Abstract
Many RNA and DNA viruses activate serine-threonine kinase AKT to increase virus replication. In contrast, measles virus (MV) infection leads to downregulation of AKT. This is thought to be beneficial for the virus because it correlates with immune suppression. To determine whether this is a sacrifice for the virus, we used a recombinant virus and transfected cells expressing constitutively active AKT and evaluated its effect on virus replication. In vitro, overexpression of AKT did not influence virus replication but did affect (cell-type dependent) virus release. In vivo, the recombinant virus did not abrogate inhibition of proliferation of spleen cells from MV-infected cotton rats.
The serine-threonine kinase AKT plays a central role in growth regulation of cells. After phosphorylation, activated AKT affects multiple cellular targets that increase metabolism, growth, and synthetic processes and suppress apoptosis (4). Viruses, as intracellular parasites, rely on the growth environment provided by the cell. In agreement with this dependency, a variety of RNA (3) and most DNA (2, 6) viruses stimulate AKT activity after infection of cells and are able to overcome control of AKT expression by cellular regulatory mechanisms. An exception is measles virus (MV), which not only does not stimulate AKT but reduces expression of phosphorylated (active) AKT after infection (1). This reduction has been shown to reduce proliferation of T cells after infection with the virus or contact with viral glycoproteins in vitro and in vivo (16, 9). In consequence, in vivo antigen-specific CD4 and CD8 T-cell responses against third-party antigens are diminished, and this is thought to be (partially) responsible for measles virus-induced immune suppression (10). However, a recent publication suggested that this might be a trade-off for the virus (17). In measles virus-infected Vero cells which had been treated with the AKT-specific inhibitor IV, the number of virus particles released into the supernatant was reduced. In contrast, intracellularly produced virus levels were not affected by AKT inhibition. When we repeated these experiments, we essentially obtained the same results (data not shown). Because AKT inhibitors severely impair the viability of cells, only a short window of time is available to distinguish between the effects of AKT inhibition and cell death on virus replication. For this reason, we planned to supplement virus-infected cells with AKT to measure its effect on virus growth. Since AKT cannot be selectively induced pharmacologically, a recombinant measles virus was constructed with an additional gene for myristoylated AKT (5) (GenBank accession no. AY781100) between the hemagglutinin gene and the polymerase gene using a molecular clone (NSE) based on the Edmonston B strain (vaccine-related) virus (14). This molecularly cloned virus expressed myristoylated AKT with a glutamine-glutamine tag in infected cells (as detected by Western blotting). After translocation to the inner plasma membrane due to the myristoylation signal, AKT was phosphorylated independently of regulatory cellular signals and led to phosphorylation of downstream glycogen synthase kinase 3 (GSK-3) (see Fig. 2). This was true in the Vero fibroblastoid cell line (African green monkey; a standard measles virus substrate; data not shown) and in the Jurkat lymphoid cell line (human CD4-positive T-cell line; see Fig. 2).
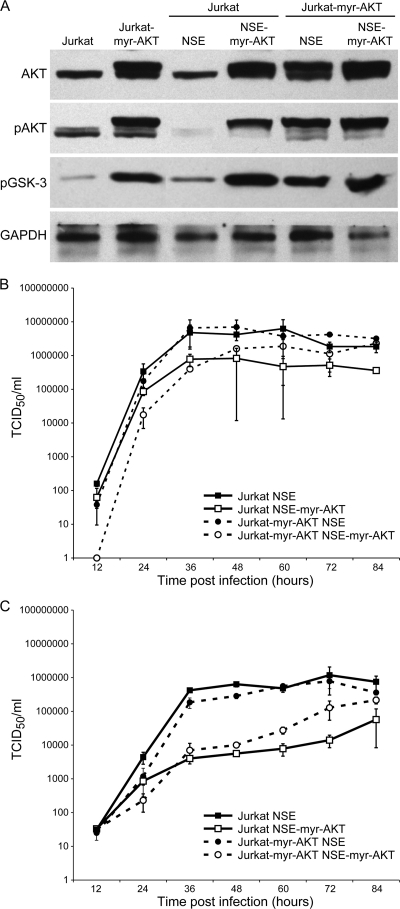
FIG. 2.
