Abstract
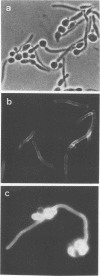
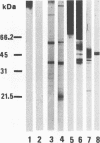
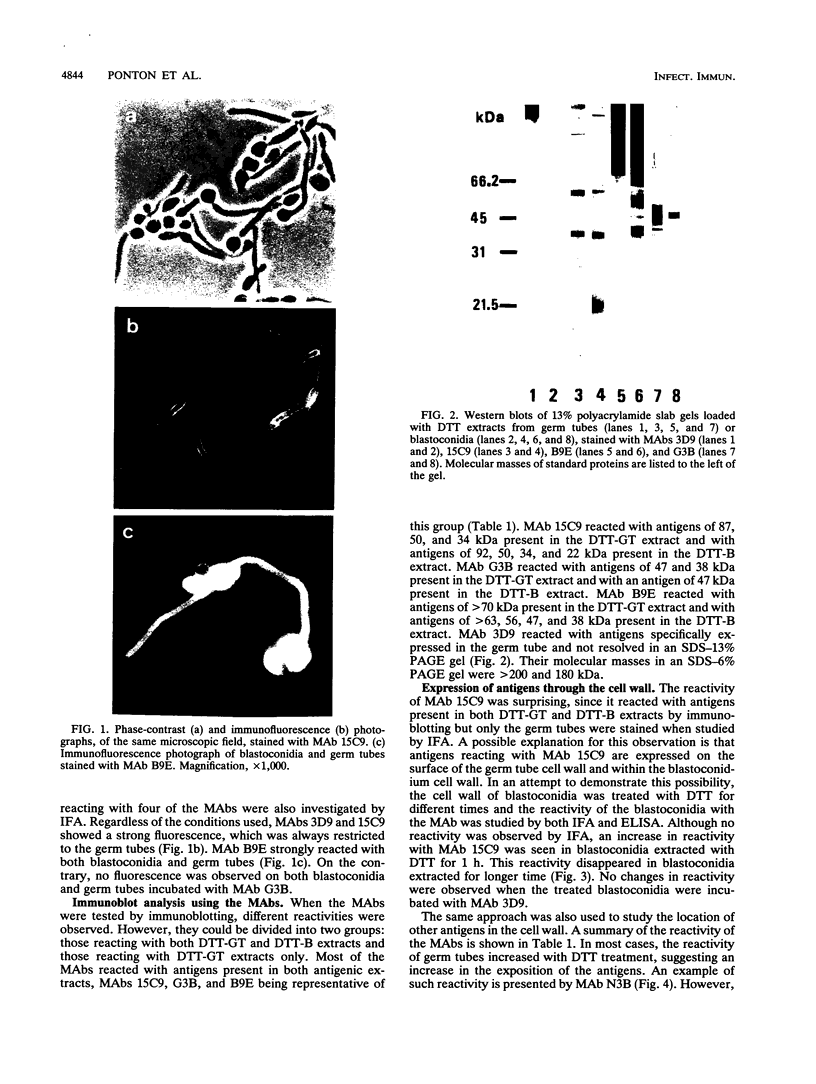
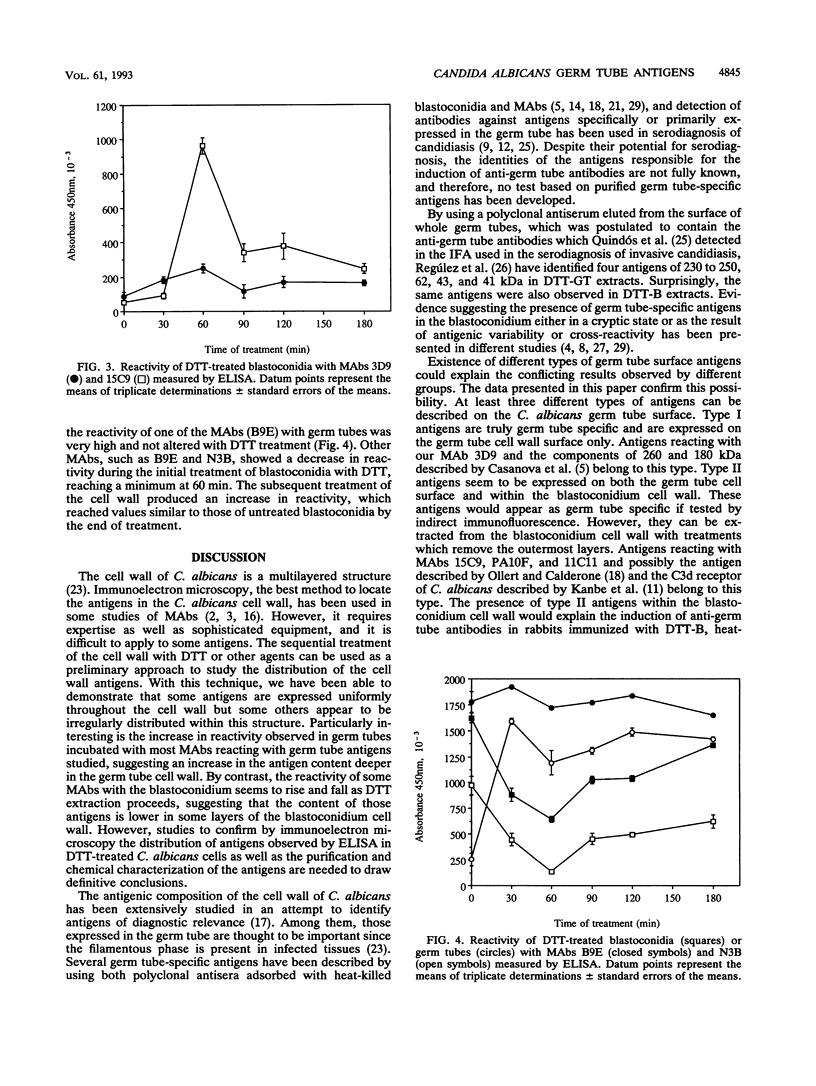
The antigenic composition of Candida albicans is very complex. In order to study the antigenic relationship between blastoconidia and germ tubes of C. albicans, we produced several monoclonal antibodies and analyzed their reactivity against cell wall antigens either in intact cells or in cells treated with dithiothreitol. Overall, four types of reactivity were found. Monoclonal antibodies 3D9 and 15C9 stained the germ tubes only when tested by indirect immunofluorescence. However, they showed a different reactivity by immunoblotting. Monoclonal antibody 3D9 reacted with antigens with molecular masses of > 200 and 180 kDa specifically expressed in the germ tube. Monoclonal antibody 15C9 reacted with antigens of 87, 50, and 34 kDa present in the germ tube extract and with antigens of 92, 50, 34, and 32 kDa present in the blastoconidium extract. The reactivity of blastoconidia treated for different times with dithiothreitol with these monoclonal antibodies was also studied by enzyme-linked immunosorbent assay. The reactivity of monoclonal antibody 3D9 did not significantly change during the cell wall extraction. However, the reactivity of monoclonal antibody 15C9 was increased for blastoconidia extracted for 60 min and decreased markedly for blastocondia extracted for 120 min. Monoclonal antibody G3B was nonreactive by indirect immunofluoresence but reacted with antigens of 47 and 38 kDa present in the germ tube extract and with an antigen of 47 kDa present in the blastoconidium extract. Monoclonal antibody B9E stained both morphological phases by indirect immunofluorescence. By immunoblotting, it reacted with antigens of > 70 kDa present in the germ tube extract and with antigens of > 63, 56, 47, and 38 kDa present in the blastoconidium extract. Based on the results presented in this study, four types of antigens are described. Type I antigens are expressed on the outermost layers of the germ tube cell wall only. Type II antigens are expressed both on the germ tube cell wall surface and within the blastoconidium cell wall. Type III antigens are found within the cell wall of both blastoconidia and germ tubes. Type IV antigens are expressed on both the blastoconidium and germ tube surface. Two types more can be hypothesized for antigens expressed on the blastoconidium cell surface and within the germ tube cell wall (type V) and for those expressed on the blastoconidium surface only (type VI).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawner D. L., Cutler J. E., Beatty W. L. Caveats in the investigation of form-specific molecules of Candida albicans. Infect Immun. 