Abstract
The PD-1/PD-L pathway plays a major role in regulating T-cell exhaustion during chronic viral infections in animal models, as well as in humans, and blockade of this pathway can revive exhausted CD8+ T cells. We examined the expression of PD-1 and its ligands, PD-L1 and PD-L2, in multiple tissues during the course of chronic viral infection and determined how the amount of PD-1 expressed, as well as the anatomical location, influenced the function of exhausted CD8 T cells. The amount of PD-1 on exhausted CD8 T cells from different anatomical locations did not always correlate with infectious virus but did reflect viral antigen in some tissues. Moreover, lower expression of PD-L1 in some locations, such as the bone marrow, favored the survival of PD-1Hi exhausted CD8 T cells, suggesting that some anatomical sites might provide a survival niche for subpopulations of exhausted CD8 T cells. Tissue-specific differences in the function of exhausted CD8 T cells were also observed. However, while cytokine production did not strictly correlate with the amount of PD-1 expressed by exhausted CD8 T cells from different tissues, the ability to degranulate and kill were tightly linked to PD-1 expression regardless of the anatomical location. These observations have implications for human chronic infections and for therapeutic interventions based on blockade of the PD-1 pathway.
Chronic viral infections are often associated with CD8+ T-cell dysfunction (30). This dysfunction, termed exhaustion, includes defects in the ability to produce antiviral cytokines, poor cytotoxicity, a loss of antigen-independent self-renewal, and the inability to vigorously re-expand following antigen exposure (30). These functional deficiencies contrast with the highly functional memory CD8+ T cells that are generated after acute infection and maintained via interleukin-7 (IL-7)- and IL-15-mediated homeostatic proliferation (30). During chronic viral infections, T-cell exhaustion often correlates with poor control of viral replication (3, 8, 38, 39). Thus, there is considerable interest in developing strategies to reverse exhaustion and restore function in virus-specific CD8+ T cells during chronic infections.
Recent studies have revealed an important role for the negative regulatory molecule PD-1 in CD8 T-cell exhaustion during chronic viral infections (29). PD-1, a member of the CD28/CTLA-4 family of costimulatory/coinhibitory receptors, contains both ITIM and ITSM motifs in the intracellular tail and can deliver negative signals, at least partly via recruitment of the phosphatase Shp-2 (29). A role for PD-1 in regulating T-cell responses to chronic viral infections was first observed using lymphocytic choriomeningitis virus (LCMV) infection of mice, where PD-1 was found to be highly expressed on exhausted CD8+ T cells from chronically infected animals but not on functional memory CD8+ T cells from mice that had cleared an acute strain of the virus (3). In vivo blockade of the PD-1 pathway led to a dramatic increase in the number of virus-specific CD8+ T cells, improved functionality of these cells, and enhanced control of viral replication (3). These observations were extended to human chronic viral infections, and a series of studies have demonstrated that human immunodeficiency virus (HIV)-, hepatitis C virus (HCV)-, and HBV-specific CD8+ T cells upregulate PD-1 in humans compared to CD8+ T cells specific for nonpersisting viruses such as influenza virus or vaccinia virus (6-8, 24, 26, 32, 33, 42). Increasing PD-1 expression also correlates with disease status during HIV infection (8, 42). In vitro blockade of PD-1-PD-L interactions can reinvigorate exhausted virus-specific T-cell responses in humans and appears to have a prominent impact on proliferative expansion and/or prevention of apoptosis in these cases (9, 24, 32). Finally, recent results from in vivo blockade in the macaque simian immunodeficiency virus (SIV) infection model demonstrated the effectiveness of blocking PD-1 in primates during chronic viral infection (36). In these studies, PD-1 blockade enhanced virus-specific T and B-cell responses, lowered viral load, and improved the survival of chronically infected animals. Thus, PD-1 has emerged as not only a major regulator of T-cell exhaustion and viral control during chronic infection but also as an important potential therapeutic target.
Despite these important studies and the clear impact of PD-1 blockade on the reversal of T-cell exhaustion, important questions remain. For example, previous work has demonstrated that PD-1 expression is not uniform on subsets of exhausted CD8 T cells (4). However, the expression of PD-1 on exhausted CD8 T cells in multiple tissues, and the relationship between PD-1 expression in these tissues to viral load, the PD-1 ligands and function has not been examined. Given the nonlymphoid accumulation of virus-specific CD8 T cells during chronic viral infections (11, 39) and the predilection of many important chronic infections for replicating in anatomically restricted locations (e.g., HCV and the liver, HIV and mucosal tissues, etc.), the dynamics of PD-1 expression by exhausted CD8 T cells outside the blood and spleen could have important therapeutic implications.
In the present study we examined these issues using the mouse model of LCMV infection. Our results demonstrate that exhausted CD8 T cells have a wide range of PD-1 expression in different tissues of chronically infected mice. Virus-specific CD8 T cells in some anatomical locations such as the liver, brain, and bone marrow (BM) expressed high PD-1 for substantially longer than virus-specific CD8+ T cells from the spleens or blood of the same mice. Although PD-1 expression in the spleen correlated well with reduced gamma interferon (IFN-γ) and tumor necrosis factor (TNF) production, the PD-1Hi virus-specific CD8+ T cells from the BM remained capable of producing antiviral cytokines ex vivo. In contrast, a strong negative correlation between PD-1 expression and cytotoxicity existed for exhausted CD8 T cells from all tissues tested. PD-L1 expression was high in the spleen, whereas in the BM antigen-presenting cell (APC) populations expressed lower amounts of PD-L1. Survival of PD-1Hi CD8+ T cells from the BM was decreased in the presence of splenic APCs, suggesting that different tissue microenvironments in vivo could selectively support the persistence of PD-1Hi exhausted CD8 T cells. Since PD-1 expression differs by anatomical location, these observations suggest that PD-1 blockade in vivo will have varying impacts on exhausted CD8 T cells from different tissues or anatomical locations. These observations have implications for human chronic infections such as HBV, HCV, and HIV.
MATERIALS AND METHODS
Mice, virus, and infections.
