Abstract
Purpose
Non-melanoma skin cancer incidence is enhanced over fifty fold in patients taking anti-rejection drugs (ARDs) following organ transplantation. Pre-clinical studies suggest that ARD treatment increases transforming growth factor β1 (TGF-β1) levels, which contribute to enhanced tumor susceptibility independent of the immune suppressive effects of ARDs. This study investigates whether TGF-β signaling is elevated in transplant patients.
Experimental Design
Immunohistochemical tissue microarray analysis was used to determine levels of TGF-β1, TGF-β2, TGF-β3, TβRII, and activated P-Smad2/3 and P-Smad1/5/8 which are phosphorylated directly by distinct TGF-β/BMP receptor complexes. We analyzed over 200 cutaneous lesions and adjacent non-lesional skin samples from 87 organ transplant recipients, and 184 cutaneous lesions and adjacent skin samples from 184 individuals who had never received ARDs.
Results
We found significantly higher levels of P-Smad2 in both non-lesional and lesional tissue from transplant recipients compared to those not exposed to ARDs (P ≤ 0.001). In contrast, P-Smad1/5/8, a marker of activation of the bone morphogenetic protein signaling pathway, was generally not expressed at higher levels in patients taking ARDs, including analysis of non-lesional skin, actinic keratoses, carcinoma in situ or squamous cell carcinoma, but was differentially expressed between keratoacanthoma from transplant recipients compared to those from non-transplant recipients (P ≤0.005).
Conclusions
Observation of elevated P-Smad2 levels in transplant recipients is consistent with the notion that elevated TGF-β signaling may contribute to malignancy in organ transplant recipients. Disparate P-Smad1/5/8 expression levels between keratoacanthoma from the two patients groups might reflect the distinct BMP-responsive cell of origin for this hair follicle-derived lesion.
INTRODUCTION
Organ transplantation, pioneered in the 1960’s, is now a routine and widespread procedure for individuals with chronic diseases of the kidney, heart, liver, lung and other organs. Forty years experience has revealed the sinister side-effect of long term treatment of organ transplant recipients (OTRs) with anti-rejection drugs (ARDs), namely a vastly elevated risk of malignancy (1, 2). The most common malignancy of OTRs is non-melanoma skin cancer (NMSC), with elevated risk factors of 39 to 100 fold in patients of European descent. Increased risk is greater for squamous cell carcinoma (SCC) than for basal cell carcinoma (BCC), with a shifting of the usual BCC: SCC ratio from 3:1 to 1:2 (3). NMSC in OTRs is often accompanied by increased numbers of warts and pre-malignant actinic keratoses (AKs). OTRs frequently develop multiple skin malignancies, and these have been reported to be more invasive than SCCs of non-OTRs and are more frequently locally recurrent after excision (4, 5). In around 10% of cases, SCCs in OTRs are metastatic, thus representing a significant health burden (1, 2).
There has been considerable debate as to the causes of elevated NMSC risk in OTRs. Most SCCs from both non-OTRs and OTRs are initiated by UV irradiation, but exposure to sunlight potentiates risk for SCC in OTRs, with an elevated relative risk of 48 fold in those with high previous sun exposure versus only 2.4 fold in OTRs with low previous sun exposure (6). Immunosuppression may enhance tumorigenesis by reducing resistance to infection by Human Papilloma Virus (HPV) (7). It has been difficult, however, to study the viral contribution to excess NMSC risk in OTRs because of the generally high incidence of detectable HPV DNA even in normal skin of non-OTRs. Nevertheless, the theory that viral infection is a major factor in elevated cancer risk in OTRs is supported by the spectrum of tumor types, other than NMSC, that are prevalent in this patient population; namely those known or suspected to have a viral etiology (8, 9). Immunosuppression may also act directly to reduce tumor immune surveillance, thus supporting malignant tumor outgrowth independent of viral status (10). Nevertheless, not all immunosuppressive drugs enhance cancer susceptibility in experimental models (11, 12). Indeed, the newer drugs, Sirolimus (rapamycin) and FTY720, have been shown to be tumor suppressing rather than tumor promoting (11–15).
It has been suggested that elevated TGF-β1 levels may contribute to the tumor promoting action of ARDs, independently of the potent immunosuppressive effects of these drugs (16, 17). TGF-βs modulate many cellular processes and TGF-β1, in particular, is a critical regulator of tissue homeostasis in the adult. These secreted cytokines exert their biological effects by binding to a cell surface heteromeric complex of type I and type II TGF-β receptors, TβRI and TβRII. Engagement of TGF-β with TβRII results in phosphorylation and activation of TβRI and consequent activation of receptor-associated Smads (R-Smads), Smad2 and Smad3, by phosphorylation of their carboxyl termini. The canonical TGF-β signaling pathway involves the nuclear translocation of P-Smads within a hexameric complex with other R-Smads, together with nuclear shuttling Smad4 (18). TGF-β can also activate non-Smad signaling pathways, such as the MAPK and JNK pathways, both indirectly and directly via TβRI and/or TβRII (19).
