Abstract
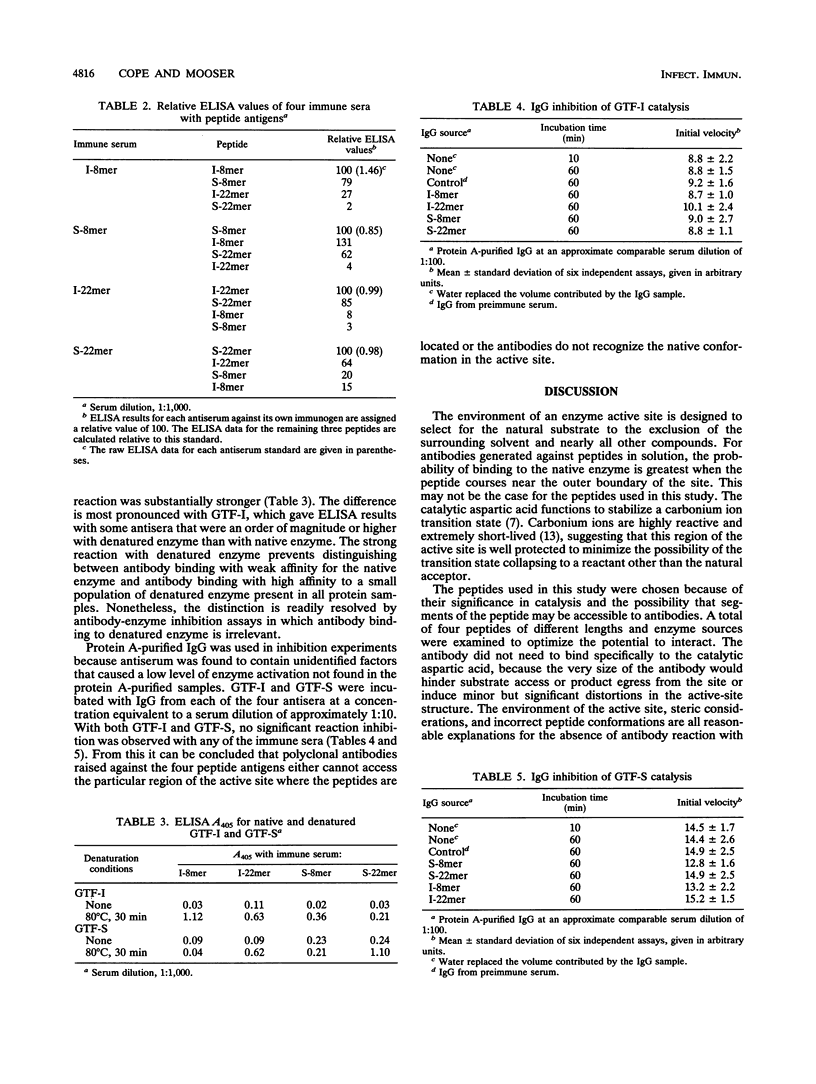
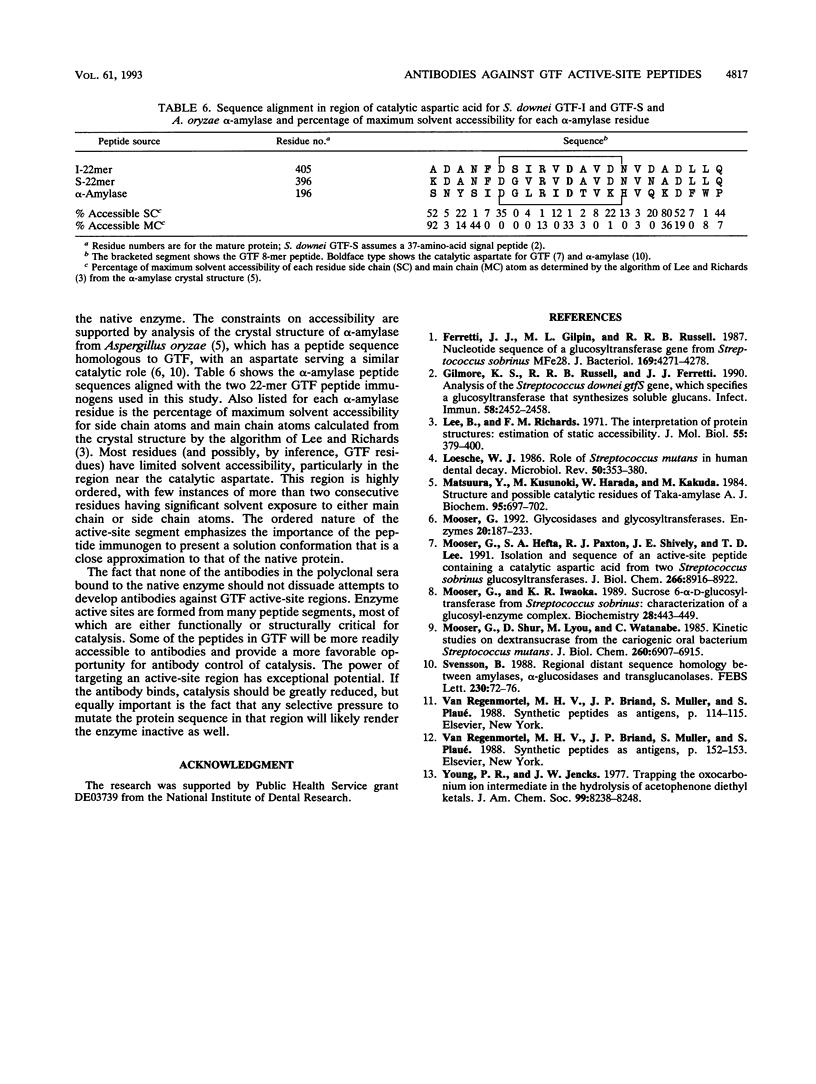
Polyclonal antibodies were raised against peptides derived from an active-site sequence common to the family of mutans streptococcal glucosyltransferases (GTFs). The sequence contains an aspartic acid residue that functions in formation of the enzyme transition state in catalysis. Two GTFs were targeted with similar but not identical sequences in this region: one that synthesizes an alpha-1,3-linked water-insoluble glucan and a homologous GTF that synthesizes an alpha-1,6-linked water-soluble glucan. For each enzyme, an 8-mer and 22-mer peptide were prepared. The two peptide lengths were chosen in order to increase the likelihood of the peptides folding in a conformation similar to that of the native enzyme. Each peptide immunogen produced high titers of antibody in rabbits, and all antisera cross-reacted with all peptides, albeit to various degrees. Native enzyme showed weak interaction with antisera, which, on the basis of enzyme denaturation experiments, likely reflects binding to a small but finite population of denatured enzyme in the sample. GTF was assayed for inhibition in the presence of protein A-purified immunoglobulin G from each antiserum. Given the mass of the antibody and catalytic importance of the peptide, any enzyme-antibody complex formation would result in enzyme inhibition. No significant inhibition was observed, which demonstrates that either polyclonal antibodies raised against each of the four peptides cannot access this active-site region, or