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). LCMV Armstrong and clone 13 strains were propagated, the titers were determined, and the strains were used as described previously (2). Briefly, LCMV titers were measured by plaque assay on Vero cell monolayers. Vero cells were plated in 35-mm wells in six-well dishes, incubated at 37°C, and used the following day when the cell monolayers were confluent. The medium was removed, and the samples to be titrated were added to the cells (0.2 ml). After adsorption for 60 min at 37°C, the cells were overlaid with 4 ml of a 50:50 mixture of 0.5% Seakem agarose (Lonza, Rockland, ME) and 2× Medium 199 (Gibco Laboratories, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum, antibiotics, and l-glutamine. The plates were incubated for 4 days at 37°C and then overlaid with 2.0 ml of 0.5% agarose-Medium 199 containing 0.05% neutral red (Gibco Laboratories). The plaques were counted the following day. B6 mice were infected intraperitoneally with LCMV Armstrong (2 × 105PFU) or intravenously with LCMV clone 13 (2 × 106 PFU). All mice were used in accordance with Institutional Animal Care and Use Committee guidelines.
Isolation of lymphocytes from tissues.
Lymphocytes were isolated from nonlymphoid tissues as described previously (39). Briefly, mice were euthanized, the hepatic veins were cut, and the livers were perfused with 5 ml of ice-cold phosphate-buffered saline (PBS). Lungs were harvested by cutting the left ventricle and injecting PBS into the right ventricle to perfuse the lungs. Livers, lungs, and kidney tissues were homogenized by using a wire screen. Homogenized lung was first incubated in 1.5 mM EDTA at 37°C for 30 min, and the liver, lung, and kidney were then incubated in 0.25 mg of collagenase B (Boehringer Mannheim)/ml and 1 U of DNase (Sigma)/ml at 37°C for 45 min. Digested tissue was applied to a 44/56% Percoll gradient and centrifuged at 850 × g for 20 min at 20°C. The intrahepatic lymphocyte population was harvested from the interface, red blood cells were lysed by using 0.83% ammonium chloride and washed, and the resulting lymphocytes counted. Brains were removed and homogenized by using a wire screen. The homogenate was adjusted to 30% Percoll and underlaid with 70% Percoll. The Percoll gradient was centrifuged at 800 × g for 20 min at 20°C. Mononuclear cells were recovered from the interface and washed once in RPMI, red blood cells were lysed by using 0.83% ammonium chloride, and the cells were washed and counted. BM lymphocytes were isolated by flushing each femur with 5 ml of cold RPMI. Spleens and lymph nodes were homogenized by using a wire screen, and red blood cells were lysed by using 0.83% ammonium chloride and washed.
Flow cytometry, intracellular cytokine staining, and CD107a assay.
Major histocompatibility complex (MHC) class I peptide tetramers were made and used as described previously (39). Antibodies to CD8, CD44, IFN-γ, TNF, PD-L2, and PD-1 (RMP1-30) were purchased from eBioscience (San Diego, CA). Antibodies to PD-1 (J43), PD-L1, and CD107a were purchased from BD Biosciences (San Diego, CA). Antibodies to MIP-1α and granzyme B were purchase from R&D Systems (Minneapolis, MN) and Caltag (Burlingame, CA), respectively. Splenocytes from LCMV-infected mice were stained as previously described. For intracellular cytokine analysis, 106 splenocytes were cultured in the absence or presence of the indicated peptide (0.2 μg/ml) and brefeldin A for 5 to 6 h at 37°C. After staining for surface antigens as described above, cells were stained for intracellular cytokines by using the Cytofix/Cytoperm kit (BD/Pharmingen). The CD107a assay was performed as previously described (5). Samples were collected by using a FACSCalibur or LSR II flow cytometer (Becton Dickinson). Staining for intracellular LCMV was carried out using the anti-NP antibody clone 1.1.3 (provided by M. Buchmeier, The Scripps Research Institute, La Jolla, CA) conjugated to A647. The staining was carried out by using a BD Cytofix/Cytoperm kit. Alexa 647 conjugation was carried out by using an Alexa Fluor labeling kit (Invitrogen) according to the manufacturer's instructions.
In vitro apoptosis assay.
Measurement of in vitro apoptosis was performed essentially as previously described (4).
Killing assay.
This is an adaptation of the VITAL killing assay described previously (12). Briefly, two sets of Ly5.1+ splenocytes were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), one with 100 nM CFSE and one with 1.25 μM CFSE. The CFSE-labeled cells were then pulsed with 2 μg of GP33-44 or SINFEKL peptide/ml, respectively, for 1 h at 37°C and then rinsed three times in RPMI with 10% fetal calf serum. The peptide pulsed targets were then incubated with magnetic bead (Miltenyi Biotec)-purified Ly5.2+ CD8+ T cells from either the spleens or BM with a 0.5 effector/target ratio (E:T) for 4, 12, or 20 h. The E:T was determined by normalizing all populations to the number of DbGP33 tetramer-positive CD8 T cells. Cells were washed and stained with Ly5.1 and Live/Dead fixable red dead cell stain kit from Molecular Probes (Eugene, OR). The killing efficiency was determined as previously described (12).
RESULTS
PD-1 expression on virus-specific CD8+ T cells varies by anatomical location.
During chronic viral infections, antigen-specific CD8+ T cells accumulate to high frequencies and absolute numbers in tissues other than the spleen, lymph nodes (LN), and blood. Nonlymphoid tissues such as the liver and BM, for example, become important reservoirs of antiviral T cells (34, 39, 41). Similar accumulation of virus-specific CD8+ T cells in the liver during HCV infection has also been observed (1, 11). Although previous studies suggested that PD-1 expression in blood and spleen differed from that observed on exhausted CD8 T cells from the liver or BM (4), the expression of PD-1 on exhausted CD8 T cells from multiple nonlymphoid tissues has not been examined. To address this question, we used the mouse model of chronic LCMV infection. In adult mice, LCMV Armstrong (Arm) is cleared by day 8 to 10 postinfection (p.i.), whereas LCMV clone 13 establishes a chronic infection with 2 to 3 months of viremia, followed by long-term persistence in some tissues (39). LCMV Arm and LCMV clone 13 differ by two amino acids, neither of which occurs in any known T-cell epitope, allowing the comparison of functional memory CD8+ T cells generated after clearance of LCMV Arm to exhausted CD8+ T cells of the same specificity found during LCMV clone 13 infection.