1990 Feb;58(2):378–383. doi: 10.1128/iai.58.2.378-383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Ultrastructural and biochemical studies of two dynamically expressed cell surface determinants on Candida albicans. Infect Immun. 1986 Jan;51(1):327–336. doi: 10.1128/iai.51.1.327-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986 Jan;51(1):337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Gil M. L., Cardeñoso L., Martinez J. P., Sentandreu R. Identification of wall-specific antigens synthesized during germ tube formation by Candida albicans. Infect Immun. 1989 Jan;57(1):262–271. doi: 10.1128/iai.57.1.262-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Torosantucci A., Boccanera M., Pellegrini G., Palma C., Malavasi F. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J Med Microbiol. 1988 Dec;27(4):233–238. doi: 10.1099/00222615-27-4-233. [DOI] [PubMed] [Google Scholar]
- Fortier B., Hopwood V., Poulain D. Electric and chemical fusions for the production of monoclonal antibodies reacting with the in-vivo growth phase of Candida albicans. J Med Microbiol. 1988 Dec;27(4):239–245. doi: 10.1099/00222615-27-4-239. [DOI] [PubMed] [Google Scholar]
- Ho Y. M., Ng M. H., Huang C. T. Antibodies to germinating and yeast cells of Candida albicans in human and rabbit sera. J Clin Pathol. 1979 Apr;32(4):399–405. doi: 10.1136/jcp.32.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. M., Ng M. H., Teoh-Chan C. H., Yue P. C., Huang C. T. Indirect immunofluorescence assay for antibody to germ tube of Candida albicans--a new diagnostic test. J Clin Pathol. 1976 Nov;29(11):1007–1010. doi: 10.1136/jcp.29.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990 Jan;3(1):32–45. doi: 10.1128/cmr.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe T., Li R. K., Wadsworth E., Calderone R. A., Cutler J. E. Evidence for expression of the C3d receptor of Candida albicans in vitro and in vivo obtained by immunofluorescence and immunoelectron microscopy. Infect Immun. 1991 May;59(5):1832–1838. doi: 10.1128/iai.59.5.1832-1838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura Y., Uesaka I. Assessment of germ tube dispersion activity of serum from experimental candidiasis: a new procedure for serodiagnosis. Infect Immun. 1974 May;9(5):788–793. doi: 10.1128/iai.9.5.788-793.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew M. A. Diagnosis of systemic Candida infections. Annu Rev Med. 1989;40:87–97. doi: 10.1146/annurev.me.40.020189.000511. [DOI] [PubMed] [Google Scholar]
- Li R. K., Cutler J. E. A cell surface/plasma membrane antigen of Candida albicans. J Gen Microbiol. 1991 Mar;137(3):455–464. doi: 10.1099/00221287-137-3-455. [DOI] [PubMed] [Google Scholar]
