Abstract
Human fibroblasts infected with human cytomegalovirus (HCMV) were more viable than uninfected cells during glucose starvation, suggesting that an alternate carbon source was used. We have determined that infected cells require glutamine for ATP production, whereas uninfected cells do not. This suggested that during infection, glutamine is used to fill the tricarboxylic acid (TCA) cycle (anaplerosis). In agreement with this, levels of glutamine uptake and ammonia production increased in infected cells, as did the activities of glutaminase and glutamate dehydrogenase, the enzymes needed to convert glutamine to α-ketoglutarate to enter the TCA cycle. Infected cells starved for glutamine beginning 24 h postinfection failed to produce infectious virions. Both ATP and viral production could be rescued in glutamine-starved cells by the TCA intermediates α-ketoglutarate, oxaloacetate, and pyruvate, confirming that in infected cells, a program allowing glutamine to be used anaplerotically is induced. Thus, HCMV infection activates the mechanisms needed to switch the anaplerotic substrate from glucose to glutamine to accommodate the biosynthetic and energetic needs of the viral infection and to allow glucose to be used biosynthetically.
Glucose (Glc) and glutamine are the two most abundant nutrients used by mammalian cells. They are necessary for the generation of energy, macromolecules, and second messengers (1, 5-7, 9-12, 16). Glucose has long been considered absolutely essential for the viability of mammalian cells because of its contribution to energy homeostasis through glycolysis and the tricarboxylic acid (TCA) cycle (Fig. 1). Recent studies demonstrated that human diploid fibroblasts are killed by glucose deprivation by a mechanism different from apoptosis (20).
FIG. 1.
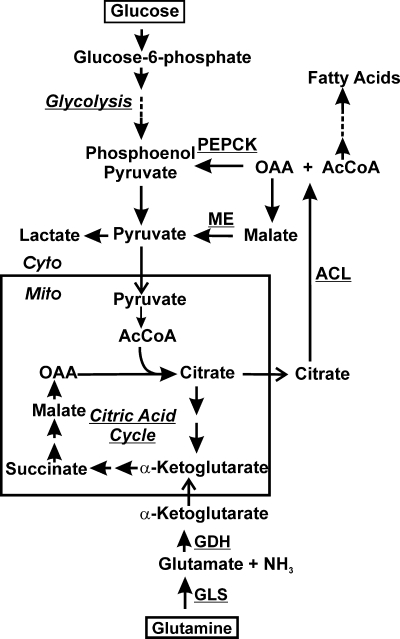
Glycolysis and the citric acid cycle showing glucose and glutamine utilization. The aspects of the cytoplasmic (Cyto) and mitochondrial (Mito) metabolism of glucose and glutamine discussed in the text are outlined. Dashed lines indicate that there are several intermediates formed (several reactions) between the ones shown. PEPCK, phosphoenolpyruvate carboxykinase; ME: malic enzyme; GDH, glutamate dehydrogenase; GLS, glutaminase; ACL, ATP citrate lyase; OAA, oxaloacetic acid; AcCoA, acetyl coenzyme A.
In 1924, Warburg observed that cancer cells metabolize glucose very differently than normal cells (18). Cancer cells converted glucose into lactate even in the presence of sufficient oxygen to support mitochondrial oxidative phosphorylation (Fig. 1). This utilization of glucose, called the Warburg effect, results in only 2 ATP molecules produced per molecule of glucose, whereas if it had proceeded through the TCA cycle and mitochondrial oxidative phosphorylation, an additional 36 ATP molecules would have been produced per molecule of glucose. Recently reported data provide an explanation for what appeared to be an inefficient utilization of glucose (7, 8, 19). In cancer cells, exogenous glutamine is used as a carbon source, which facilitates the cell's ability to use glucose biosynthetically instead of breaking it down completely for energy. This is accomplished by glutamine being converted to α-ketoglutarate via glutaminase (GLS) and glutamate dehydrogenase (GDH) (Fig. 1). This process of replenishing TCA cycle intermediates is called anaplerosis. Thus, glutamine anaplerotically fills the TCA cycle (Fig. 1), providing NADH for oxidative phosphorylation as well as TCA cycle intermediates, which serve as important biosynthetic precursors (7, 8). In contrast, normal cells are believed to use only a small amount of consumed glutamine for macromolecular biosynthesis and energy; thus, glucose and glutamine metabolism are dramatically altered in tumor cells (8, 16).
While glutamine starvation in many cell types has little impact on cell viability, it has been shown to induce cell death in cancer cell lines that overexpress the oncogene c-myc (20). These cells also showed decreased levels of ATP production correlating with decreased concentrations of TCA cycle intermediates; both are predictable consequences of glutamine starvation if glutamine is being used anaplerotically. In agreement with this finding, the effects of glutamine starvation could be reversed by the addition of the TCA cycle intermediates pyruvate (Pyr) and oxaloacetate (OAA) (Fig. 1).
Human cytomegalovirus (HCMV) is a slow-growing betaherpesvirus that exerts a large energetic and biosynthetic demand on cells to ensure successful viral replication. Recent mass spectrometry-based metabolic flux studies indicated global metabolic upregulation in infected cells (14, 15). This included greatly increased glycolysis in which the vast majority of glucose-derived acetyl coenzyme A (AcCoA) went to support fatty acid synthesis (Fig. 1) to make membranes needed by the virus. Thus, there is a great decrease in the amount of glucose-derived carbon entering the TCA cycle. In other words, the virus induces a modified Warburg effect so that glucose-derived carbon can be used biosynthetically. These metabolomic data also suggest that glutamine may be used to anaplerotically fill the TCA cycle.