C57BL/6 mice were infected with either LCMV Arm or LCMV clone 13 and lymphocytes were isolated from eight tissues at multiple time points postinfection. A kinetic analysis of PD-1 expression on virus-specific CD8 T cells from eight tissues of chronically infected mice is shown in Fig. 1. PD-1 expression was initially upregulated by day 5 p.i. after LCMV Arm or clone 13 infection but began to decline by day 8 p.i. after LCMV Arm infection and remained low thereafter (Fig. 1C) (3, 40). During clone 13 infection PD-1 expression on exhausted CD8 T cells from the spleen and blood was high at days 8 and 30 p.i. but began to decline by 2 to 3 months p.i. (Fig. 1). Interestingly, however, the expression of PD-1 during chronic LCMV infection was not uniform in all tissues. Rather, virus-specific CD8+ T cells from some tissues, such as BM, liver, brain, and kidney were high for PD-1 at all time points, including during and after control of viremia (Fig. 1A and C).
FIG. 1.
Kinetics and pattern of PD-1 expression by virus specific CD8+ T cells in different anatomical locations. (A) After infection with LCMV clone 13, lymphocytes were isolated from the eight tissues indicated, and PD-1 expression was determined on DbGP33 tetramer-positive CD8 T cells at the indicated times postinfection or on naive (CD44Lo) CD8 T cells by flow cytometry. The numbers on the right for each histogram series indicate the MFI of the corresponding histogram. (B) Examples of DbGP33 tetramer versus PD-1 staining for the spleen, liver, and BM on day 90 p.i. (C) Summary data from panel A. PD-1 expression was examined on DbGP33 CD8+ T cells isolated from eight anatomical locations of LCMV Armstrong and LCMV clone 13-infected mice on days 8, 30, and 90 p.i. (n = 3 to 6 mice/tissue/time point).
The presence of infectious virus is not the sole determinant of PD-1 expression.
Viral load has been correlated with PD-1 expression in a number of studies (8, 31, 35, 42). To determine whether PD-1 expression on LCMV specific CD8+ T cells correlated with the presence of viral replication in the tissues analyzed in Fig. 1, we determined viral load in each tissue at multiple time points postinfection by plaque assay after infection with clone 13. The presence of infectious virus was highest at day 8 p.i. in all tissues (Fig. 2A). The viral load in the spleen, lungs, liver, BM and blood declined substantially by 1 month p.i. and was undetectable by conventional plaque assay by 3 months p.i. (Fig. 2A). While the levels of replicating virus also declined in the LN, kidneys, and brain, infectious virus was still detectable in the LN and brains of some mice and in the kidneys of all mice 90 days p.i. (Fig. 2A).
FIG. 2.
Kinetics of control of viral replication varies by anatomical location. (A) Viral load was determined by plaque assay in the indicated tissues of LCMV clone 13-infected mice on day 8, 30, and 90 p.i. Dashed line indicates the approximate limit of detection determined by serum volume, tissue weight, or by assaying the BM from an entire femur. (B) Heat map visualization of the comparison between tissue PD-1 MFI on DbGP33+ CD8 T cells (see Fig. 1) and tissue viral load from panel A. Some Arm viral load data are derived from an earlier study (39). Tissues where PD-1 expression remains high and the viral load is low are highlighted in yellow.
In tissues where virus levels remained high such as the kidneys and brain, PD-1 levels also remained high (Fig. 2B). However, while PD-1 levels declined in tissues such as the spleen, blood, and lungs where virus levels declined, PD-1 remained elevated in the liver and BM even though the kinetics of viral infection were similar to those in the spleen, blood, and lungs (Fig. 2B). Thus, although the presence of infectious virus was associated with high PD-1 expression on virus-specific CD8+ T cells from some tissues, in other locations such as the liver and BM PD-1 expression remained high on virus-specific CD8+ T cells despite undetectable levels of infectious virus.
Arenaviruses, including LCMV, can persist in infected cells in vitro and in vivo in the absence of the production of infectious virus (23, 27, 37). It was therefore possible that persistent LCMV antigen was present even in the absence of infectious virus. In order to explore this possibility, the spleen and BM were chosen as representative tissues in which PD-1 levels either decreased (spleen) or remained high (BM) following the control of viral replication during LCMV clone 13 infection despite equally low levels of plaquable virus. The spleen and BM were also chosen because the majority of resident cells in each tissue are of hematopoietic origin and thus lend themselves to analysis by flow cytometry. LCMV-infected cells from the spleen and BM were detected by intracellular staining with an anti-LCMV NP antibody. Although no LCMV NP was detected in the spleen (or BM [data not shown]) of LCMV Arm immune animals, persistently infected cells were found in both the spleen and the BM of LCMV clone 13-infected animals on day 90 p.i. when no plaquable virus was detected (Fig. 3A). LCMV NP+ cells from both the spleen and the BM shared a similar phenotype (CD8− B220− CD11bHi CD11cInt), suggesting a reservoir of noninfectious virus in macrophages or possibly DC. These results indicate that in the absence of infectious virus, viral antigens can still be present. Since LCMV NP protein was detected in both spleen and BM cells of the same phenotype, these observations alone did not explain the PD-1 expression pattern of exhausted CD8 T cells from these locations. However, there was a relative increase in the frequency of LCMV NP+ cells in the BM over time, whereas the frequency of LCMV NP+ cells in the spleen remained constant. The reasons for the persistence of LCMV NP+ cells in the absence of production of viral progeny are not currently clear but could relate to an inability of plaque assays to detect all infectious virus, an ongoing low-level innate immune response to the virus or production of antiviral antibodies that might prevent the generation or detection of progeny virus. With respect to the impact on T cells, an increase in the percentage of persistently infected cells in the BM could result in more frequent encounter of exhausted CD8 T cells with antigen and be a factor influencing the sustained PD-1 expression seen on LCMV specific CD8+ T cells in the BM of chronically infected mice.
FIG. 3.
Persistent expression of viral proteins in LCMV clone 13-infected mice in the absence of plaque-forming virus. (A) Cells were isolated from the spleen and BM of LCMV clone 13-infected mice at day 90 p.i. and stained for intracellular LCMV NP expression. (B) The relative frequency of LCMV NP expressing cells in the spleen and BM on days 8, 30, and 90 p.i. is indicated. The data represent three mice/time point and are representative of two (days 8 and 30) or three (day 90) independent experiments.
