Abstract
Cytokines are potentially useful in vaccination as adjuvants or modulators of the type of response induced. The work below describes the expression of a cloned cytokine gene for murine interleukin-4 (mIL-4) by a live vaccine vector, an attenuated aroA strain (SL7207) of Salmonella typhimurium, in a murine model system. SL7207 was used as a carrier for two different high-level expression vectors. Both resulting strains, designated SL7207(pOmpAmIL-4) and SL7207(pKKmIL-4), expressed the cloned gene product as monitored by both immunological and biological assays. However, SL7207(pOmpAmIL-4) produced mIL-4 at higher levels and was more stable in vitro than SL7207(pKKmIL-4). When SL7207(pOmpAmIL-4) was used as a live vaccine in BALB/c mice, this strain grew and survived at higher levels than the parental attenuated strain or empty plasmid-carrying strain in spleens, livers, and intestines. This difference in growth and survival did not appear to be caused by alterations in specific lymphocyte-mediated anti-Salmonella immune responses such as delayed-type hypersensitivity or serum antibody as measured by enzyme-linked immunosorbent assay; such alterations have been induced by IL-4 administration in other in vivo systems, and the lack of effect here may reflect the fact that IL-4 is not secreted from the bacteria in large quantities, most of the cytokine being in the cytoplasmic-membrane-bound fraction. Conversely, the ability of mouse macrophages to kill the bacteria in vitro was inhibited by bacterial production of mIL-4. This reduction in macrophage killing activity suggests that bacterial production of mIL-4 may be detrimental to host defense against Salmonella infection and may explain the enhanced bacterial growth and survival in vivo.
Full text
PDF
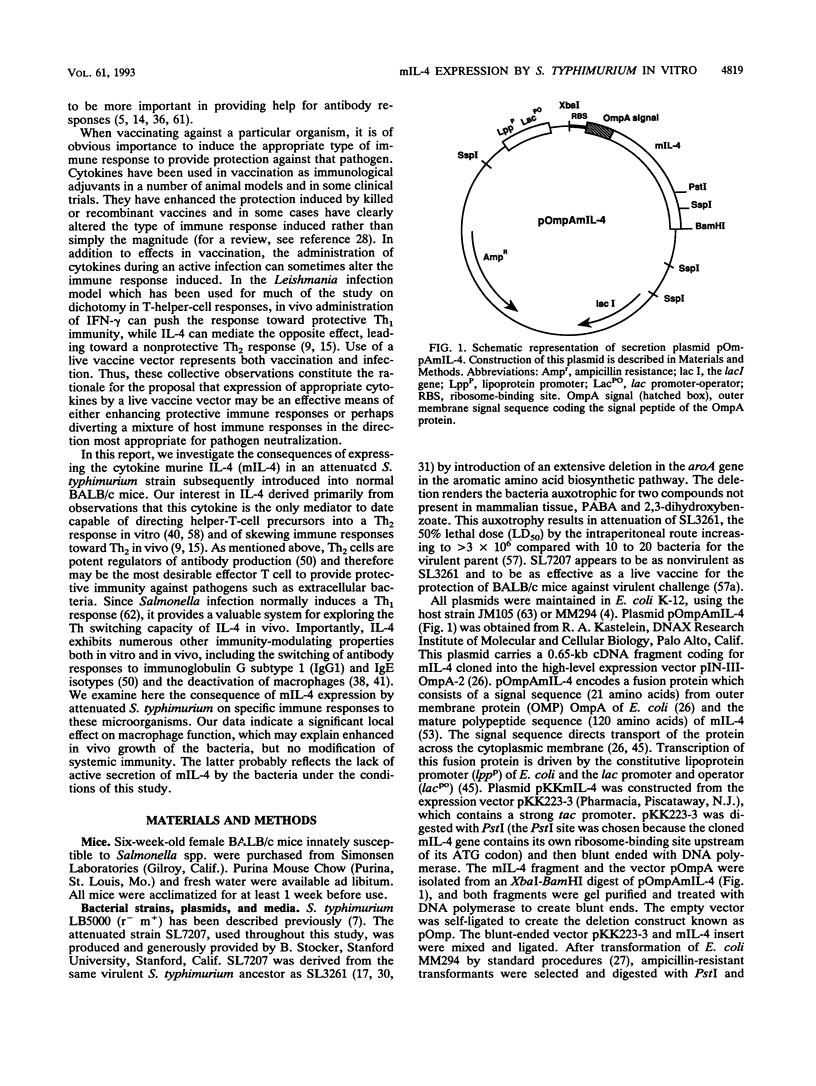


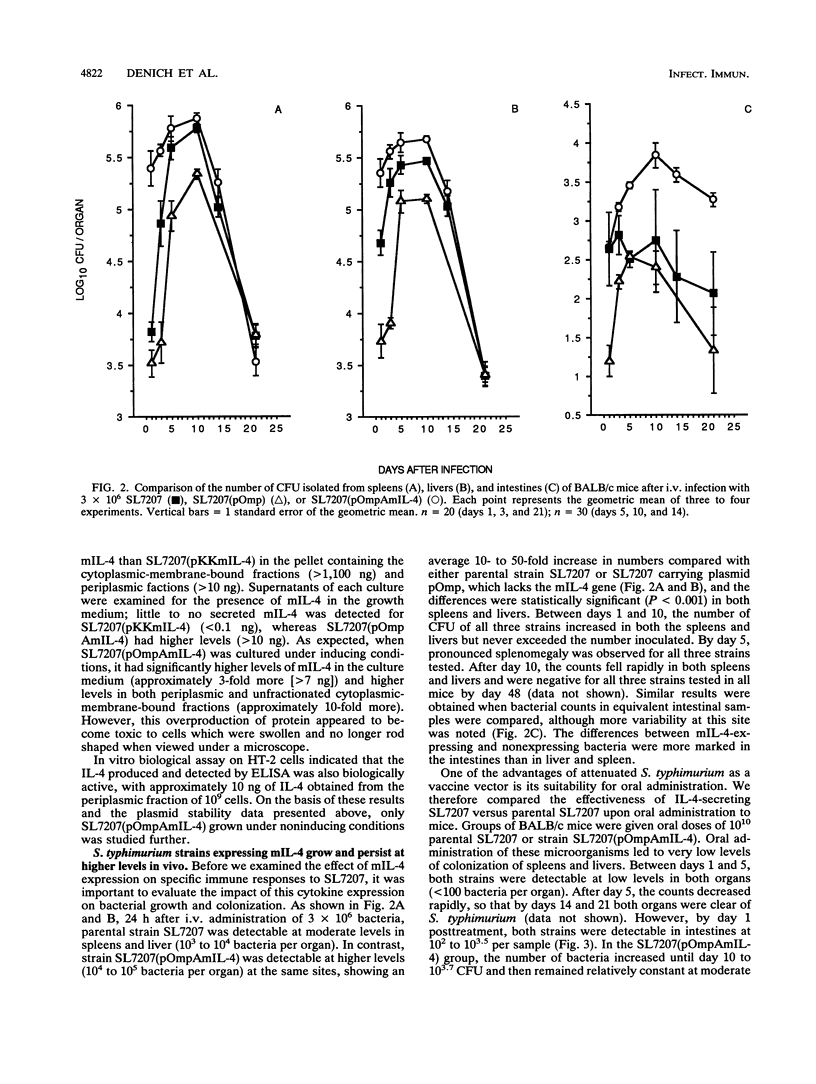



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom W. H., Liano D., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988 Apr 1;167(4):1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier M. J., Chatfield S. N., Dougan G., Nowicka U. T., O'Callaghan D., Beesley J. E., Milano S., Cillari E., Liew F. Y. Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992 Feb 15;148(4):1176–1181. [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Clements J. D. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect Immun. 1990 May;58(5):1159–1166. doi: 10.1128/iai.58.5.1159-1166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Cárdenas L. Vaccines against enterotoxigenic bacterial pathogens based on hybrid Salmonella that express heterologous antigens. Res Microbiol. 1990 Sep-Oct;141(7-8):981–993. doi: 10.1016/0923-2508(90)90138-g. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Powell C. J., Dubois D. R., Hartman A., Summers P. L., Eckels K. H. Expression of the envelope antigen of dengue virus in vaccine strains of Salmonella. Res Microbiol. 1990 Sep-Oct;141(7-8):855–858. doi: 10.1016/0923-2508(90)90121-6. [DOI] [PubMed] [Google Scholar]