No influence of AKT on viral growth in the lymphoid cell line Jurkat. (A) Total AKT, pAKT S473, and pGSK-3 expression from NSE- and NSE-myr-AKT-infected (MOI of 0.02) Jurkat and Jurkat-myr-AKT cells. Myr-AKT is seen as a doublet; the upper band is the myristoylated form, which is not affected by MV. Kinetics of intracellular progeny accumulation (B) and progeny release (C) are shown for 84 h postinfection from Ed-NSE- and NSE-myr-AKT-infected (MOI of 0.02) Jurkat and Jurkat-myr-AKT cells. Biologically relevant titers of more than 10-fold were observed at 36, 48, and 60 h (P at least < 0.01) postinfection.
In Vero cells, different growth parameters were investigated in comparing the parental NSE and recombinant NSE-myr-AKT virus. These parameters included the polymerase elongation rate, the rate of viral transcription and replication, glycoprotein production, and intracellular virus production and release from cells. The measurement of the polymerase elongation rate, viral transcription, and genome replication was carried out as published previously (see reference 12 for procedure and primers). Cell-free virus was purified from the supernatant of MV-infected Vero cells by sedimentation on a discontinuous sucrose gradient as described previously (8). RNA from infected Vero cells (multiplicity of infection [MOI] of 1) was isolated using the Qiagen RNeasy kit (Qiagen), reverse transcribed, and amplified by quantitative PCR (qPCR) using FastStart DNA Master SYBR green I reagents in the Light Cycler 480 system from Roche Applied Science (Meylan, France). All experiments were carried out in duplicate, and data were analyzed using the Light Cycler 480 software and were expressed as copy numbers per 2 μg of RNA.
The elongation rate for the parental NSE virus was comparable to previously published data (1.33 nucleotides [nt]/s). In comparison, the polymerase elongation rate of NSE-myr-AKT was reduced (0.66 nt/s). Viral transcription and genome replication were slightly lower for NSE-myr-AKT (Fig. 1 and data not shown). This slight difference did not translate into measurable differences in the level of glycoproteins or virus replication. The expression of both the hemagglutinin and fusion proteins was identical 24 h after infection by flow cytometry (data not shown). To obtain kinetic growth curves, Vero cells in 6-well plates were infected with NSE or NSE-myr-AKT (MOI of 0.02), and tissue culture supernatants and cell lysates were harvested at 0, 12, 24, 36, 48, 60, and 72 h postinfection. Virus titers were quantified by the 50% tissue culture infectious dose (TCID50) assay. For both viruses, the levels of both intracellular virus and virus released from cells were similar over time (Fig. 1).
FIG. 1.
Measles virus replication after AKT overexpression in the fibroblast cell line Vero. Accumulation of N mRNA (A) during primary transcription from Vero cells infected with NSE or NSE-myr-AKT infected (MOI of 1.0). The kinetics of genome accumulation (B) and progeny release (C) at 72 h postinfection from NSE- and NSE-myr-AKT-infected (MOI of 0.02) Vero cells did not differ. The slight differences seen in accumulation of N mRNA and genome were observed in two independent experiments but did not reach statistical significance. Viral titers also were not statistically significant different.
In order to test whether the complete lack of stimulation of virus growth by myr-AKT in Vero cells was cell (type) specific, the level of virus replication in Jurkat cells was also assessed. Jurkat cells have the additional advantage that transfected variants which overexpress myr-AKT exist (Jurkat-myr-AKT) (7). Whereas contact with virus leads to downregulation of AKT and inhibits proliferation of Jurkat cells, this inhibition is overcome in Jurkat cells transfected with myr-AKT (1). The levels of AKT, pAKT, and pGSK-3 in uninfected Jurkat and Jurkat-myr-AKT cells or cells infected with NSE or NSE-myr-AKT were determined by Western blotting with polyclonal antibodies against AKT, pAKT serine 473, and pGSK-3 (Cell Signaling). After infection of Jurkat cells with measles virus, the expression of endogenous phosphorylated AKT is strongly reduced (Fig. 2A). The myristoylated form is not affected by infection whether it is expressed in transfected cells or by the recombinant virus. After infection of both Jurkat cells and Jurkat-myr-AKT cells with either NSE or NSE-myr-AKT, no difference in intracellular virus levels could be detected (Fig. 2). The level of progeny virus released by the cells was reduced after NSE-myr-AKT infection in comparison to results with NSE infection, irrespective of the cell line used (Jurkat cells or Jurkat-myr-AKT cells). To test whether these effects were specific for measles virus, Jurkat and Jurkat-myr-AKT cells were infected with vaccinia virus (Copenhagen strain). Vaccinia virus is a DNA virus and has been shown to induce upregulation of pAKT, and virus replication is reduced after inhibition of AKT phosphorylation. Infection of Jurkat-myr-AKT led to a reduction of viral titers at 24 h (4 × 105 PFU/ml) and 48 h (1.5 × 106 PFU/ml) in comparison to results with Jurkat cells (5.7 × 105 PFU/ml after 24 h; 1.2 × 107 PFU/ml after 48 h). These data indicate that increases in pAKT, which lead to activation of IRF-3 as part of the antiviral response (4), changes in microtubule stabilization (11), and actin filament remodeling (13) can reach a level detrimental to virus replication in general (at least in Jurkat-myr-AKT cells).