PD-L1 expression differs between the spleen and bone marrow.
A second factor that could influence PD-1 expression in different tissues is the expression of the PD-1 ligands, PD-L1 and PD-L2. The interaction of PD-1 with PD-L1 is associated with decreased cell survival (17). In order to determine whether a difference in the expression of the ligands for PD-1, PD-L1, and PD-L2 was associated with different anatomical locations, we examined the expression of PD-L1 and PD-L2 in the spleen and BM. As shown in Fig. 4A, PD-L1 expression was similar on T cells and CD11c+ DCs from the spleen and BM. However, CD11b+ and CD11bInt cells, as well as CD19+ B220+ cells, had higher PD-L1 expression in the spleen compared to the BM, and this difference persisted to at least day 90 p.i. (Fig. 4A and B). PD-L2 expression, in contrast, was relatively low on most cell types at the time points examined and showed only subtle differences between the spleen and BM (Fig. 4C and D). To investigate how PD-L1 compared on LCMV-infected cells in spleen versus BM, we next gated on LCMV NP+ cells and examined PD-L1 levels. PD-L1 expression was lower on LCMV NP+ cells from the BM compared to the spleen (Fig. 4E). Thus, tissue-specific PD-L1 expression on infected cells may contribute to the difference in PD-1 expression observed on exhausted CD8+ T cells from various anatomical locations.
FIG. 4.
PD-ligand expression varies by anatomical location and time p.i. (A) PD-L1 expression on phenotypically defined cell types from the spleens and the BM of clone 13-infected mice at day 30 p.i. FMO, fluorescence minus one negative control. (B) Longitudinal analysis of PD-L1 expression in the spleen and BM expressed as the MFI. (C) PD-L2 expression on phenotypically defined cell types from the spleen and BM of clone 13-infected mice at day 30 p.i. (D) Longitudinal analysis of PD-L2 expression in the BM and the spleen expressed as MFI. (E) PD-L1 expression on virus-infected cells (anti-LCMV NP+, primarily CD4− B220− CD8aLo CD11bHi CD11cInt) in the spleen and BM (n = 3 to 5 for each time point for panels A to E).
APCs from different tissues influence the survival of exhausted CD8+ T cells.
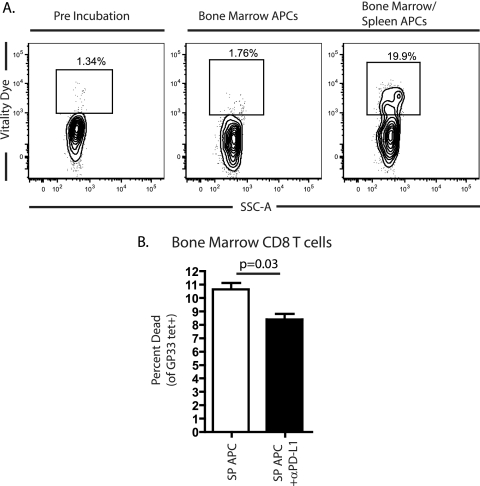
PD-1 expression is associated with increased susceptibility to spontaneous apoptosis in vitro, and cross-linking PD-1 on exhausted CD8+ T cells further increases the rate of cell death (4, 24, 25). Our results show that despite similar viral load, exhausted CD8 T cells from different tissues vary in expression of PD-1. In addition, tissue-specific expression of PD-L1 was inversely correlated with PD-1 expression in the same tissues (Fig. 1 and 4). We hypothesized that PD-1Hi exhausted CD8+ T cells might have a survival advantage in tissues with lower PD-L1 expression. To explore this possibility, we cultured PD-1Hi exhausted CD8+ T cells from the BM of chronically infected mice (∼3 months p.i.) with either BM CD8-depleted APCs alone or BM APCs plus spleen-derived APCs and monitored survival of the PD-1Hi exhausted CD8 T cells by using vital dye exclusion. (Note that “APCs” here simply refers to all non-CD8 T cells in the tissue that could present MHC class I-restricted peptides to CD8 T cells.) As shown in Fig. 5, the addition of APCs from the spleen that expressed higher levels of PD-L1 led to an 11-fold increase in death among the exhausted PD-1Hi BM-derived CD8+ T cells. Furthermore, the inclusion of a blocking antibody against PD-L1 in these cocultures of BM CD8 T cells and CD8 T-cell-depleted splenic APCs decreased the killing of the BM-derived exhausted CD8 T cells. This effect was statistically significant but did not restore the level of apoptosis to that observed with BM APCs, suggesting perhaps inefficient blockade in vitro or a role for other factors such as additional inhibitory receptors. These data suggest, however, that lower expression of PD-L1 on APCs in the BM could provide PD-1Hi exhausted CD8 T cells a survival advantage in this location compared to the spleen, where higher PD-L1 expression will more efficiently engage PD-1 and likely lead to cell death.
FIG. 5.
Survival of PD-1Hi exhausted CD8+ T cells was influenced by tissue-resident APCs. (A) PD-1Hi exhausted CD8+ T cells from the BM of LCMV clone 13-infected mice (∼3 months p.i.) were cultured for 5 h with BM-derived CD8-depleted “APCs” alone or in combination with splenic CD8-depleted APC- from the same infected mice. The percentage of dead/dying PD-1Hi CD8+ T cells was determined by using an amine reactive dye (vital dye). (B) The assay in panel A was repeated with or without the addition of 10 μg of αPD-L1/ml. Similar results were observed using vital dye exclusion and staining for active caspase-3 (data not shown). The data represent the findings in two to four independent experiments.
Cytokine production by exhausted CD8 T cells from different tissues.