We have investigated the impact of glucose and glutamine on HCMV replication. We have found that under conditions of glucose deprivation, infected cells are more viable than mock-infected cells. Thus, we hypothesized that the infected cells use glutamine anaplerotically. In agreement with this prediction, glutamine was found to be necessary for ATP production in infected cells but not in uninfected cells. Furthermore, cells starved of glutamine beginning 24 h postinfection (hpi) failed to produce infectious virions. HCMV-induced glutaminolysis was indicated by increased glutamine uptake and ammonia production corresponding to increased activities of glutaminase and glutamate dehydrogenase. These enzymes convert glutamine to α-ketoglutarate (α-KG) for anaplerotic use in the TCA cycle. The anaplerotic use of glutamine in the TCA cycle was also demonstrated by the finding that both ATP production and viral growth could be rescued by replacing glutamine with the TCA cycle intermediate α-ketoglutarate, oxaloacetate, or pyruvate. Thus, our data suggest that in HCMV-infected cells, as in many tumor cells, a program is activated whereby glutamine utilization increases specifically to maintain the TCA cycle, allowing glucose to be used biosynthetically.
MATERIALS AND METHODS
Cells and viruses.
Primary or life-extended human foreskin fibroblasts (HFFs) (3) were propagated and maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, Glutamax, and antibiotics. Cells were infected with purified HCMV (Towne) at a multiplicity of infection (MOI) of 3.
Nutrient starvation.
For glucose and glutamine depletion studies, the base medium used was Dulbecco's modified Eagle's medium lacking glucose, glutamine, pyruvate, and phenol red (catalog number D5030-10X1L; Sigma). This was supplemented with 10% dialyzed fetal bovine serum (FBS), antibiotics, and 1 g/liter glucose and/or 2 mM glutamine as need for the experiment. The dialyzed FBS used was commercially available dialyzed serum (HyClone), which we exhaustively dialyzed against saline to ensure that glucose and glutamine had been removed. The dialyzed FBS was thoroughly tested to ensure that it maintains the ability to support cell growth under normal conditions. Cells grown in replete medium (1 g/liter glucose plus 2 mM glutamine) were washed three times with glutamine- or glucose-free medium and refed in the appropriate medium as needed for the experiment. For experiments where citric acid cycle intermediates were added to the medium, the concentrations were 7 mM dimethyl-α-ketoglutaric acid, 4 mM oxaloacetic acid, and 4 mM pyruvate (all from Sigma).
Virus infection time course studies.
HFFs, grown in 6-well plates, were infected as described above or mock infected. For continuous glutamine/glucose starvation, cells were immediately washed in, and fed with, glutamine-free or glucose-free medium, and samples were harvested at various times postinfection (0 to 120 h). For delayed glutamine or glucose starvation, infections were allowed to proceed for various times in replete medium and then washed and fed with either glutamine-free or glucose-free medium. The cells were then incubated for an additional 12 or 24 h before harvesting for various assays or virus titration using the 50% tissue culture infective dose (TCID50) method.
Cell viability.
Attached cells were removed by trypsinization, and the suspensions were places into a centrifuge tube along with the culture medium containing any detached cells. The cells were pelleted by light centrifugation, and the pellets were suspended in 0.5 ml phosphate-buffered saline (PBS). A total of 0.2 ml of cell suspension was added to 0.3 ml PBS plus 0.5 ml of 0.4% trypan blue solution, mixed, allowed to stand for 5 min, and then counted with a hemacytometer.
Glutamine consumption and ammonia production.
Glutamine consumption was measured by using the Nova Biomedical BioProfile Flex analyzer. Briefly, cells were grown to approximately 80% confluence and then mock or HCMV infected and placed in replete medium. The controls were plated with an equal volume of medium but no cells, incubated identically as the cell-containing plates. Medium was removed from the plates for analysis immediately or frozen at −80°C for no more than 5 days prior to analysis. Glutamine concentrations (measured in mmol/liter) were determined for each sample. Glutamine consumption is equal to the glutamine concentration remaining in the medium from the cell samples subtracted from the glutamine concentration in the medium control. Concentrations were divided by the number of cells and presented on a per-cell basis. Ammonia production was also measured by using the BioProfile Flex analyzer. Ammonia measurements were made simultaneously with the glutamine measurements for each time point. Ammonia concentrations (measured in mmol/liter) were determined for each sample and medium control; ammonia production was calculated by subtracting the concentration of the medium control from the concentration of the sample. Concentrations were divided by the number of cells and presented on a per-cell basis.
ATP quantitation.
The ATP concentration was measured by using the ATP determination kit protocol (Invitrogen), a luciferase-based quantitation method. Briefly, cells were infected or mock infected as indicated above and treated as indicated for the individual experiments. Cells were collected and then lysed in boiling water (0.5 ml per sample). After lysis, cellular debris was removed by centrifugation (14,000 × g for 10 min). For each ATP assay, 10 μl of lysate was used per assay. Reactions were conducted in triplicate for each condition/sample. Cells from duplicate plates were counted; using the cell count, the ATP concentration per cell was calculated.
Western analysis.