antibodies do not recognize the native enzyme conformation. The advantages and challenges of generating antibodies against enzyme active-site peptides are discussed in the context of the crystal structure of Aspergillus oryzae alpha-amylase, which has a homologous peptide segment which serves the same catalytic function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore K. S., Russell R. R., Ferretti J. J. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect Immun. 1990 Aug;58(8):2452–2458. doi: 10.1128/iai.58.8.2452-2458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Kusunoki M., Harada W., Kakudo M. Structure and possible catalytic residues of Taka-amylase A. J Biochem. 1984 Mar;95(3):697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]
- Mooser G., Hefta S. A., Paxton R. J., Shively J. E., Lee T. D. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J Biol Chem. 1991 May 15;266(14):8916–8922. [PubMed] [Google Scholar]
- Mooser G., Iwaoka K. R. Sucrose 6-alpha-D-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl-enzyme complex. Biochemistry. 1989 Jan 24;28(2):443–449. doi: 10.1021/bi00428a006. [DOI] [PubMed] [Google Scholar]
- Mooser G., Shur D., Lyou M., Watanabe C. Kinetic studies on dextransucrase from the cariogenic oral bacterium Streptococcus mutans. J Biol Chem. 1985 Jun 10;260(11):6907–6915. [PubMed] [Google Scholar]
- Svensson B. Regional distant sequence homology between amylases, alpha-glucosidases and transglucanosylases. FEBS Lett. 1988 Mar 28;230(1-2):72–76. doi: 10.1016/0014-5793(88)80644-6. [DOI] [PubMed] [Google Scholar]