In the current study, we examined endogenous markers of active TGF-β signaling, specifically levels of phosphorylated R-Smads, in tumor tissue and adjacent normal skin from OTRs and non-OTRs. Our findings support the hypothesis, generated from numerous pre-clinical studies, that potentiation of TGF-β signaling in response to ARDs might contribute to elevated SCC incidence in human OTRs.
MATERIAL AND METHODS
Human Tissue Samples
This study was conducted according to the principles of the World Medical Association Declaration of Helsinki, and was approved by the Institutional Review Board on Human Research at UCSF. Clinical samples from surgical tumor excisions were obtained from patients after informed consent. All OTRs for whom there were drug histories had been on a drug regimen of cyclosporine and/or azathioprine and/or prednisone and/or mycophenolic acid and/or FK506, as illustrated in Fig. 1S. None of the patients had received extensive treatment with Sirolimus or FTY720. 223 skin lesions: 16 actinic keratoses (AKs), 34 keratoacanthoma (KAs), 96 squamous cell carcinoma (SCC) and 77 carcinoma in situ (CIS) from 87 OTRs were compared to 184 skin lesions: 15 AKs, 41 KAs, 87 SCC and 41 CIS from 184 non-OTRs. Of these samples, 181 were represented by more than one independent core on the array. 184 samples were excised with sufficient adjacent non-lesional skin for analysis; consisting of 74 samples from 30 OTRs, and 110 samples from105 non-OTRs.
Preparation of Tissue Microarray
Tumor type was confirmed by hematoxylin and eosin staining of formalin-fixed, paraffin-embedded sections. 0.6 mm cylindrical cores from each region of interest were arranged randomly with respect to tissue type and patient group within the tissue microarray block. 5μM sections harboring 200–300 punches per microscope slide were cut onto Superfrost plus™ slides (Fisher Scientific, Houston, TX).
Antibodies
The following commercially-available primary antibodies were used for immunohistochemical staining: α-TGF-β1 (TB21, Anogen, Mississauga, ON, Canada) 1:100 dilution; α-P-Smad1-Ser463/465/Smad5-Ser463/465/Smad8-Ser463/465 (#9511, Cell Signaling, Danvers, MA) 1:10 dilution; α-TβRII (sc-400, Santa Cruz Biotechnology, Santa Cruz, CA) 1:25 dilution; α-P-Smad2 (Ser465/467) from (#3101, Cell Signaling, Danvers, MA) 1:100 dilution; α-P-Smad2/3 (Ser 433/435) (#sc-11769, Santa Cruz Biotechnology, Santa Cruz, CA); α-TGF-β2(sc-90, Santa Cruz Biotechnology, Santa Cruz, CA) 1:125. A polyclonal anti-peptide antibody raised against amino acids 50–60 of mature TGF-β3 was kindly provided by Kathy Flanders (20), and used at a 1:450 dilution.
Immunohistochemistry
Tissue sections were de-paraffinized in xylene and rehydrated through a series of ethanols. Endogenous peroxidase was blocked by incubation in DAKO dual endogenous enzyme block (Carpinteria, CA). Antigen retrieval was performed by heating in 0.01M citrate buffer pH 6, for 6 minutes at 121°C. Primary antibody was diluted in DakoCytomation antibody diluent (Carpinteria, CA), and applied to sections for 1 hour at room temperature. Slides were then washed twice for 5 minutes each in Tris Buffer Saline with 0.01% Tween-20, and treated with DAKO EnVision+ dual link peroxidase system (Carpinteria, CA) for 30 minutes at room temperature, followed by development using the DAKO chromagen system (Carpinteria, CA) for 10 minutes, and counterstaining in hematoxylin for 1 min.
Scoring of Tissue Cores
Micro-arrays were examined using an Olympus BX60 microscope with UPlanFl 20X/0.50 ocular, and images were captured using an Olympus DP71 camera with DP controller software version 3.1.1.267 (Olympus, Japan). Scoring of each immunohistochemical stain was performed independently by two investigators who were blinded to patient category. Tissue cores were scored by staining intensity within the epithelial tissue. If expression was heterogeneous, then the assigned score was that observed in ≥ 75% of the epithelium. Within each immunohistochemical staining experiment ~80–90% of tissue cores were informative, with 10–20% being lost from the slide during processing. P-Smad2, P-Smad2/3 and P-Smad1/5/8 staining were scored for nuclear intensity, and TGF-β1, TGF-β2, TGF-β3 and TβRII were scored for cytoplasmic/membrane intensity, with numerical scores of 0, 1, 2 and 3 representing negative, low, medium and high staining intensity.