- Edwards M. F., Stocker B. A. Construction of delta aroA his delta pur strains of Salmonella typhi. J Bacteriol. 1988 Sep;170(9):3991–3995. doi: 10.1128/jb.170.9.3991-3995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., De Cueninck B. J., Resavy D., Shockman G. D., Carey R. B., Swenson R. M. Quantitative determination in human sera of vaccine-induced antibody to type-specific polysaccharides of group B streptococci using an enzyme-linked immunosorbent assay. J Infect Dis. 1983 May;147(5):847–856. doi: 10.1093/infdis/147.5.847. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Sultzer B. M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984 Sep;150(3):425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- Fairweather N. F., Chatfield S. N., Makoff A. J., Strugnell R. A., Bester J., Maskell D. J., Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonella carrier. Infect Immun. 1990 May;58(5):1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Lyness V. A., Maskell D. J. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect Immun. 1987 Nov;55(11):2541–2545. doi: 10.1128/iai.55.11.2541-2545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Fitch F. W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990 Jun 1;144(11):4110–4120. [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heath A. W., Playfair J. H. Cytokines as immunological adjuvants. Vaccine. 1992;10(7):427–434. doi: 10.1016/0264-410x(92)90389-2. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Genes aroA and serC of Salmonella typhimurium constitute an operon. J Bacteriol. 1985 Jul;163(1):355–361. doi: 10.1128/jb.163.1.355-361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D., Attridge S., van den Bosch L., Hackett J. A chromosomal integration system for stabilization of heterologous genes in Salmonella based vaccine strains. Microb Pathog. 1988 Dec;5(6):407–418. doi: 10.1016/0882-4010(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Iizawa Y., Czuprynski C. Effects of administration of murine recombinant IL-4 on the resistance of mice to Listeria monocytogenes infection. Immunol Lett. 1992 Apr;32(2):185–189. doi: 10.1016/0165-2478(92)90113-3. [DOI] [PubMed] [Google Scholar]
- Jin F. S., Youle R. J., Johnson V. G., Shiloach J., Fass R., Longo D. L., Bridges S. H. Suppression of the immune response to immunotoxins with anti-CD4 monoclonal antibodies. J Immunol. 1991 Mar 15;146(6):1806–1811. [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989 Feb;57(2):609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L., MacDonald G., West J., Woods A., Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL 4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol. 1987 Mar 15;138(6):1674–1679. [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Kumaratilake L. M., Ferrante A. IL-4 inhibits macrophage-mediated killing of Plasmodium falciparum in vitro. A possible parasite-immune evasion mechanism. J Immunol. 