- Ollert M. W., Calderone R. A. A monoclonal antibody that defines a surface antigen on Candida albicans hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990 Mar;58(3):625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P. A., Walsh T. J. Fungal infections in the pediatric cancer patient. Semin Oncol. 1990 Jun;17(3 Suppl 6):6–9. [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Analysis of cell wall extracts of Candida albicans by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot techniques. Infect Immun. 1986 Sep;53(3):565–572. doi: 10.1128/iai.53.3.565-572.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Identification of two germ-tube-specific cell wall antigens of Candida albicans. Infect Immun. 1986 Dec;54(3):864–868. doi: 10.1128/iai.54.3.864-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D., Hopwood V., Vernes A. Antigenic variability of Candida albicans. Crit Rev Microbiol. 1985;12(3):223–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- Quindós G., Pontón J., Cisterna R. Detection of antibodies to Candida albicans germ tube in the diagnosis of systemic candidiasis. Eur J Clin Microbiol. 1987 Apr;6(2):142–146. doi: 10.1007/BF02018195. [DOI] [PubMed] [Google Scholar]
- Quindós G., Pontón J., Cisterna R., Mackenzie D. W. Value of detection of antibodies to Candida albicans germ tube in the diagnosis of systemic candidosis. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):178–183. doi: 10.1007/BF01963834. [DOI] [PubMed] [Google Scholar]
- Regulez P., Arilla M. C., Bikandi J., Quindós G., Cisterna R., Ponton J. Identification of antigens reacting with anti-Candida albicans germ tube antibodies. Eur J Epidemiol. 1992 May;8(3):356–361. doi: 10.1007/BF00158568. [DOI] [PubMed] [Google Scholar]
- Smail E. H., Jones J. M. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect Immun. 1984 Jul;45(1):74–81. doi: 10.1128/iai.45.1.74-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Kenny G. E. Characterization of antigens specific to the surface of germ tubes of Candida albicans by immunofluorescence. Infect Immun. 1984 Mar;43(3):850–855. doi: 10.1128/iai.43.3.850-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Kenny G. E. Enzymatic release of germ tube-specific antigens from cell walls of Candida albicans. Infect Immun. 1985 Sep;49(3):609–614. doi: 10.1128/iai.49.3.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Tam M. R., Nichols E. J., Kenny G. E. Antigenic differences in the surface mannoproteins of Candida albicans as revealed by monoclonal antibodies. Infect Immun. 1988 Mar;56(3):601–606. doi: 10.1128/iai.56.3.601-606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]