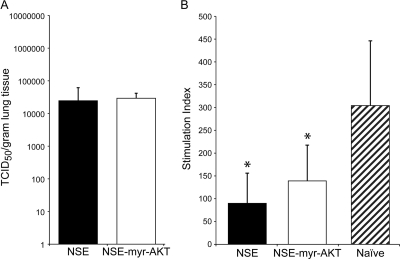
In order to determine the importance of AKT overexpression in vivo for virus growth, measles virus-specific immunity, and immune suppression, cotton rats were inoculated intranasally with either virus; lungs, mediastinal lymph node (MDLN) cells, and spleen cells were analyzed on day 4 after infection. For this analysis, female inbred cotton rats (Harlan, Indianapolis, IN) were infected intranasally (i.n.). To determine virus titers from lungs, lung tissue was homogenized and tested by the TCID50 method (15). NSE and NSE-myr-AKT viruses grew to comparable titers in cotton rat lungs (Fig. 3).
FIG. 3.
In vivo growth of measles virus is not influenced by AKT expression. There is no difference in viral lung titers between NSE- and NSE-myr-AKT-infected cotton rats at 4 days postinfection (A). Splenocyte proliferation is inhibited for both NSE- and NSE-myr-AKT-infected animals compared to that for naive cotton rats (5 animals per group). The differences between infected and naive animals were statistically significant (*, P < 0.05).
A hallmark of measles virus-induced immune suppression is the inhibition of mitogen-stimulated lymphocyte proliferation ex vivo. To test proliferation inhibition, proliferation of spleen cells from infected and naive animals was measured by the 3H-thymidine incorporation assay (9). After concanavalin A stimulation of spleen cells (as described in reference 9), proliferation was inhibited compared to that for naive animals independently of the virus used for infection. The most likely explanation for the lack of a difference in immune suppression is that pAKT is expressed in infected endothelial cells and macrophages. These cells still express MV glycoproteins on the cell surface, which, upon contact with T cells, leads to inhibition of AKT phosphorylation (9). In consequence, MV-specific T-cell responses induced after infection should not differ between the two viruses. To measure T-cell responses, an enzyme-linked immunospot (ELISPOT) assay was employed as described previously with modifications (18). Ninety-six-well Polysorb plates (Nunc) were coated with 100 μl of 3.3 μg/ml of goat anti-cotton rat gamma interferon (IFN-γ) (R&D Systems) capturing antibody per well overnight. Lymph node or spleen cells (in triplicate) were added and stimulated with 10 μg/ml of gradient-purified UV-inactivated measles virus antigen for 48 h at 37°C. After washing, the plate was incubated with a biotinylated goat anti-cotton rat IFN-γ detection antibody (R&D Systems) and subsequently with an alkaline phosphatase-conjugated rabbit antigoat antibody (Sigma). Finally, spots were developed by adding 3% agarose solution containing AMP-5-bromo-4-chloro-3-indolylphosphate (BCIP) substrate, as described previously for the B-cell ELISPOT assay (10). The local immune response is generated in the MDLN, and the number of IFN-γ-secreting T cells/106 lymph node cells in MDLN was 344 ± 75 after NSE infection and 315 ± 51 after NSE-myr-AKT infection. In the spleen, a secondary lymphoid organ, lower numbers of IFN-γ-secreting T cells were found (156 ± 22/106 spleen cells after NSE infection and 171 ± 16/106 spleen cells after infection with NSE-myr-AKT). These data indicate that the two viruses induced comparable T-cell responses after infection.