We next examined the function of exhausted CD8 T cells from the spleen, BM, and liver. As expected, virus-specific CD8+ T cells from the spleen of LCMV Arm immune mice were highly functional, and the majority of GP33-specific CD8+ T cells were able to coproduce IFN-γ/TNF or IFN-γ/MIP-1α (Fig. 6A). While LCMV-specific CD8+ T cells from the spleen and BM of chronically infected mice were functionally compromised, when directly compared to each other, virus-specific CD8 T cells from the BM retained slightly better per cell function based on coproduction of IFN-γ/TNF or IFN-γ/MIP-1α (Fig. 6). In the spleen PD-1 expression was negatively correlated with IFN-γ production; PD-1Hi LCMV-specific CD8+ T cells in the spleen produced less IFN-γ/cell compared to the PD-1Lo CD8+ T cells in this tissue (Fig. 6C and D). In contrast, in the BM, PD-1Hi exhausted CD8 T cells retained the ability to make IFN-γ in vitro, and there was no significant difference between the mean fluorescence intensity (MFI) of IFN-γ production between the PD-1Hi and PD-1Lo virus-specific CD8+ T cells in the BM (Fig. 6D). Exhausted CD8 T cells from the liver were intermediate between the spleen and BM for both PD-1 expression and cytokine production.
FIG. 6.
PD-1Lo and PD-1Hi subsets of virus-specific CD8+ T-cell differ in their functional capacity during chronic LCMV infection. (A) CD8+ T cells from the spleen (PD-1Lo/Int) and from the BM (PD-1Hi) from clone 13-infected mice day 45 p.i. were stimulated for 5 h with the GP33-41 peptide. IFN-γ, TNF, and MIP-1α production were examined by flow cytometry. The numbers indicate the percentages of responding cells coproducing the two indicated cytokines. (B) Summary data on the coproduction of IFN-γ and TNF for multiple mice (n = 3). (C) Lymphocytes from the spleens, livers, and BM isolated from clone 13-infected mice at day 45 p.i. were stimulated for 5 h with the GP33-41 peptide and stained for PD-1 and IFN-γ. The numbers indicate the percentages of IFN-γ+ cells that were PD-1Lo or PD-1Hi. (D) The MFI of IFN-γ for PD-1Lo or PD-1Hi virus-specific CD8 T cells from the spleen, liver, or BM on day 45 p.i.
The APC populations from different tissues did not appear to impact CD8 T-cell functionality in vitro as much as they did cell death. Mixing splenocytes with BM APCs did not significantly alter the functionality of the spleen-derived or BM-derived LCMV-specific CD8+ T cells, although upon the addition of spleen-derived APCs there was a trend toward reduced MFI of IFN-γ from BM CD8 T cells consistent with higher PD-L1 on the spleen-derived APCs (Fig. 7A and data not shown). To determine whether the PD-1 pathway directly regulated cytokine production in the short term, in vitro GP33 peptide stimulation was performed for 5 h in the absence or presence of an anti-PD-L1 blocking antibody and the percent increase in IFN-γ-producing CD8+ T cells was determined for LCMV-specific CD8+ T cells derived from the spleen versus the BM. Short-term anti-PD-L1 treatment did not improve the functionality of the BM-derived CD8+ T cells in vitro. However, short-term PD-1/PD-L1 blockade did show a trend to increase the frequency of IFN-γ producers derived from the spleen by ∼15%, although this difference was not statistically significant (Fig. 7B). Thus, it is possible that cytokine production by exhausted CD8+ T cells could be regulated by the PD-1/PD-L1 pathway either by a direct impact on cytokine production or through increased survival of cytokine-producing CD8+ T cells. However, the impact in the short term is modest, in contrast to the robust improvement observed after 2 weeks of blockade in vivo (3-5, 36).
FIG. 7.
Anatomical differences in functional capacity are not dramatically influenced by resident APCs. (A) Splenocytes from LCMV clone 13-infected CD45.1 mice (day 90 p.i.) were mixed with BM lymphocytes from LCMV clone 13-infected CD45.2 mice (day 90 p.i.), stimulated with GP33 peptide, and the intracellular cytokines were examined after 5 h as in Fig. 6. (B) Splenocytes and BM lymphocytes from LCMV clone 13-infected mice (day 90 p.i.) were stimulated with GP33-41 peptide in the presence or absence of αPD-L1 antibody, and the production of IFN-γ was assessed by ICS after 5 h.
Exhausted CD8+ T cells from different tissues vary in cytotoxic potential.
In addition to cytokine production, cytotoxicity is a crucial function of CD8 T cells that correlates with long-term control of chronic viral infections (19, 20, 43). To directly examine cytotoxicity ex vivo, we performed a VITAL killing assay for exhausted CD8 T cells from the different tissues of chronically infected mice (12). As expected, virus-specific CD8+ T cells generated after LCMV Arm infection (day 10 p.i.) were highly cytolytic in this assay (Fig. 8A). When the cytotoxic activity of virus-specific CD8+ T cells from three tissues of chronically infected mice was compared, virus-specific CD8 T cells from the spleen had the best per-cell killing, followed by CD8 T cells from the liver. Little cytotoxic activity was observed for BM-derived virus-specific CD8+ T cells (Fig. 8A). This hierarchy of cytotoxic activity correlated well with the expression of PD-1 by these virus-specific CD8+ T cells. We next assessed whether including anti-PD-L1 during the 20-h killing assay could improve cytotoxicity. Unlike the effect of this blocking antibody in vivo (3-5, 10), short-term in vitro blockade had minimal effect, and cytotoxicity was not improved for T cells from any tissue (Fig. 8E). Thus, while short-term PD-L1 blockade did not reactivate the cytolytic machinery, there was a strong correlation between higher PD-1 expression and decreased ex vivo cytotoxic potential for exhausted CD8 T cells, suggesting an in vivo relationship between PD-1 expression and cytotoxic potential.
FIG. 8.
PD-1Hi CD8+ T cells have diminished cytotoxic potential despite high expression of granzyme B. (A) A VITAL killing assay was performed using GP33-41-pulsed targets and purified CD8+ T cells from the spleens, livers, or BM of LCMV clone 13-infected mice at day 90 p.i. Splenocytes from LCMV Arm-infected mice (day 10 p.i.) served as a positive control. The assay was performed in the presence or absence of αPD-L1 antibody with an E:T for DbGP33-specific CD8 T cells of 0.5 for 4, 12, or 20 h (20-h time point shown; data are representative of three independent experiments). (B and C) DbGP33+ CD8+ T cells from the spleens, livers, and BM isolated from clone 13-infected mice at day 45 p.i were stained for granzyme B and PD-1. The MFI values of granzyme B for DbGP33+ CD8 T cells from the indicated tissues are shown. (D and E) Lymphocytes from the spleens, livers, and BM were stimulated in vitro with GP33-41 peptide, and CD107a degranulation was assessed in combination with staining for PD-1. Degranulation for DbGP33+ CD8 T cells from the three tissues is indicated.
