi, S. Akinaga, K. Matsumoto, K. Irie, S. V. Sharma, H. Nakano, T. Mizukami, and M. Harai. 1998. Radicicol leads to selective depletion of raf kinase and disrupts K-ras-activated aberrant signalling pathway. J. Biol. Chem. 273:822-828. [DOI] [PubMed] [Google Scholar]
- 69.Spegelaere, P., B. van Hille, N. Spruyt, S. Faisst, J. J. Cornelis, and J. Rommelaere. 1991. Initiation of transcription from the minute virus of mice P4 promoter is stimulated in rat cells expressing a c-Ha-ras oncogene. J. Virol. 65:4919-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart, M. 2007. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell. Biol. 8:195-208. [DOI] [PubMed] [Google Scholar]
- 71.Strong, J. E., G. Wong, S. E. Jones, A. Grolla, S. Theriault, G. P. Kobinger, and H. Feldmann. 2008. Stimulation of Ebola virus production from persistent infection through activation of the Ras/MAPK pathway. Proc. Natl. Acad. Sci. U. S. A. 105:17982-17987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tattersall, P., and J. Bratton. 1983. Reciprocal productive and restrictive virus-cell interaction of immunosuppressive and prototype strains of minute virus of mice. J. Virol. 46:944-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Telerman, A., M. Tuynder, T. Dupressoir, B. Robaye, F. Sigaux, E. Shaulian, M. Oren, J. Rommelaere, and R. Amson. 1993. A model of tumor suppression using H-1 parvovirus. Proc. Natl. Acad. Sci. U. S. A. 90:8702-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toolan, H., and N. Ledinko. 1965. Growth and cytopathogenicity of H-viruses in human and simian cell cultures. Nature 208:812-813. [DOI] [PubMed] [Google Scholar]
- 75.Tran, E. J., and S. R. Wente. 2006. Dynamic nuclear pore complexes: life on the edge. Cell 125:1041-1053. [DOI] [PubMed] [Google Scholar]
- 76.Tsao, J., M. S. Chapman, M. Agbandje, W. Séller, K. Smith, H. Wu, M. Luo, T. J. Smith, M. G. Rossmann, R. W. Compans, and C. R. Parrish. 1991. The three-dimensional structure of canine parvovirus and its functional implications. Science 251:1456-1464. [DOI] [PubMed] [Google Scholar]
- 77.Wan, P. T., M. J. Garnett, S. M. Roe, S. Lee, D. Niculescu-Duvaz, V. M. Good, C. M. Jones, C. J. Marshall, C. J. Springer, D. Barford, and R. Marais. 2004. Mechanism of activation of the RAF-ERK signalling pathway by oncogenic mutations of B-RAF. Cell 116:855-867. [DOI] [PubMed] [Google Scholar]
- 78.Wellbrock, C., M. Karasarides, and R. Marais. 2004. The Raf proteins take centre stage. Nat. Rev. Mol. Cell. Biol. 5:875-885. [DOI] [PubMed] [Google Scholar]
- 79.Williams, N. G., H. Paradis, S. Agarwal, D. L. Charest, S. L. Pelech, and T. M. Roberts. 1993. Raf-1 and p21v-ras cooperate in the activation of mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 90:5772-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams, N. G., T. M. Roberts, and P. Li. 1992. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc. Natl. Acad. Sci. U. S. A. 89:2922-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xia, K., N. K. Mukhopadhyay, R. C. Inhorn, D. L. Barber, P. E. Rose, R. S. Lee, R. P. Narsimhan, A. D. D'Andrea, J. D. Griffin, and T. M. Roberts. 1996. The cytokine-activated tyrosine kinase JAK2 activates Raf-1 in a p21ras-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 93:11681-11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan, W., and C. R. Parrish. 2001. Canine parvovirus capsid assembly and differences in mammalian and insect cells. Virology 279:546-557. [DOI] [PubMed] [Google Scholar]
- 83.Zheng, Y., J. Li, D. L. Johnson, and J. H. Ou. 2003. Regulation of hepatitis B virus replication by the ras-mitogen-activated protein kinase signaling pathway. J. Virol. 77:7707-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]