Statistical Analyses
Statistical analyses were undertaken using Stata v10.0 (College Station, Texas) or R (http://www.r-project.org). Numerical scores from replicate cores were shown to be fairly concordant using a weighted kappa test (Weighted Kappa values: P-Smad2 = 0.33; P-Smad2/3 = 0.33; P-Smad1/5/8= 0.36; TGF-β1 = 0.17; TβRII= 0.25; TGF-β2 = 0.28; TGF-β3 = 0.32). This is considered highly significant (P values all < 10−3), especially considering tumor heterogeneity. Scores from these paired cores were averaged such that each tumor or skin sample only contributed one observation for subsequent statistical analysis.
Scores from at least two independent investigators blinded to sample identities were shown to be concordant using a weighted kappa test. A score of 0.21–0.4 indicates fair concordance, 0.41–0.6 indicates moderate concordance and 0.61–0.8 indicates substantial concordance. Age distributions between the non-OTR and OTR groups were compared by standard t-test. The difference in staining intensities between non-OTR and OTR groups was assessed using Kruskal Wallis rank sum test. Statistical significance was set at a P-value of less than 0.05. The effect of age on P-Smad2 intensity in SCC tumors was assessed by locally weighted linear regression (LOWESS).
RESULTS
Elevated-activation of P-Smad2 in cutaneous lesions of OTRs
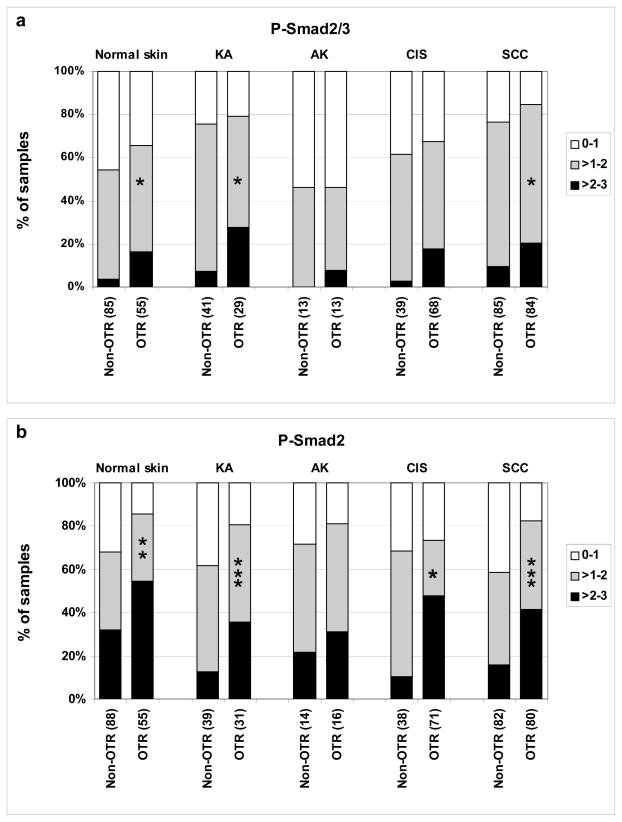
Levels of nuclear-localized R-Smads have been directly correlated with the magnitude of transcriptional responses to TGF-β signaling (21). Therefore, to determine whether elevated TGF-β signaling might be associated with enhanced rates of NMSC in OTRs, we examined nuclear phosphorylated R-Smads within skin lesions from OTRs and non-OTRs (Fig. 1). First we utilized an anti-P-Smad2/3 antibody raised against the phosphorylated carboxy-terminal peptide of Smad3 that recognizes the activated, phosphorylated forms of both Smad2 and Smad3. Within all the lesions examined, there was a trend towards higher levels of P-Smad2/3 staining in cores from OTRs compared to those from non-OTRs which was significant for KA and SCC (Fig. 2a).