Cells were lysed by using radioimmunoprecipitation assay (RIPA) buffer. Proteins were quantitated by using the Bio-Rad protein assay (Bio-Rad, Hercules, CA), and 25 μg of each sample was separated by SDS-PAGE (12% polyacrylamide gels). Proteins were transferred onto nitrocellulose membranes, which were blocked in 5% nonfat milk diluted in 1× Tris-buffered saline (TBS) containing 0.5% Tween 20 (1× TBST) for 30 min at room temperature. Primary antibodies were diluted in 5% bovine serum albumin (BSA) in 1× TBST and incubated at 4°C overnight. The membranes were then washed three times for 10 min in 1× TBST. Secondary horseradish peroxidase-conjugated antibodies were diluted in 5% nonfat milk in 1× TBST and incubated for 1 h at room temperature. The membranes were washed in 1× TBST three times for 10 min. The membranes were incubated in Lumi-Light Western blotting substrate (Roche Diagnostics, Indianapolis, IN). Membranes were exposed to film and developed. Antibodies used were as follows: glutamate dehydrogenase antibody ab55061 (Abcam), glutaminase antibody ab60709 (Abcam), and actin antibody MAB1501 (Chemicon International).
Enzyme assays.
The enzymatic activities of glutaminase, glutamate dehydrogenase, malic enzyme (ME), and phosphoenolpyruvate (PEP) carboxykinase (PEPCK) were determined by spectrophotometric assays previously described by Bergmeyer (2). For each assay enzymatic activity was determine by the conversion of NADH to NAD measured by the change in absorbance at 340 nm over time. For each enzyme assay, concentrations were based on 200-μl reaction mixture volumes in a microtiter plate. Each reaction mixture contained no less than 50 μg of cell lysate protein.
To determine glutaminase activity, cells were lysed in a solution containing 50 mM Tris, 150 mM KH2PO4-K2HPO4, 1 mM EDTA, and 1% Triton X-100 (pH 7.4). After three freeze-thaw cycles the lysates were sonicated briefly to achieve optimal lysis. Glutaminase activity was measured via coupling to glutamate dehydrogenase (GDH)-mediated NADH production. Briefly, glutaminase deaminates glutamine to glutamate, and GDH then converts glutamate to α-ketoglutarate. The second reaction requires the reduction of NAD+ to NADH; thus, it results in an increase in the absorbance at 340 nm. Cell lysates (50 μg) were placed into 0.05 M acetate buffer (pH 5.0) containing 2 mM l-glutamine, and the mixture was incubated for 1 h at 37°C. This reaction mixture, which produced glutamate, was added to 100 μl 2× GDH buffer (100 mM triethanolamine [TEA] [pH 7.4], 2 mM EDTA, 4 mM MgCl2, 60 mM β-mercaptoethanol, and 0.2% Triton X-100) supplemented with 0.36 mM NAD+, 1.0 mM ADP (an activator of GDH), and 48 U of glutamate dehydrogenase (Sigma), giving the reaction mixture a final volume of 200 μl. The production of NADH was monitored to determine the enzyme activity after a 40-min incubation at 25°C.
For glutamate dehydrogenase activity, cells were lysed in a solution containing 50 mM TEA (pH 7.4), 1 mM EDTA, 2 mM MgCl2, 30 mM β-mercaptoethanol, and 0.1% Triton X-100. After three freeze-thawing cycles, the lysates were briefly sonicated. Cell lysates (50 μg) were added to GDH buffer containing 1 mM glutamate, 0.18 mM NAD+, and 0.5 mM ADP, and the absorbance was monitored at 340 nm following a 40-min incubation at 25°C. As indicated in the glutaminase-GDH-coupled system, the conversion of glutamate to α-ketoglutarate requires the reduction of NAD+ to NADH, resulting in an increase in the absorbance at 340 nm.
PEPCK activity was determined for cells lysed in a solution containing 50 mM TEA (pH 7.4), 300 mM sucrose, 1 mM EDTA, and 0.1% Triton X-100 using three freeze-thaw cycles and brief sonication. The conversion of oxaloacetate to PEP was monitored by coupling the reaction to the pyruvate kinase (PK)-lactate dehydrogenase (LDH)-coupled enzyme reaction. Briefly, the PEP produced from the PEPCK reaction will be converted to pyruvate by PK, and that pyruvate is reduced by LDH to produce lactate. During the LDH reaction, NADH is converted to NAD+, resulting in a decrease in the absorbance at 340 nm. Again, 50 μg of cell lysate was added to buffer containing 10 mM HEPES (pH 7.5), 10 mM MgCl2, 20 mM KCl, 5 mM ATP, 10 mM OAA, 0.18 mM NADH, and 2 U PK and 16 U LDH (both from Sigma). The absorbance at 340 nm was monitored for 15 min at 25°C.
ME was assayed by measuring the conversion of malate to pyruvate as indicate by the production of NADPH created during the reaction. Malate is converted to pyruvate by ME in the presence of Mn2+ and NADP+, leading to the generation of CO2 and NADPH. The increase in the NADPH concentration results in an increase in the absorbance at 340 nm. Cells were lysed by freeze-thawing in a solution containing 50 mM TEA (pH 7.4), 1 mM EDTA, 2 mM MgCl2, 30 mM β-mercaptoethanol, and 0.1% Triton X-100. Cell lysates (50 μg) were placed into a solution containing 670 mM TEA (pH 7.4), 5 mM MnCl2, 3.5 mM malate, and 0.18 mM NADP+. The absorbance at 340 nm was measured after 10 min at 25°C.