Usually, the amount of AKT expressed by cells is tightly regulated to maintain the amount necessary for cell survival. However, many RNA and DNA viruses profit from increased levels of pAKT expression and have devised strategies to increase it (3, 6, 2). Our data indicate that measles virus does not rely on increased levels of phosphorylated AKT for virus replication. However, there is some evidence that pAKT might influence virus release (17; this work). Measles virus reduces pAKT levels in infected cells or cells contacted by virus significantly (1). This reduction of pAKT in T cells leads to proliferation inhibition, which might be partially responsible for measles virus-induced immune suppression. In summary, this leads to the conclusion that the reduced amount of pAKT is no trade-off for the virus but apparently is an evolutionary strategy developed to contain immune responses.
Acknowledgments
This investigation was partially supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32RR007073 (to M.C.) from the National Center for Research Resources.
The plasmid expressing the bovine myristoylated AKT was kindly provided by Susheela Tridandapani, the Ohio State University, Columbus, OH. Jurkat cells expressing myristoylated AKT were a kind gift from Lawrence P. Kane, University of Pittsburgh, Pittsburgh, PA.
Footnotes
Published ahead of print on 25 November 2009.
REFERENCES
- 1.Avota, E., A. Avots, S. Niewiesk, L. P. Kane, U. Bommhardt, and V. S.-S. S. ter Meulen. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725-731. [DOI] [PubMed] [Google Scholar]
- 2.Buchkovich, N. J., Y. Yu, C. A. Zampieri, and J. C. Alwine. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat. Rev. Microbiol. 6:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooray, S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 85:1065-1076. [DOI] [PubMed] [Google Scholar]
- 4.Ehrhardt, C., and S. Ludwig. 2009. A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway. Cell Microbiol. 11:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganesan, L. P., G. Wei, R. A. Pengal, L. Moldovan, N. Moldovan, M. C. Ostrowski, and S. Tridandapani. 2004. The serine/threonine kinase Akt promotes Fc gamma receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J. Biol. Chem. 279:54416-54425. [DOI] [PubMed] [Google Scholar]
- 6.Ji, W. T., and H. J. Liu. 2008. PI3K-Akt signaling and viral infection. Recent Pat. Biotechnol. 2:218-226. [DOI] [PubMed] [Google Scholar]
- 7.Kane, L. P., V. S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-kappaB by the Akt/PKB kinase. Curr. Biol. 9:601-604. [DOI] [PubMed] [Google Scholar]
- 8.Naniche, D., G. Varior-Krishnan, G. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niewiesk, S., I. Eisenhuth, A. Fooks, J. C. S. Clegg, J.-J. Schnorr, S. Schneider-Schaulies, and V. ter Meulen. 1997. Measles virus-induced immune suppression in the cotton rat (Sigmodon hispidus) model depends on viral glycoproteins. J. Virol. 71:7214-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niewiesk, S., M. Götzelmann, and V. ter Meulen. 2000. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proc. Natl. Acad. Sci. U. S. A. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onishi, K., M. Higuchi, T. Asakura, N. Masuyama, and Y. Gotoh. 2007. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells 12:535-546. [DOI] [PubMed] [Google Scholar]
- 12.Plumet, S., and D. Gerlier. 2005. Optimized SYBR green real-time PCR assay to quantify the absolute copy number of measles virus RNAs using gene specific primers. J. Virol. Methods 128:79-87. [DOI] [PubMed] [Google Scholar]
- 13.Qian, Y., L. Corum, Q. Meng, J. Blenis, J. Z. Zheng, X. Shi, D. C. Flynn, and B. H. Jiang. 2004. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am. J. Physiol. Cell Physiol. 286:C153-C163. [DOI] [PubMed] [Google Scholar]
- 14.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dötsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles virus from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hygiene 27:493-497. [Google Scholar]
- 16.Schlender, J., J. J. Schnorr, T. Cattomen, R. Cattaneo, M. A. Billeter, V. Ter Meulen, and S. Schneider-Schaulies. 1996. Surface interaction of measles virus glycoproteins is necessary and sufficient for the induction of proliferative inhibition of human peripheral blood mononuclear cells. Proc. Natl. Acad. Sci. U. S. A. 93:13194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun, M., S. M. Fuentes, K. Timani, D. Sun, C. Murphy, Y. Lin, A. August, M. N. Teng, and B. He. 2008. Akt plays a critical role in replication of nonsegmented negative-stranded RNA viruses. J. Virol. 82:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan, X., J. L. Hurwitz, S. Krishnamurthy, T. Takimoto, K. Boyd, R. A. Scroggs, S. Surman, A. Portner, and K. S. Slobod. 2007. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine 25:8782-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]