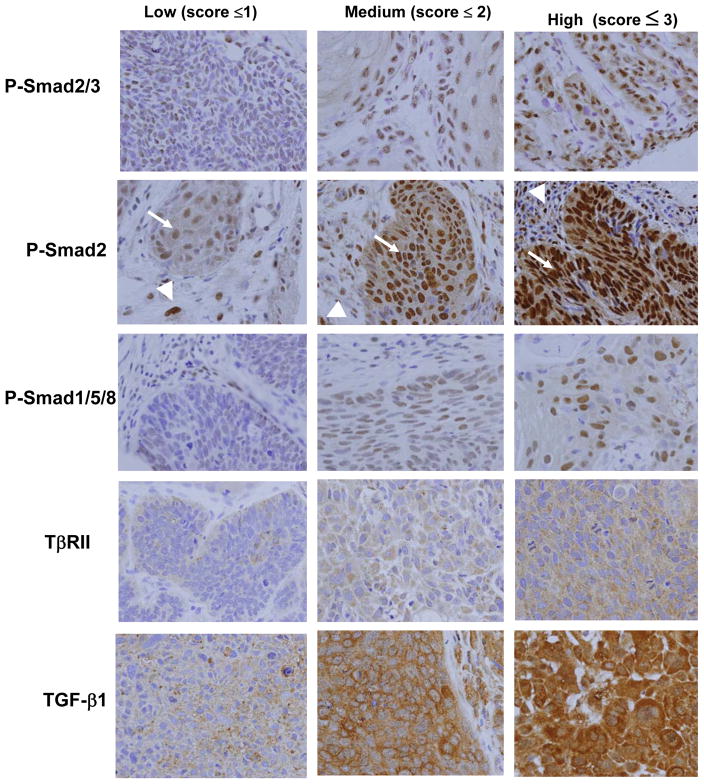
Figure 1. Immunohistochemical staining intensities of TGF-β superfamily pathway members in cutaneous samples from NMSC patients.
The tissue microarrays were stained with the indicated antibodies, and scored as to the relative intensity of epithelial staining. Photomicrographs of cores from the same microarray were taken at 40 × magnification under identical light exposure conditions, to show the range of intensities observed for each antibody. Note that at all three staining intensities for P-Smad2 there are highly positive nuclei within the tumor stroma (arrowheads), contrasting with the variable staining intensities of the carcinoma cells (arrows).
Figure 2. Elevated P-Smad2 and 3 staining in cutaneous samples from OTRs.
Tissue arrays were stained for P-Smad2/3 (a) or P-Smad2 (b), and the individual cores scored for staining intensity. The percentage of specimens in each staining category was plotted for OTRs and non-OTRs. The number of independent patient specimens contributing to the data are shown on the x axis, and significant differences between OTRs and non-OTRs are indicated by asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001, Kruskal Wallis). Double blind scoring was substantially concordant for P-Smad2/3 (0.63) and P-Smad2 (0.61) as assessed by weighted kappa test.
To validate and extend these findings, a second antibody specific to P-Smad2 was used to assess P-Smad2 nuclear expression levels (Fig. 1). P-Smad2 staining showed the same trend as that seen with the anti-P-Smad2/3 antibody, namely higher staining in OTR compared to non-OTRs lesions. However, the differential was even greater than that for P-Smad2/3, in CIS, KA and SCC (Fig. 2).
Elevated-activation of P-Smad2 in non-lesional skin from OTRs
To determine whether the increased expression of P-Smad2 was specific to lesional tissue, or was a generalized cutaneous effect, non-lesional skin adjacent to KA, CIS and SCC were examined for levels of staining for these markers. Indeed, the non-lesional skin from OTRs had higher staining than that of non-OTRs for both P-Smad2/3 and P-Smad2 (Fig. 2), suggesting a systemic effect of ARDs.
Importantly, elevated P-Smad2 and P-Smad2/3 levels in both lesional and non-lesional tissues of OTRs compared to non-OTRs was in striking contrast to data obtained from the same tissue microarrays stained with over twenty other antibodies (K. Ridd and B. Bastian personal communication), including those for TGF-β1, TGF-β2, TGF-β3, TβRII and P-Smad1/5/8 (see Figs. 3, 4, 4S).
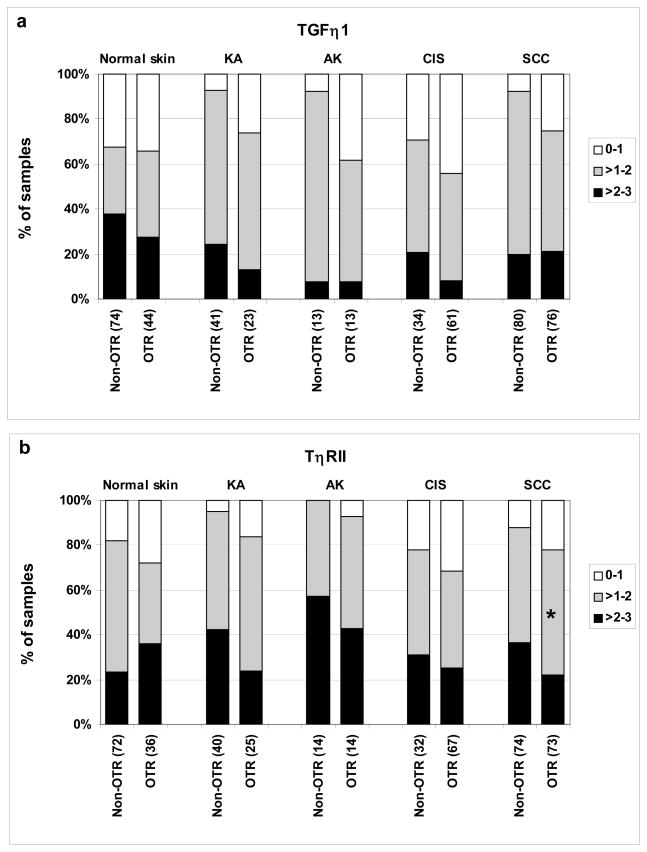
Figure 3. TGF-β1 and TβRII levels are not elevated in SCCs of OTRs compared to non-OTRs.