RESULTS
Infected human fibroblasts (HFs) are more viable in the absence of glucose than uninfected human fibroblasts.
In these experiments, the replete medium used was Dulbecco's modified Eagle's medium lacking glucose, glutamine, pyruvate, and phenol red (catalog number D5030; Sigma), which was supplemented with 10% dialyzed fetal bovine serum (FBS), antibiotics, 1 g/liter glucose, and 2 mM glutamine. The dialyzed serum is commercially available dialyzed serum, which we then exhaustively dialyzed against saline to ensure that glucose and glutamine had been removed.
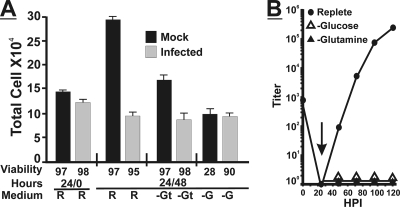
Figure 2A shows the results of cell growth and viability determinations using mock- or HCMV-infected HFs under conditions of glucose and glutamine deprivation. Cells were grown to 50% confluence in 6-well plates in replete medium (10% FBS) and then either HCMV infected (MOI of 3) or mock infected and maintained in replete medium containing 10% dialyzed FBS for 24 h. At this point the cells from infected and mock-infected plates were counted, and their viability was determined for the start of the experiment (24/0; 24 h in replete medium, 0 h additional incubation); the total numbers of cells and their viabilities (at least 97%) were similar between mock and infected cells. The medium on other plates was changed to either fresh replete medium or medium lacking glucose or glutamine; in all cases the medium contained 10% dialyzed FBS. These cells were allowed to grow for an additional 48 h before counting and determining viability (24/48). The mock-infected cells grew in complete medium, as indicated by the increased cell number compared to the 24/0-h time point, and the viability was high. Infected cells in replete medium did not grow, as expected for HCMV-infected cells (17), with counts remaining similar to the 24/0-h cell count, and the viability remained high. In the absence of glutamine, infected cells did not grow, while mock-infected cells appeared to have expanded modestly; viability remained high in both cases. Neither infected nor mock-infected cells grew in the absence of glucose. The viability of mock-infected cells fell to 28%, as expected from data from previously studies (20) showing that human fibroblasts die in the absence of glucose. However, the infected cells remained viable (90%) in the absence of glucose. Thus, infected cells had an advantage that allowed them to remain viable under conditions of glucose deprivation.
FIG. 2.
Glutamine is necessary for virus replication and cell viability of HCMV-infected cells. (A) HF cells were plated and grown to 50% confluence in replete medium and then mock (black bars) or HCMV infected (MOI of 3) (gray bars) for 24 h in replete medium. At this point (24/0), one set of cells was counted, and the viability was determined by using trypan blue exclusion staining. Other sets of cells had the medium changed to glucose-free (−G), glutamine-free (−GT), or replete (R) medium. Forty-eight hours after the medium change (24/48), cell counts and viability were determined. Error bars are based on standard deviations of the means for 3 determinations for three replicate experiments. The standard deviation of the mean of cell viability determinations was no greater than ±5% based on three determinations for three replicate experiments. (B) Confluent monolayers of HF cells were infected and grown as described above (A). At 24 hpi (vertical arrow), the medium was changed to replete (R), glutamine-free (−glutamine), or glucose-free (−glucose) medium. Cells were harvested every 24 h up to 120 hpi, and titers of infectious virions in each sample were determined by using the TCID50 method.
Infectious virions are not produced under glucose-free or glutamine-free conditions.
We hypothesized from the above-described results that the increased viability in infected cells during glucose deprivation may be due to an increased anaplerotic use of glutamine as described above for tumor cells (see the introduction). As shown in Fig. 2B, we determined the levels of growth of HCMV under glucose-free or glutamine-free conditions. HFs were mock infected or infected with HCMV (MOI of 3) for 24 h in replete medium to allow the infections to get established and prepare the cells to be able to survive under glucose-free conditions. Remember that glutamine is a nonessential amino acid, and the cells can make it; therefore, in these experiments, glutamine free indicates that no exogenous glutamine was added.
Figure 2B shows that in replete medium, a normal growth curve was generated. In the absence of glucose, or in the absence of exogenously added glutamine, viruses were not produced. It is not surprising that under glucose-free conditions, no infections virions were detected. However, the complete lack of infectious virion formation under glutamine-free conditions was unexpected. This suggests that by 24 hpi, a supply of exogenous glutamine is essential for HCMV growth despite the presence of glucose and normal cell viability.
Levels of glutamine uptake and ammonia production increase in HCMV-infected cells.