Recent studies indicate a critical importance of cytotoxicity in long-term control of chronic infections, including HIV (13, 19, 20). To investigate further the reasons for differences in the cytotoxic potential of exhausted CD8+ T cells from the spleen, liver, and BM, we examined granzyme B expression and degranulation (CD107a assay). Granzyme B expression was highest on DbGP33+ CD8+ T cells from the BM and lowest on those from the liver (Fig. 8B). In all three tissues, the PD-1Hi subset of LCMV specific CD8+ T cells from chronically infected mice expressed higher granzyme B compared to PD-1Lo CD8+ T cells in the same tissue, indicating that, whereas PD-1 does not have a direct relationship with granzyme B expression across different tissues, PD-1Hi T cells in any given tissue expressed higher amounts of granzyme B than PD-1Lo exhausted CD8 T cells (Fig. 8C). Degranulation, as measured by the CD107a assay, was high in the liver and spleen and low in the BM, suggesting an inverse relationship between degranulation and granzyme B expression in exhausted CD8+ T cells (Fig. 8D and E). Within a given tissue, PD-1 expression was also inversely correlated with the MFI of CD107a after in vitro stimulation; PD-1Lo LCMV-specific CD8+ T cells from the liver and spleen appeared to degranulate more efficiently following short-term in vitro stimulation (Fig. 8E). The inverse correlation observed between degranulation and granzyme B expression in tissue specific exhausted CD8+ T cells populations, as well as between PD-1Hi and PD-1Lo subsets, suggests that granule contents might be more efficiently released by PD-1Lo exhausted CD8+ T cells and that this impact is tissue independent. It is also possible that inefficient degranulation underlies the poor cytotoxicity of PD-1Hi exhausted CD8 T cells. Future studies are necessary to determine the molecular connections between PD-1 signaling and degranulation, as well as to examine other aspects of the cytolytic machinery, such as the expression of other granzymes and perforin.
DISCUSSION
T-cell exhaustion often occurs during chronic viral infections and prevents optimal control of viral replication. Recent interest has focused on PD-1 as a key inhibitory receptor pathway negatively regulating the function of exhausted CD8 T cells. Blocking the PD-1 pathway in vitro or in vivo revives exhausted CD8 T cells in chronically infected mice, primates, and humans (16, 29), identifying this pathway as a key therapeutic target. However, we have recently identified anatomically and phenotypically defined subsets of exhausted CD8 T cells that differ in responsiveness to PD-1 pathway blockade (4). In the current studies we found that PD-1 expression by exhausted CD8 T cells varied dramatically in different tissues. Our data indicate that differences in tissue-specific PD-1 expression are regulated by levels of infectious virus, antigen load in the absence of infectious virus, and PD-L1 expression. These observations also suggest that anatomical differences in these factors could provide survival niches for subsets of exhausted CD8 T cells with different properties.
Chronic infections often result in the anatomical accumulation of virus-specific T cells. In the LCMV system substantial accumulation of tetramer-positive CD8 T cells can occur in the liver, BM, and brain during chronic infection (39). At some time points, numerically more antigen-specific CD8 T cells are found in the livers than in the spleens of LCMV clone 13-infected mice (39), indicating that these sites are important quantitative reservoirs of antiviral CD8 T cells. Similar observations have been made for HCV infection in humans where virus-specific CD8 T cells can dramatically accumulate in the infected liver (11). During SIV or HIV infection there is also an enrichment in virus-specific CD8 T cells in sites such as the intestinal mucosa (28). An interesting aspect of the present study is that virus-specific CD8 T cells in different anatomical locations differed dramatically in their expression of PD-1. One potential reason for PD-1 expression changes in tissues is the amount of ongoing viral replication in different sites. In HCV infection, for example, virus-specific CD8 T cells from the liver, the site of viral replication, express higher PD-1 than CD8 T cells of the same specificity in the blood (22, 26). During chronic LCMV infection, virus-specific CD8 T cells in sites of long-term viral replication such as the brain and kidney expressed the highest PD-1. However, there were other tissues, such as the BM and liver, where viral infection appeared to be controlled with kinetics similar to the spleen and blood and yet antigen-specific CD8 T cells remained PD-1Hi in these locations. Although we were able to implicate the amount of viral antigen in the absence of production of infectious virus as one potential difference between the spleen and BM that could contribute to high PD-1 expression in this location, our results also suggest a role for other factors. PD-L1 expression was lower in the BM. Mixing BM-derived exhausted CD8 T cells with splenic APCs resulted in increased death of PD-1Hi exhausted CD8 T cells from the BM, and this effect was at least partially PD-L1 dependent. Thus, it is possible that lower PD-L1 expression in some anatomical locations favors the survival of exhausted CD8 T cells expressing higher levels of PD-1. However, these results do not exclude other pathways, including additional inhibitory receptors (5) or inflammatory signals (18), in influencing PD-1 expression and/or preferential localization of PD-1Hi exhausted CD8 T cells in some locations.