Tissue micro-arrays were stained for TGF-β1 (a) or TβRII (b) and the individual cores were scored as to staining intensity. The percentage of samples in each staining category was plotted for OTRs and non-OTRs. The number of independent patient specimens contributing to the data are shown on the x axis, and significant differences between OTRs and non-OTRs are indicated by asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001, Kruskal Wallis). Double blind scoring was substantially concordant for TGF-β1 (0.63) and moderately concordant for TβRII (0.49) as assessed by weighted kappa test.
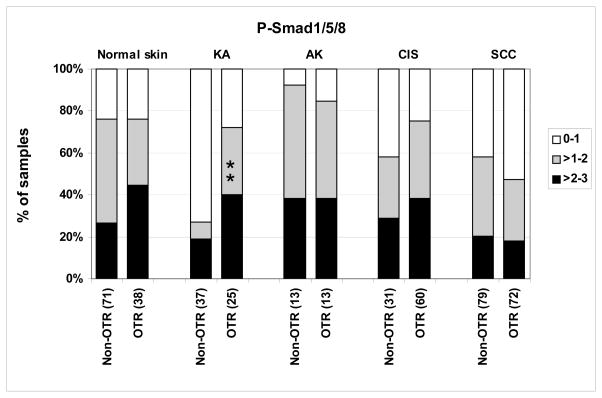
Figure 4. P-Smad1/5/8 levels are elevated specifically in KAs from OTRs versus non-OTRs.
Tissue microarrays were stained for P-Smad1/5/8 and individual cores were scored as to staining intensity. The percentage of samples in each staining category was plotted for OTRs and non-OTRs. The number of independent patient specimens contributing to the data are shown on the x axis, and significant differences between OTRs and non-OTRs are indicated by asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001, Kruskal Wallis).
P-Smad2 expression levels in SCC do not correlate with age, gender or sun exposure
Studies have shown that Smad2 expression is altered by several parameters, including patient gender and age, or body site of the cutaneous lesion (22–24). In this study we found no differential expression of P-Smad2 in SCCs excised from sun-exposed sites compared to those from sun-protected sites, no correlation between gender and P-Smad2 intensity and P-Smad2 staining did not correlate with patient age in either of the two patient groups (Fig. 2S). In conclusion, the only significant factor correlating with P-Smad2 activation was transplant status (Table 1S).
TGF-β1 and TβRII immunostaining are not enhanced in response to ARD therapy
Given the increased activation of Smad2 in cutaneous samples from the OTR population and the previous observations of enhanced circulating plasma TGF-β1 levels in response to ARDs (25–27), we investigated whether OTRs had increased immunostaining for the ligand, TGF-β1, and its receptor, TβRII (Fig. 1). In contrast to the observed increase in Smad2 activation in OTRs versus non-OTRs, we found no increase in TGF-β1 levels between these groups, if anything there was a trend for decreased staining, which was not significant (Fig. 3a). We then examined expression levels of the other TGF-β ligands, TGF-β2 and TGF-β3 (Fig. 4S), to determine whether these might account for increased pSmad2 in SCC from OTRs. However, both ligands showed the same staining intensities in OTRs and non-OTRs. TβRII showed a slight but significant reduction in expression in SCCs of OTRs compared to non-OTRs (P = 0.03) (Fig. 3b), but in no other lesional type, nor in non-lesional skin.
The BMP/Smad pathway is activated by ARDs in KA
The BMP-Smad1/5/8 pathway represents another arm of intra-cellular signaling of the TGF-β superfamily, initiated by BMPs and acting via specific BMP type I and type II receptors (21). Since the TGF-β and BMP signaling pathways are intimately linked, particularly through Smad4, and these two signaling pathways often inhibit one another (21), we investigated the possibility that ARD-stimulated skin tumors show alterations in BMP signaling.
α-P-Smad1/5/8 staining in tissue arrays (Fig. 1) was very similar between OTRs and non-OTRs when comparing AKs, CIS, SCC and non-lesional skin (Fig. 4), suggesting that ARD-induced NMSC involves the induction of the TGF-β/Smad2 pathway, but not the BMP-Smad1/5/8 pathway. In contrast, KA from non-OTRs had a notably lower level of nuclear P-Smad1/5/8 staining compared to other lesional types and to non-lesional skin (P = 0.0006), whereas this effect was over-ridden by ARDs in OTRs (Fig. 4).