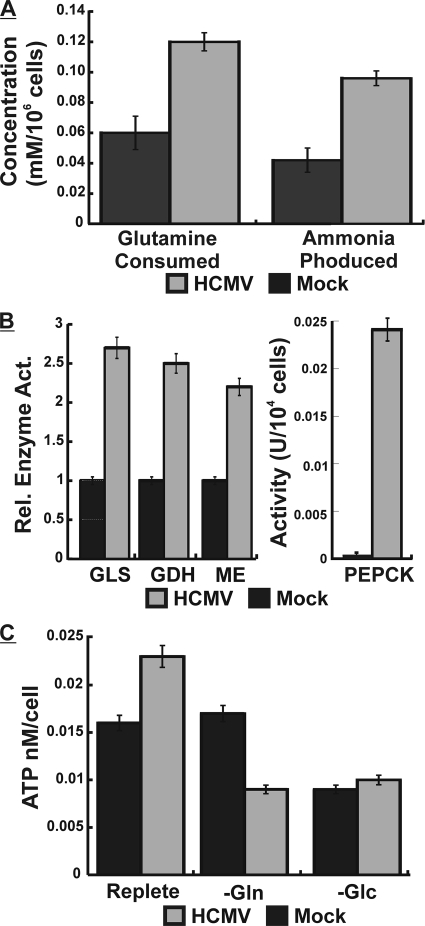
To explore the utilization of glutamine in infected cells, we mock or HCMV infected cells using replete medium and 10% FBS. At 24 hpi, an early time in infection, glutamine consumption and ammonia production were measured as described in Materials and Methods. Figure 3A shows that glutamine consumption had already increased at least 2-fold, and this was accompanied by an equivalent increase in ammonia production. Taken together, these results indicate that glutamine uptake and deamination to glutamate (glutaminolysis) (Fig. 1) are increased in infected cells.
FIG. 3.
Glutamine metabolism is altered during HCMV infection. (A) Cells were mock (black bars) or HCMV (gray bars) infected for 24 h in replete medium, at which time the medium was removed and analyzed for glutamine consumption and ammonia production by using a Nova Biomedical Flex analyzer. Error bars are based on standard deviations of the means for 5 separate measurements. (B) Cells were mock (black bars) or HCMV (gray bars) infected for 24 h and then extracted and assayed for glutaminase (GLS), glutamate dehydrogenase (GDH), malic enzyme (ME), and phosphoenolpyruvate carboxykinase (PEPCK), as described in Materials and Methods. Error bars are standard deviations of the means for 3 determinations from 3 separate experiments. (C) Mock-infected (black bars) or HCMV-infected (gray bars) cells were grown for 24 h in replete medium and then placed into either fresh replete, glutamine-free (−Gln), or glucose-free (−Glc) medium for 12 h, at which time intracellular ATP levels (nm/cell) were measured by a luciferase assay as described in Materials and Methods. Error bars are standard deviations of the means for 5 separate determinations.
Glutaminase and glutamate dehydrogenase enzymatic activities increase during infection.
To further prove that increased glutaminolysis and conversion to α-ketoglutarate were occurring during infection, HFs were mock or HCMV infected for 24 h in replete medium as described above and then extracted and assayed for the enzymatic activities of glutaminase (GLS), the enzyme that deaminates glutamine to glutamate, and glutamate dehydrogenase (GDH), which converts glutamate to α-ketoglutarate, which can enter the TCA cycle (Fig. 1). Figure 3B shows that GLS and GDH enzyme activities are at least 2.5 times greater in infected cells at 24 hpi, strongly suggesting that the infected cells are using glutaminolysis to metabolize the increased amounts of glutamine to form α-ketoglutarate.
Glutamine starvation causes ATP depletion in HCMV-infected cells.
If glutamine is entering the TCA cycle, then glutamine starvation should cause a loss of ATP production. Thus, we monitored intracellular ATP concentrations. Confluent cells in replete medium were mock or HCMV infected for 24 h, and the medium was then changed to fresh replete medium or glutamine-free or glucose-free medium; all media contained 10% dialyzed FBS. Twelve hours later, the cells were extracted for ATP determinations. Figure 3C shows that in replete medium, HCMV-infected cells contained about 30% more ATP than mock-infected cells. Glutamine depletion had little effect on ATP levels in mock-infected cells; however, in infected cells, ATP levels were lowered by 60% compared to infected cells in replete medium. The removal of glucose lowered ATP levels by 50% in mock-infected cells and by 50 to 60% in infected cells. These data suggest that in infected cells, in addition to glucose, glutamine becomes a significant nutrient for energy generation.
The effects of glutamine starvation in infected cells can be rescued by TCA cycle intermediates.
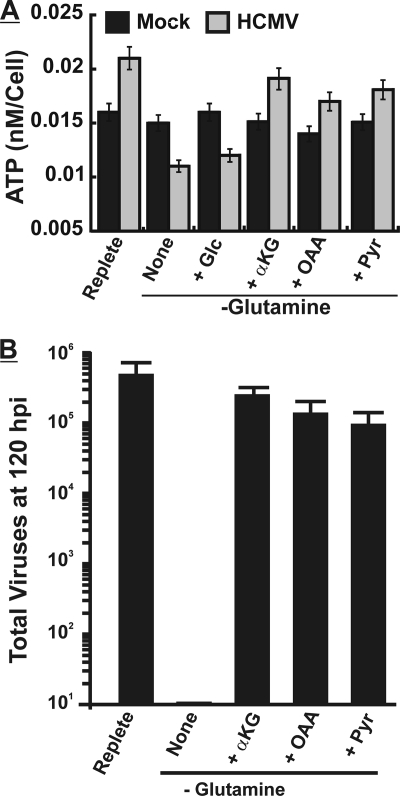
The data described above suggest that glutamine enters the TCA cycle via conversion to α-ketoglutarate, increasing the production of ATP. If this is the case, then TCA cycle intermediates should rescue ATP production and viral growth under glutamine-free conditions. Confluent HFs were mock or HCMV infected for 24 h and then changed to fresh replete medium, glutamine-free medium, or glutamine-free medium supplemented with either 7 mM dimethyl-α-ketoglutarate (α-KG), 4 mM oxaloacetate (OAA), 4 mM pyruvate (Pyr), or 5 mM glucose (Glc).