There is now considerable evidence that blocking the PD-1 pathway can have a positive impact on the function of exhausted CD8 T cells in vitro and in vivo (16, 29). However, the precise mechanisms for this positive effect remain poorly understood, and the exact T-cell pathways and functions impacted by blocking PD-1 signaling are not well defined. There is little doubt that blocking the PD-1 pathway on exhausted CD8 T cells enhances proliferative expansion, very likely by enhancing division, as well as reducing apoptosis (3-5, 8, 24, 25, 32). However, the impact of PD-1 blockade on direct effector functions is less clear. Several studies suggest an impact of PD-1 pathway blockade on short-term cytokine production (i.e., ca. 6 to 30 h) (14, 42). Such an effect that occurs prior to any cell division suggests that PD-1 pathway blockade can relieve a proximal block in signaling leading to more efficient transcription/translation of cytokine genes. However, other studies have found that a major impact of PD-1 pathway blockade on effector function occurs after exhausted CD8 T cells have undergone proliferative expansion (3-5, 8, 9, 24, 25, 32, 36, 42). These studies suggest that either selective expansion and/or survival or reprogramming of exhausted CD8 T cells must occur before enhanced per cell function is achieved. These concepts are consistent with the notion that T-cell exhaustion is a state of differentiation (40) and that relieving a proximal signaling defect by PD-1 blockade might not on its own be sufficient to restore function. Our data are most consistent with the latter ideas, since blocking the PD-1 pathway using αPD-L1 in short-term intracellular cytokine staining (ICS) or cytotoxicity assays in vitro did not dramatically improve the function of exhausted CD8 T cells. It is worth pointing out that function was not improved for either the PD-1Hi exhausted CD8 T cells from the BM or the PD-1Int/Lo exhausted CD8 T cells from the spleen. Exhaustion of this latter subset is reversible in vivo (4). In addition, the PD-1Hi exhausted CD8 T cells from the BM were capable of producing cytokines, although these cells were still poorly functional compared to memory CD8 T cells from Arm immune mice. Given this disconnect between PD-1 expression and short-term cytokine production, it is unlikely that PD-1 signaling alone on exhausted CD8 T cells is the proximal cause for poor IFN-γ/TNF production. However, this interpretation does not exclude a role for PD-1 signaling in fostering the development of poor functionality since exhausted CD8 T cells differentiate from effector CD8 T cells during chronic viral infection.
Exhausted CD8 T cells from the spleen, liver, and BM are progressively less cytotoxic ex vivo consistent with increasing expression of PD-1 on these T cells. Higher granzyme B expression and reduced degranulation (CD107a staining) suggest that exhausted CD8 T cells from the BM in particular have a deficiency in releasing cytotoxic granules. However, again, at least in short-term assays, αPD-L1 did not enhance cytotoxicity, suggesting that even though cytotoxic potential correlates with PD-1, once virus-specific CD8 T cells are functionally exhausted, recovery of cytotoxicity requires reversal of this state of differentiation via cell division (or selective expansion of less exhausted subsets of CD8 T cells). Recent work indicates a crucial role for preservation of cytotoxicity during chronic viral infections in long-term control of viral replication (13, 19, 20, 43). The more severe defects in cytotoxicity in exhausted CD8 T cells from tissues such as the BM and liver could therefore provide a niche for pathogen persistence in these locations. Based on PD-1 expression it is likely that PD-1Hi CD8 T cells from the brain and kidney are also poorly cytotoxic. It will be interesting to examine this connection between anatomical location, PD-1 expression, and cytotoxicity in human chronic viral infections such as HIV, HCV, and HBV.
An implication of the current observations is that exhausted CD8 T cells from various tissues will respond differently to therapeutic intervention based on PD-1 pathway blockade. This is indeed what has been observed in previous adoptive-transfer studies where splenic PD-1Int/Lo exhausted CD8 T cells responded to αPD-L1 in vivo, while PD-1Hi exhausted CD8 T cells from the liver or BM responded poorly (4). In vitro studies with HCV specific CD8 T cells from the liver or blood suggested a similar situation exists in humans (22). Thus, our results have important implications for therapeutic interventions in humans based on the PD-1 pathway. In considering the potential of PD-1 pathway blockade to reverse CD8 T-cell exhaustion in different chronic infections, the tissue localization and PD-1 expression pattern of those exhausted CD8 T cells should be considered. Our results suggest that the virus-specific CD8 T cells that accumulate outside of the blood and spleen could be relatively unresponsive subsets, at least to PD-1 pathway blockade. These results suggest that during infections such as HCV, HBV, and HIV, where virus-specific CD8 T cells accumulate in the liver or intestinal mucosa, the profile of PD-1 expression in the peripheral blood might be insufficient to predict how an individual will respond to a PD-1 pathway-based therapeutic. For example, there could be PD-1Int/Lo virus-specific T cells in the blood, and yet the virus-specific T cells in relevant tissue (i.e., liver for HCV, gut for HIV) might be PD-1Hi and refractory to PD-1 or PD-L1 blockade alone. Alternatively, the presence of PD-1Int/Lo exhausted CD8 T cells that are responsive to inhibitory receptor blockade in any anatomical site might predict a positive outcome to such an intervention. During HCV infection where many subjects often have detectable exhausted CD8 T cells in the liver, but not the periphery, the detection of any circulating virus-specific CD8 T cells might be a useful indicator of the therapeutic potential of PD-1 pathway blockade (22). Our finding that PD-1Hi CD8+ T cells display differential functionality based on their tissue of origin suggests that other factors in addition to PD-1 are involved in maintaining T-cell exhaustion. We have previously identified additional inhibitory receptors pathways such as LAG-3, 2B4, and CD160 that could be involved (5, 40), and other studies have found a role for CTLA-4 (15, 21). Future work will be required to determine whether these inhibitory receptors and/or other pathways also have tissue-specific roles in CD8 T-cell exhaustion. PD-1-based therapeutic interventions hold considerable promise, and our results suggest continued optimism in this regard, especially if the reasons for differential responsiveness of phenotypically or anatomically defined subsets of exhausted CD8 T cells can be defined.
Acknowledgments
We thank Wherry lab members for insightful comments.
This study was supported by the National Institutes of Health (NIH; AI071309, AI56299, and HHSN26620050030C), the Foundation for the NIH through the Grand Challenges in Global Health Initiative, The Dana Foundation, and The Ellison Medical Foundation.
E.J.W. has a patent licensing arrangement for blocking the PD-1/PD-L1 pathway.
Footnotes
Published ahead of print on 2 December 2009.