DISCUSSION
The major conclusion from this study is that patients on long term ARD therapy have increased P-Smad2 signaling within benign KA, CIS, malignant SCC, as well as in adjacent non-lesional skin. Although a tumor suppressive role for this enhanced signaling pathway in OTRs cannot be excluded, this observation, together with a wealth of pre-clinical data on TGFβ activities in skin tumorigenesis, would support a role for activation of the TGF-β/Smad2 pathway in outgrowth and progression of SCC in OTRs (16). In mouse skin, over-expression of TGF-β1 acts as a suppressor of the early outgrowth of benign tumors (28). As carcinomas progress, oncogene activation blunts this negative growth response and tumor cells acquire enhanced cellular plasticity in response to increased TGF-β1, resulting in an elevated incidence of NMSC and a more invasive phenotype (28, 29). TGF-β also acts on the tumor microenvironment to stimulate tumor progression (30). The hypothesis that ARDs might enhance tumorigenesis by increasing TGF-β1 levels is therefore plausible.
In an immunodeficient SCID–beige mouse model, injection of anti-TGF-β blocking antibodies inhibited a CsA–stimulated increase in the number of metastases resulting from injection of A549 adenocarcinoma cells (16). Since the host mice lacked both T and NK cells, it was concluded that this was a direct effect of TGF-β acting on the tumor cell per se rather than on the immune system. This interpretation was supported by the fact that A549 cells treated with CsA in vitro undergo morphological changes characteristic of invasive cells, and that these changes are completely prevented by the use of TGF-β blocking antibodies (16). Similar findings have been made in a rat model of metastatic colon cancer using a small molecule inhibitor of TβRI (31).
In contrast to earlier reports on SCC from non-OTRs (32–34), we found that the majority of SCC from both non-OTRs and OTRs showed active P-Smad2 signaling. The reason for this discrepancy between previous studies and ours could be multifold. Guasch et al. (34) restricted their studies to genital SCC which might have a very different etiology from that of cutaneous SCC. Other studies utilized antibodies against total Smads rather than P-Smads (33), or used smaller samples sizes (32). Nonetheless, consistent with findings by Hoot et al. (33), P-Smad2 staining was slightly but significantly (P =0.005) decreased in SCC compared to normal adjacent skins of non-OTRs (Figs. 2 and 3S), an effect that was overridden in OTRs.
In contrast to P-Smad2, staining intensities of TGF-β1 and TβRII were relatively unaffected by OTR status, if anything they showed a tendency for decreased levels in SCC from OTRs. This observation, regarding ligand level, is surprising in light of several reports of increased systemic TGFβ-1 levels in response to ARDs in humans (25–27, 35) as well as in mice (16, 17, 31). It might be that examination of steady-state levels of the ligand by immunohistochemistry is misleading. In vivo, once activated, TGF-β1 is very rapidly cleared (36), as such, TGF-β1 protein levels might appear depleted at the sites of activation. Similarly, with respect to decreased TβRII staining in OTRs, the activation of TGF-β receptors results in their degradation via the endosomal pathway (37). It is therefore conceivable that rapid receptor turnover during chronic activation might lead to lower immunohistochemically detectable levels of TβRII in OTRs, despite enhanced signaling.
It is possible that other TGF-β superfamily ligands that activate P-Smad2 may be elevated in OTRs. TGF-β2 and TGF-β3, which act through the same TβRI/TβRII complex, were ruled out by immunostaining. However, activins, nodal and myostatin can also activate P-Smad2 via their own distinct serine threonine receptor kinase complexes (38). Another possibility is that increased nuclear localization of P-Smad2 may be caused by changes in nuclear shuttling and turnover of this signaling molecule. P-Smad2 shuttling and stability is regulated by interaction with other transcription factors and by ubiquitylation (18, 21), as well as by phosphorylation of the centrally located Smad “linker region”, for example by the ERK/MAPK pathway (39, 40). Activation by TGF-β1/TβRII is therefore not required in order to explain elevation of P-Smad2 levels. Nevertheless, reports of elevated systemic TGF-β1 levels in response to ARD treatment (25–27, 41) would suggest that this ligand contributes some component to enhanced P-Smad2 activation.