Cells were harvested for ATP determinations 12 h after the medium was changed as described in the legend of Fig. 3C. Figure 4A reiterates the finding that in replete medium ATP levels increased in infected cells in comparison to mock-infected cells and that the removal of glutamine severely affects ATP levels in infected cells but not in mock-infected cells. The addition of either dimethyl-α-ketoglutarate, oxaloacetate, or pyruvate rescued much of the ATP production in glutamine-starved, infected cells (Fig. 4A). In mock-infected cells, the metabolites made no difference in ATP concentrations. The addition of extra glucose to glutamine-free medium (5 mM added plus the 5.5 mM normally in the media) was done to replace the carbon lost by glutamine removal (Fig. 4A). This did not rescue ATP production, indicating that the loss of ATP is a glutamine-specific effect and not due to the lowering of the total carbon supply.
FIG. 4.
Citric acid cycle intermediates rescue ATP production and viral growth in HCMV-infected cells deprived of glutamine. (A) Cells were mock (black bars) or HCMV (gray bars) infected for 24 h in replete medium and then placed into either fresh replete (R) or glutamine-free (None) medium or glutamine-free medium supplemented with glucose (+Glc) (an additional 5 mM), 4 mM pyruvate (+Pyr), 4 mM oxaloacetic acid (+OAA), or 7 mM dimethyl-α-ketoglutaric acid (+αKG). Twenty-four hours after the medium change, ATP levels were determined. Error bars are standard deviations of the means for 5 separate determinations. (B) Confluent monolayers of HF cells were infected (MOI of 3) for 24 hpi, and the medium was then changed to replete medium, glutamine-free medium (None), or glutamine-free medium supplemented with 4 mM pyruvate (+Pyr), 4 mM oxaloacetic acid (+OAA), or 7 mM dimethyl-α-ketoglutaric acid (+αKG). At 120 hpi viruses were harvested, and titers were determined as described in Materials and Methods. Error bars are standard deviations of the means for 3 parallel determinations.
Another set of cells was similarly treated and harvested at 120 hpi to determine total viral production. Figure 4B reiterates the finding that infectious virus production is severely inhibited under glutamine-free conditions, but this is rescued by α-KG, OAA, and Pyr.
The data in Fig. 4 show that TCA cycle intermediates can substantially rescue the effects of glutamine starvation in infected cells; this suggests that in infected cells, glutamine is converted to α-ketoglutarate, where it enters the citric acid cycle to produce OAA (Fig. 1). This can account for the infected cell's decreased reliance on glucose for the citric acid cycle and the essential requirement for glutamine. Thus, HCMV induces a shift in anaplerotic substrates during infection.
The activities of pyruvate-producing enzymes are increased in infected cells.
The ability of pyruvate to rescue ATP and virus production during glutamine starvation is unexpected unless, in replete medium, infected cells can derive pyruvate from glutamine. This could occur through glutamine-derived α-ketoglutarate proceeding through the TCA cycle to citrate. Mitochondrial citrate can be transported to the cytoplasm, where ATP citrate lyase (ACL) converts it to AcCoA and OAA (Fig. 1). This is the means by which cytoplasmic AcCoA is generated for fatty acid synthesis, which was previously shown to be critically important for the success of an HCMV infection (15). The resulting OAA can be returned to pyruvate in two ways (Fig. 1): phosphoenolpyruvate carboxykinase (PEPCK) converts OAA to phosphoenolpyruvate (PEP), which can be converted to pyruvate, or OAA can be converted to malate, and malic enzyme (ME) converts it to pyruvate, which can reenter the mitochondrion. Figure 3B shows that PEPCK and ME activities are significantly increased by 24 hpi, suggesting that pyruvate synthesis from cytoplasmic OAA is very important for the viral infection and indicates a means for pyruvate to be derived from glutamine in the infected cells. This will be further considered below in the Discussion. The activation of PEPCK is particularly remarkable since it is normally induced in a very tissue-specific manner (4) since it controls the rate-limiting step of gluconeogenesis; it is inactive in normal, uninfected HFs (Fig. 3B).
HCMV infection alters the regulation of cellular glutamine metabolism in a temporal manner.
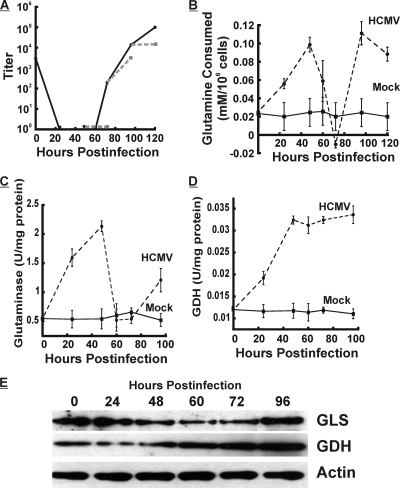
To better assess the impact of glutamine starvation on viral growth, we allowed the viral infection to proceed for various times in replete medium and then changed the medium to glutamine-free medium for 24 h prior to extraction for the titration of infectious virions. Figure 5A shows the comparisons: the glutamine-deprived period of the infection is shown as a dashed gray line to be compared with the growth curve in replete medium, shown as a solid black line. The removal of glutamine at 48 hpi and harvest at 72 hpi severely inhibited virus production in comparison to virus production in replete medium harvested at 72 hpi. However, glutamine starvation at between 72 and 96 h of infection did not impact viral growth as severely. Conversely, glutamine removal between 96 and 120 hpi again inhibited infectious-virion formation. These data suggest that in infected cells, the essential requirement for glutamine changes temporally during infection.