1992 Jul 1;149(1):194–199. [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990 Sep 1;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Gibson C. W., Eisenstein T. K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985 Mar;91(1):75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Louis J. A. Immunology of leishmaniasis. Curr Opin Immunol. 1992 Aug;4(4):413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- Maskell D. J., Sweeney K. J., O'Callaghan D., Hormaeche C. E., Liew F. Y., Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987 Mar;2(3):211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Tite J., Dougan G. Immune responses in BALB/c mice following immunization with aromatic compound or purine-dependent Salmonella typhimurium strains. Immunology. 1990 Feb;69(2):184–189. [PMC free article] [PubMed] [Google Scholar]
- Otsuka T., Villaret D., Yokota T., Takebe Y., Lee F., Arai N., Arai K. Structural analysis of the mouse chromosomal gene encoding interleukin 4 which expresses B cell, T cell and mast cell stimulating activities. Nucleic Acids Res. 1987 Jan 12;15(1):333–344. doi: 10.1093/nar/15.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Ballou W. R., Baron L. S., Majarian W. R., Brey R. N., Hockmeyer W. T., Young J. F., Cryz S. J., Ou J., Lowell G. H. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988 Apr 15;240(4850):336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- Schödel F., Will H. Expression of hepatitis B virus antigens in attenuated Salmonellae for oral immunization. Res Microbiol. 1990 Sep-Oct;141(7-8):831–837. doi: 10.1016/0923-2508(90)90118-a. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- Taylor M. K., Cohen J. J. Cell-mediated cytotoxicity. Curr Opin Immunol. 1992 Jun;4(3):338–343. doi: 10.1016/0952-7915(92)90086-t. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Gao X. M., Hughes-Jenkins C. M., Lipscombe M., O'Callaghan D., Dougan G., Liew F. Y. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. III. Delivery of recombinant nucleoprotein to the immune system using attenuated Salmonella typhimurium as a live carrier. Immunology. 1990 Aug;70(4):540–546. [PMC free article] [PubMed] [Google Scholar]
- Tonkonogy S. L., McKenzie D. T., Swain S. L. Regulation of isotype production by IL-4 and IL-5. Effects of lymphokines on Ig production depend on the state of activation of the responding B cells. J Immunol. 1989 Jun 15;142(12):4351–4360. [PubMed] [Google Scholar]
- Yang D. M., Fairweather N., Button L. L., McMaster W. R., Kahl L. P., Liew F. Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990 Oct 1;145(7):2281–2285. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- al-Ramadi B. K., Chen Y. W., Meissler J. J., Jr, Eisenstein T. K. Immunosuppression induced by attenuated Salmonella. Reversal by IL-4. J Immunol. 1991 Sep 15;147(6):1954–1961. [PubMed] [Google Scholar]
- al-Ramadi B. K., Greene J. M., Meissler J. J., Jr, Eisenstein T. K. Immunosuppression induced by attenuated Salmonella: effect of LPS responsiveness on development of suppression. Microb Pathog. 1992 Apr;12(4):267–278. doi: 10.1016/0882-4010(92)90045-p. [DOI] [PubMed] [Google Scholar]