The observation of reduced levels of the activated BMP pathway components in KA compared to adjacent skin of non-OTRs, and the increased activation of BMP-Smads in KA in response to long term ARD therapy, is particularly intriguing. KA are benign lesions that are thought to arise from the hair follicle (42). It is well established that the BMP signaling pathway plays a major role in follicular stem cell maintenance and the adult hair follicle cycle (43–45). BMP ligands, BMP2, BMP4 and BMP6, in particular, are known to be elevated in the stem/progenitor niche of the hair follicle, where they signal through the BMPR1A receptor to limit stem cell expansion and to induce hair cell differentiation (43, 44, 46). Horsley et al. (44) recently demonstrated that BMPs maintain bulge stem cells through the transcriptional activation of NFATc1, which acts to limit stem cell proliferation. NFATc1 activation and nuclear localization is regulated by the phosphatase, Calcineurin, which in turn, is inhibited by the most commonly used ARD, CsA. Thus ARDs would release stem cells from the BMP-NFAT1c mediated proliferative constraint. The activation of BMP signaling seen within KA from OTRs compared to non-OTRs may therefore result from preferential expansion of a BMP-responsive follicular stem/progenitor compartment that occurs when the consequent down-stream negative growth response is short-circuited by CsA inhibition of NFATc1 activity.
In conclusion, this study together with a wealth of published preclinical data suggests that ARD-enhanced TGF-β signaling might contribute to enhanced skin malignancy in the OTR patient population. Several specific TGF-β inhibitory drugs are now in clinical trials for various applications (47), and the established anti-hypertensive drug, losartan, has been shown to reduce circulating TGF-β1 levels (48, 49). Such drugs should be used with caution due to possible inhibition of the tumor suppressive arm of TGFβ signaling that could potentially result in more benign lesions, such as KA. However, moderate down-regulation of excessive P-Smad2 levels in OTRs by the use of topical TGF-β inhibitors (50) might be useful to reduce cutaneous malignancy and further tumor progression. It might even be considered worthwhile to administer losartan for difficult to manage OTRs with many SCCs.
Supplementary Material
Acknowledgments
This study was funded by NIH P01 grant AR050440 to EE, BB and RJA. We thank Chris Walker for heroic coordination of patient sample collection; Honrado Lopez for assistance with TGF-β2 and -β3 immunohistochemical staining; the Helen Diller Family Comprehensive Cancer Center Biostatistical Core for assistance with statistical analysis; Kathy Flanders for the α-TGF-β3 antibody; the patients, primary skin care physicians, and pathologists for their generous assistance, and the journals that published our requests for samples – American Journal of Transplantation, Archives of Dermatology, Dermatologic Surgery, Skin and Aging and especially Cutis - and organizations that assisted in the dissemination of these requests, including the International Skin Cancer Consortium and the National Kidney Foundation.
References
- 1.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–67. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 2.Ajithkumar TV, Parkinson CA, Butler A, Hatcher HM. Management of solid tumours in organ-transplant recipients. Lancet Oncol. 2007;8:921–32. doi: 10.1016/S1470-2045(07)70315-7. [DOI] [PubMed] [Google Scholar]
- 3.Ramsay HM, Fryer AA, Hawley CM, Smith AG, Nicol DL, Harden PN. Factors associated with nonmelanoma skin cancer following renal transplantation in Queensland, Australia. J Am Acad Dermatol. 2003;49:397–406. doi: 10.1067/s0190-9622(03)00902-2. [DOI] [PubMed] [Google Scholar]
- 4.Euvrard S, Kanitakis J, Pouteil-Noble C, et al. Aggressive squamous cell carcinomas in organ transplant recipients. Transplant Proc. 1995;27:1767–8. [PubMed] [Google Scholar]
- 5.Adamson R, Obispo E, Dychter S, et al. High incidence and clinical course of aggressive skin cancer in heart transplant patients: a single-center study. Transplant Proc. 1998;30:1124–6. doi: 10.1016/s0041-1345(98)00178-x. [DOI] [PubMed] [Google Scholar]
- 6.Bavinck JN, De Boer A, Vermeer BJ, et al. Sunlight, keratotic skin lesions and skin cancer in renal transplant recipients. Br J Dermatol. 1993;129:242–9. doi: 10.1111/j.1365-2133.1993.tb11841.x. [DOI] [PubMed] [Google Scholar]
- 7.Harwood CA, Surentheran T, Sasieni P, et al. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Dermatol. 2004;150:949–57. doi: 10.1111/j.1365-2133.2004.05847.x. [DOI] [PubMed] [Google Scholar]
- 8.Busnach G, Piselli P, Arbustini E, et al. Immunosuppression and cancer: A comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplant Proc. 2006;38:3533–5. doi: 10.1016/j.transproceed.2006.10.144. [DOI] [PubMed] [Google Scholar]
- 9.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. Jama. 2006;296:2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 10.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 11.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 12.Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation. 2002;73:1565–72. doi: 10.1097/00007890-200205270-00008. [DOI] [PubMed] [Google Scholar]