FIG. 5.
HCMV infection alters the regulation of cellular glutamine metabolism in a temporal manner. (A) Infection time course done using replete medium (solid line) or where glutamine was removed 24 h prior to harvest (dashed lines, 48 to 72 hpi, 72 to 96 hpi, and 96 to 120 hpi). The titers of infections virions were determined by the TCID50 method. (B to E) cells in replete medium were mock or HCMV infected. To ensure that nutrients were not limiting during the course of this experiment, the replete medium was changed 24 h prior to each measurement. At various time points, glutamine consumption (B), glutaminase activity (C), and glutamate dehydrogenase (GDH) activity were determined as described in Materials and Methods. (E) Western analysis of the levels of glutaminase (GLS), glutamate dehydrogenase (GDH), and actin at each time point during infection.
To examine temporal variations, we measured glutamine consumption during the infection time course. This experiment was done by using replete medium. To ensure that nutrients were not limiting during the course of this experiment, the replete medium was changed 24 h prior to each measurement. Figure 5B shows that the level of glutamine consumption increases through 48 hpi but thereafter decreases to the point that by 72 hpi, consumption is less than what the cell can produce. However, between 72 and 96 hpi, the level of consumption sharply rises again. Glutamine consumption in mock-infected cells remained constant, at a low level, over the time course.
Given the temporal variation of glutamine consumption, we investigated the enzymatic activities involved in glutaminolysis during infection. We found that glutaminase (Fig. 5C) exhibited temporal variations in activity in a pattern similar to that of glutamine consumption. Glutamine dehydrogenase (Fig. 5D) activity rose coordinately with glutaminase activity and remained high during the remainder of the infection.
Figure 5E shows Western analysis of glutaminase and glutamate dehydrogenase levels during an HCMV infection time course performed using replete medium. GDH levels increased steadily during the course of the infection; however, GLS protein levels varied temporally, as did its enzyme activity. These data suggest that glutamine consumption and glutaminase activity are regulated coordinately.
DISCUSSION
We have shown that HCMV infection causes major alterations in cellular metabolism. Previous studies have shown that the level of glucose consumption is increased in HCMV-infected cells within the first 24 h of infection, leading to speculation that increased glycolysis may be the mechanism for energy production during infection (13). However, we have found that in the absence of glucose, HCMV-infected cells show an increased viability compared to that of uninfected cells. This observation was explained by the finding that glutamine anaplerotically maintains the citric acid cycle for ATP production and TCA cycle biosynthetic intermediates. This allows glucose to be diverted for use in synthetic processes (Fig. 1). This was previously shown to occur in tumor cells (7). In addition, recent metabolic flux profiling data, based on mass spectrometric analysis of metabolites in HCMV-infected cells, suggested that a primary use of glucose is in nucleotide (pentose phosphate pathway) and fatty acid syntheses (14, 15). These mass spectrometric data also suggest the anaplerotic utilization of glutamine, but these data do not predict the critical nature of glutamine that we have observed.
We show that during the course of HCMV infection, the level of glutamine consumption increases, and the infected cells become dependent upon glutamine for ATP production and viral production. HCMV begins to induce the switching of anaplerotic substrates from glucose to glutamine within the first 24 h of infection, and this increased significantly by 48 h postinfection. The finding that glutamine is the major anaplerotic substrate during infection was confirmed by the rescue of ATP synthesis and viral growth when stoichiometrically equal amounts of the citric acid cycle intermediates α-ketoglutarate, oxaloacetic acid, and pyruvate were added individually to glutamine-free medium. The finding that the conversion of glutamine to α-ketoglutarate is increased in infected cells is supported by the observation of increased glutaminase and glutamate dehydrogenase activities. Our data suggest that the inhibition of glutamine uptake or glutaminolysis may be an effective antiviral therapy for HCMV infection.
The ability of pyruvate to rescue ATP and virus production during glutamine starvation is unexpected and implies that under replete conditions, infected cells can derive pyruvate from glutamine. This could occur when glutamine-derived α-ketoglutarate proceeds through the TCA cycle to citrate (Fig. 1). At this point citrate can be transported from the mitochondrion to the cytoplasm, where ATP citrate lyase (ACL) converts it AcCoA and OAA (Fig. 1). This is the means by which cytoplasmic AcCoA is generated for fatty acid synthesis, which is critically important for the success of an HCMV infection (15). As shown in Fig. 1, the resulting OAA can be returned to pyruvate by way of PEPCK or ME; both of these enzyme activities are significantly increased during infection. Either of these enzymes complete a pathway for deriving pyruvate from glutamine.
The export of citrate from the mitochondria is a shuttle mechanism that usually involves citrate being exported, and malate, generated from cytoplasmic OAA, is shuttled back. However, our data suggest that OAA and malate are converted to pyruvate by PEPCK and ME in the cytoplasm of the infected cell; thus, the virus appears to induce a citrate-pyruvate shuttle. This may be advantageous because a by-product of the ME reaction is NADPH; thus, the conversion of cytoplasmic malate to pyruvate would provide a source of cytoplasmic NADPH, which would be important not only biosynthetically (e.g., fatty acid synthesis) but also to combat oxidative stress.