REFERENCES
- 1.Accapezzato, D., V. Francavilla, M. Paroli, M. Casciaro, L. V. Chircu, A. Cividini, S. Abrignani, M. U. Mondelli, and V. Barnaba. 2004. Hepatic expansion of a virus-specific regulatory CD8+ T-cell population in chronic hepatitis C virus infection. J. Clin. Invest. 113:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn, S. D., H. Shin, G. J. Freeman, and E. J. Wherry. 2008. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 39:15016-15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn, S. D., H. Shin, W. N. Haining, T. Zou, C. J. Workman, A. Polley, M. R. Betts, G. J. Freeman, D. A. Vignali, and E. J. Wherry. 2009. Coregulation of CD8+ T-cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boettler, T., E. Panther, B. Bengsch, N. Nazarova, H. C. Spangenberg, H. E. Blum, and R. Thimme. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80:3532-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boni, C., P. Fisicaro, C. Valdatta, B. Amadei, P. Di Vincenzo, T. Giuberti, D. Laccabue, A. Zerbini, A. Cavalli, G. Missale, A. Bertoletti, and C. Ferrari. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. Depierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Golden-Mason, L., B. Palmer, J. Klarquist, J. A. Mengshol, N. Castelblanco, and H. R. Rosen. 2007. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 81:9249-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha, S. J., S. N. Mueller, E. J. Wherry, D. L. Barber, R. D. Aubert, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2008. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 205:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. U. S. A. 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermans, I. F., J. D. Silk, J. Yang, M. J. Palmowski, U. Gileadi, C. McCarthy, M. Salio, F. Ronchese, and V. Cerundolo. 2004. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J. Immunol. Methods 285:25-40. [DOI] [PubMed] [Google Scholar]
- 13.Hess, C., M. Altfeld, S. Y. Thomas, M. M. Addo, E. S. Rosenberg, T. M. Allen, R. Draenert, R. L. Eldrige, J. van Lunzen, H. J. Stellbrink, B. D. Walker, and A. D. Luster. 2004. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet 363:863-866. [DOI] [PubMed] [Google Scholar]
- 14.Jeong, H. Y., Y. J. Lee, S. K. Seo, S. W. Lee, S. J. Park, J. N. Lee, H. S. Sohn, S. Yao, L. Chen, and I. Choi. 2008. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T-cell immunity in chronic hepatitis C infection. J. Leukoc. Biol. 83:755-764. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann, D. E., D. G. Kavanagh, F. Pereyra, J. J. Zaunders, E. W. Mackey, T. Miura, S. Palmer, M. Brockman, A. Rathod, A. Piechocka-Trocha, B. Baker, B. Zhu, S. Le Gall, M. T. Waring, R. Ahern, K. Moss, A. D. Kelleher, J. M. Coffin, G. J. Freeman, E. S. Rosenberg, and B. D. Walker. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8:1246-1254. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, D. E., and B. D. Walker. 2009. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 182:5891-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keir, M. E., M. J. Butte, G. J. Freeman, and A. H. Sharpe. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinter, A. L., E. J. Godbout, J. P. McNally, I. Sereti, G. A. Roby, M. A. O'Shea, and A. S. Fauci. 2008. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 181:6738-6746. [DOI] [PubMed] [Google Scholar]
- 19.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T-cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 20.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamoto, N., H. Cho, A. Shaked, K. Olthoff, M. E. Valiga, M. Kaminski, E. Gostick, D. A. Price, G. J. Freeman, E. J. Wherry, and K. M. Chang. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T-cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5:e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamoto, N., D. E. Kaplan, J. Coleclough, Y. Li, M. E. Valiga, M. Kaminski, A. Shaked, K. Olthoff, E. Gostick, D. A. Price, G. J. Freeman, E. J. Wherry, and K. M. Chang. 2008. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 134:1927-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldstone, M. B., and M. J. Buchmeier. 1982. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature 300:360-362. [DOI] [PubMed] [Google Scholar]
- 24.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T-cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrovas, C., D. A. Price, J. Mattapallil, D. R. Ambrozak, C. Geldmacher, V. Cecchinato, M. Vaccari, E. Tryniszewska, E. Gostick, M. Roederer, D. C. Douek, S. H. Morgan, S. J. Davis, G. Franchini, and R. A. Koup. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 110:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2006. Liver infiltrating lymphocytes in chronic human HCV infection display an exhausted phenotype with high PD-1 and low CD127 expression. J. Virol. 81:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez, M., M. J. Buchmeier, M. B. Oldstone, and P. W. Lampert. 1983. Ultrastructural localization of viral antigens in the CNS of mice persistently infected with lymphocytic choriomeningitis virus (LCMV). Am. J. Pathol. 110:95-100. [PMC free article] [PubMed] [Google Scholar]
- 28.Shacklett, B. L., J. W. Critchfield, A. L. Ferre, and T. L. Hayes. 2009. Mucosal T-cell responses to HIV: responding at the front lines. J. Intern. Med. 265:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe, A. H., E. J. Wherry, R. Ahmed, and G. J. Freeman. 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8:239-245. [DOI] [PubMed] [Google Scholar]
- 30.Shin, H., and E. J. Wherry. 2007. CD8 T-cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19:408-415. [DOI] [PubMed] [Google Scholar]
- 31.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 33.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398-11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Most, R. G., K. Murali-Krishna, J. G. Lanier, E. J. Wherry, M. T. Puglielli, J. N. Blattman, A. Sette, and R. Ahmed. 2003. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology 315:93-102. [DOI] [PubMed] [Google Scholar]
- 35.Velu, V., S. Kannanganat, C. Ibegbu, L. Chennareddi, F. Villinger, G. J. Freeman, R. Ahmed, and R. R. Amara. 2007. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 81:5819-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velu, V., K. Titanji, B. Zhu, S. Husain, A. Pladevega, L. Lai, T. H. Vanderford, L. Chennareddi, G. Silvestri, G. J. Freeman, R. Ahmed, and R. R. Amara. 2008. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsh, R. M., Jr., and M. J. Buchmeier. 1979. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology 96:503-515. [DOI] [PubMed] [Google Scholar]
- 38.Wherry, E. J., J. N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wherry, E. J., S. J. Ha, S. M. Kaech, W. N. Haining, S. Sarkar, V. Kalia, S. Subramaniam, J. N. Blattman, D. L. Barber, and R. Ahmed. 2007. Molecular signature of CD8+ T-cell exhaustion during chronic viral infection. Immunity 27:670-684. [DOI] [PubMed] [Google Scholar]
- 41.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, J. Y., Z. Zhang, X. Wang, J. L. Fu, J. Yao, Y. Jiao, L. Chen, H. Zhang, J. Wei, L. Jin, M. Shi, G. F. Gao, H. Wu, and F. S. Wang. 2007. PD-1 upregulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors, but not in long-term nonprogressors. Blood 109:4671-4678. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in downregulation of antigen-specific T cells during persistent viral infection. J. Virol. 76:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]