- 13.Law BK, Chytil A, Dumont N, et al. Rapamycin potentiates transforming growth factor beta-induced growth arrest in nontransformed, oncogene-transformed, and human cancer cells. Mol Cell Biol. 2002;22:8184–98. doi: 10.1128/MCB.22.23.8184-8198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wulff BC, Kusewitt DF, VanBuskirk AM, Thomas-Ahner JM, Duncan FJ, Oberyszyn TM. Sirolimus reduces the incidence and progression of UVB-induced skin cancer in SKH mice even with co-administration of cyclosporine A. J Invest Dermatol. 2008;128:2467–73. doi: 10.1038/jid.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T, Takahara S, Hatori M, et al. A novel immunosuppressive drug, FTY720, prevents the cancer progression induced by cyclosporine. Cancer Lett. 2002;181:165–71. doi: 10.1016/s0304-3835(01)00799-6. [DOI] [PubMed] [Google Scholar]
- 16.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–4. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 17.Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76:597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 19.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 20.Flanders KC, Thompson NL, Cissel DS, et al. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989;108:653–60. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 22.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- 23.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–51. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han KH, Choi HR, Won CH, et al. Alteration of the TGF-beta/SMAD pathway in intrinsically and UV-induced skin aging. Mech Ageing Dev. 2005;126:560–7. doi: 10.1016/j.mad.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Coupes BM, Newstead CG, Short CD, Brenchley PE. Transforming growth factor beta 1 in renal allograft recipients. Transplantation. 1994;57:1727–31. [PubMed] [Google Scholar]
- 26.Shin GT, Khanna A, Ding R, et al. In vivo expression of transforming growth factor-beta1 in humans: stimulation by cyclosporine. Transplantation. 1998;65:313–8. doi: 10.1097/00007890-199802150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Citterio F, Pozzetto U, Romagnoli J, et al. Plasma levels of transforming growth factor-beta1 in renal transplant recipients receiving different immunosuppressive regimens. Transplant Proc. 2004;36:698–9. doi: 10.1016/j.transproceed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Cui W, Fowlis DJ, Bryson S, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–42. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 29.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–94. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 30.Lu SL, Reh D, Li AG, et al. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004;64:4405–10. doi: 10.1158/0008-5472.CAN-04-1032. [DOI] [PubMed] [Google Scholar]
- 31.Ohsawa I, Murakami T, Uemoto S, Kobayashi E. In vivo luminescent imaging of cyclosporin A-mediated cancer progression in rats. Transplantation. 2006;81:1558–67. doi: 10.1097/01.tp.0000209448.50238.de. [DOI] [PubMed] [Google Scholar]
- 32.Han G, Lu SL, Li AG, et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–23. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoot KE, Lighthall J, Han G, et al. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–32. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–27. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanna AK, Plummer MS, Hilton G, Pieper GM, Ledbetter S. Anti-transforming growth factor antibody at low but not high doses limits cyclosporine-mediated nephrotoxicity without altering rat cardiac allograft survival: potential of therapeutic applications. Circulation. 2004;110:3822–9. doi: 10.1161/01.CIR.0000150400.15354.7D. [DOI] [PubMed] [Google Scholar]
- 36.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990;86:1976–84. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 38.Harradine KA, Akhurst RJ. Mutations of TGFbeta signaling molecules in human disease. Ann Med. 2006;38:403–14. doi: 10.1080/07853890600919911. [DOI] [PubMed] [Google Scholar]
- 39.Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–7. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 40.Sekimoto G, Matsuzaki K, Yoshida K, et al. Reversible Smad-dependent signaling between tumor suppression and oncogenesis. Cancer Res. 2007;67:5090–6. doi: 10.1158/0008-5472.CAN-06-4629. [DOI] [PubMed] [Google Scholar]
- 41.Khanna A, Kapur S, Sharma V, Li B, Suthanthiran M. In vivo hyperexpression of transforming growth factor-beta1 in mice: stimulation by cyclosporine. Transplantation. 1997;63:1037–9. doi: 10.1097/00007890-199704150-00026. [DOI] [PubMed] [Google Scholar]
- 42.Fisher BK, Elliott GB. On the Origin of Keratoacanthoma: Reflections on an Unusual Lesion. Can Med Assoc J. 1965;93:272–3. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, He XC, Tong WG, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–39. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 44.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–4. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–23. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006;6:565–78. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 48.Campistol JM, Inigo P, Jimenez W, et al. Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy. Kidney Int. 1999;56:714–9. doi: 10.1046/j.1523-1755.1999.00597.x. [DOI] [PubMed] [Google Scholar]
- 49.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santiago B, Gutierrez-Canas I, Dotor J, et al. Topical application of a peptide inhibitor of transforming growth factor-beta1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol. 2005;125:450–5. doi: 10.1111/j.0022-202X.2005.23859.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.