Our data also show that glutamine metabolism during the course of infection is regulated temporally. Under our experimental conditions, glutamine consumption increases until 48 hpi and thereafter decreases to the point that by 72 hpi, consumption is less than what the cell can production (glutamine is a nonessential amino acid, and the cell can make it). However, between 72 and 96 hpi, the level of consumption sharply rises again. The observation that glutaminase levels and activities rise and fall with similar kinetics suggests that the virus coordinately regulates the temporal consumption of glutamine, at least in part, by regulating glutaminase levels. At this point the reason for this temporal regulation is unclear but may reflect the differing metabolic needs of the viral infection as the infection progresses from the synthetic phase to the virion assembly phase to the egress phase.
Why does HCMV require such an increase in glutamine metabolism? These and other data (14, 15) suggest that HCMV does not want glucose to be metabolized for energy in the citric acid cycle but instead maintains it for synthetic purposes such as fatty acid synthesis (Fig. 1). Thus, the maintenance of the citric acid cycle and ATP levels must be transferred to glutamine, which becomes the major anaplerotic substrate. Tumor cells have been shown to utilize a similar mechanism, and they become addicted to glutamine such that glutamine withdrawal causes cell death (19, 20). We did not see infected-cell death in the absence of glutamine, suggesting a difference between the mechanisms used by infected cells and tumor cells to achieve the anaplerotic utilization of glutamine.
Acknowledgments
We thank members of Craig Thompson's laboratory at the University of Pennsylvania for sharing their reagents, equipment, and helpful comments. Additionally, we acknowledge Yongjun Yu, Carisa Tilton, Amy Clippinger, Nick Buchkovich, and Sherri Adams for their helpful comments, support, and guidance.
This work was supported by the Abramson Family Cancer Research Institute and Public Health Service grant R01-CA028379-29 awarded to J.C.A. by the National Cancer Institute.
Footnotes
Published ahead of print on 25 November 2009.
REFERENCES
- 1.Baggetto, L. G. 1992. Deviant energetic metabolism of glycolytic cancer cells. Biochimie 74:959-974. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeyer, H. U. 1984. Methods of enzymatic analysis, vol. III and IV. Verlag Chemie, Weinheim, Germany.
- 3.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakravarty, K., H. Cassuto, L. Reshef, and R. W. Hanson. 2005. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit. Rev. Biochem. Mol. Biol. 40:129-154. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, E. H., P. Barkhan, and A. J. Hale. 1963. Observations on the proliferation of human leucocytes cultured with phytohaemagglutinin. Br. J. Haematol. 9:101-111. [DOI] [PubMed] [Google Scholar]
- 6.Curi, R., C. J. Lagranha, S. Q. Doi, D. F. Sellitti, J. Procopio, T. C. Pithon-Curi, M. Corless, and P. Newsholme. 2005. Molecular mechanisms of glutamine action. J. Cell. Physiol. 204:392-401. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis, R. J., A. Mancuso, E. Daikhin, I. Nissim, M. Yudkoff, S. Wehrli, and C. B. Thompson. 2007. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U. S. A. 104:19345-19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis, R. J., N. Sayed, D. Ditsworth, and C. B. Thompson. 2008. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 18:54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edinger, A. L., and C. B. Thompson. 2002. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 13:2276-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulda, S., and K. M. Debatin. 2007. HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell Cycle 6:790-792. [DOI] [PubMed] [Google Scholar]
- 11.Hammerman, P. S., C. J. Fox, and C. B. Thompson. 2004. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends Biochem. Sci. 29:586-592. [DOI] [PubMed] [Google Scholar]
- 12.Hedeskov, C. J. 1968. Early effects of phytohaemagglutinin on glucose metabolism of normal human lymphocytes. Biochem. J. 110:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landini, M. P. 1984. Early enhanced glucose uptake in human cytomegalovirus-infected cells. J. Gen. Virol. 65:1229-1232. [DOI] [PubMed] [Google Scholar]
- 14.Munger, J., S. U. Bajad, H. A. Coller, T. Shenk, and J. D. Rabinowitz. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2:1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munger, J., B. D. Bennett, A. Parikh, X.-J. Feng, J. McArdle, H. A. Rabitz, T. Shenk, and J. D. Rabinowitz. 2008. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newsholme, E. A., B. Crabtree, and M. S. Ardawi. 1985. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci. Rep. 5:393-400. [DOI] [PubMed] [Google Scholar]
- 17.Stinski, M. F., and D. T. Petrik. 2008. Functional roles of the human cytomegalovirus essential IE86 protein. Curr. Top. Microbiol. Immunol. 325:133-152. [DOI] [PubMed] [Google Scholar]
- 18.Warburg, O., K. Posener, and E. Negelein. 1924. Über den Stoffwechsel der Carcinomzelle. Biochem. Z. 152:319-344. [Google Scholar]
- 19.Wise, D. R., R. J. DeBerardinis, A. Mancuso, N. Sayed, X.-Y. Zhang, H. K. Pfeiffer, I. Nissim, E. Daikhin, M. Yudkoff, S. B. McMahon, and C. B. Thompson. 2008. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U. S. A. 105:18782-18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuneva, M., N. Zamboni, P. Oefner, R. Sachidanandam, and Y. Lazebnik. 2007. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178:93-105. [DOI] [PMC free article] [PubMed] [Google Scholar]