Abstract
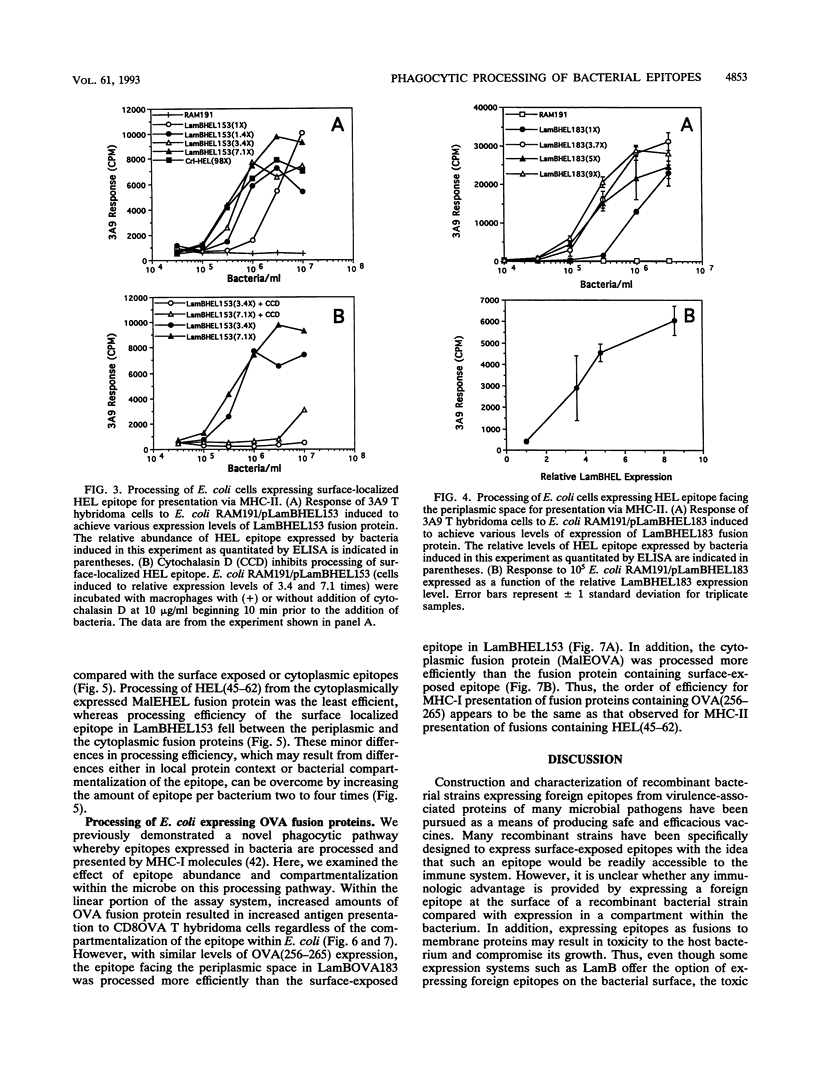
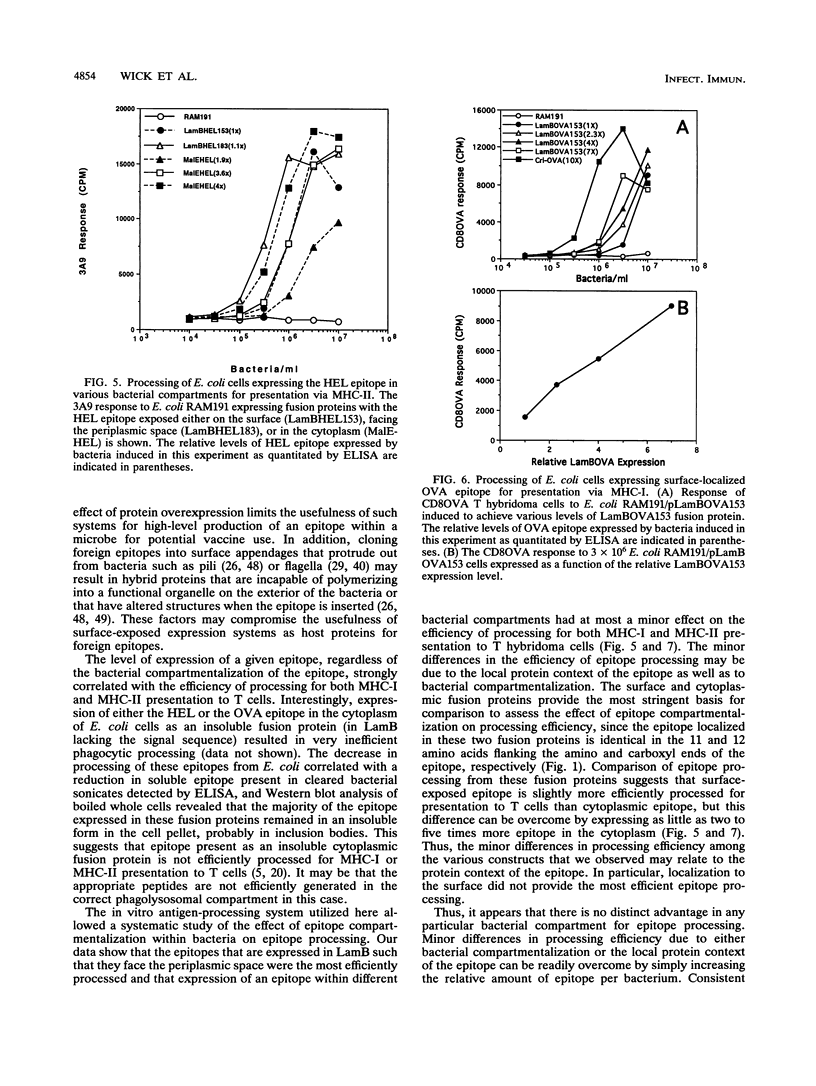
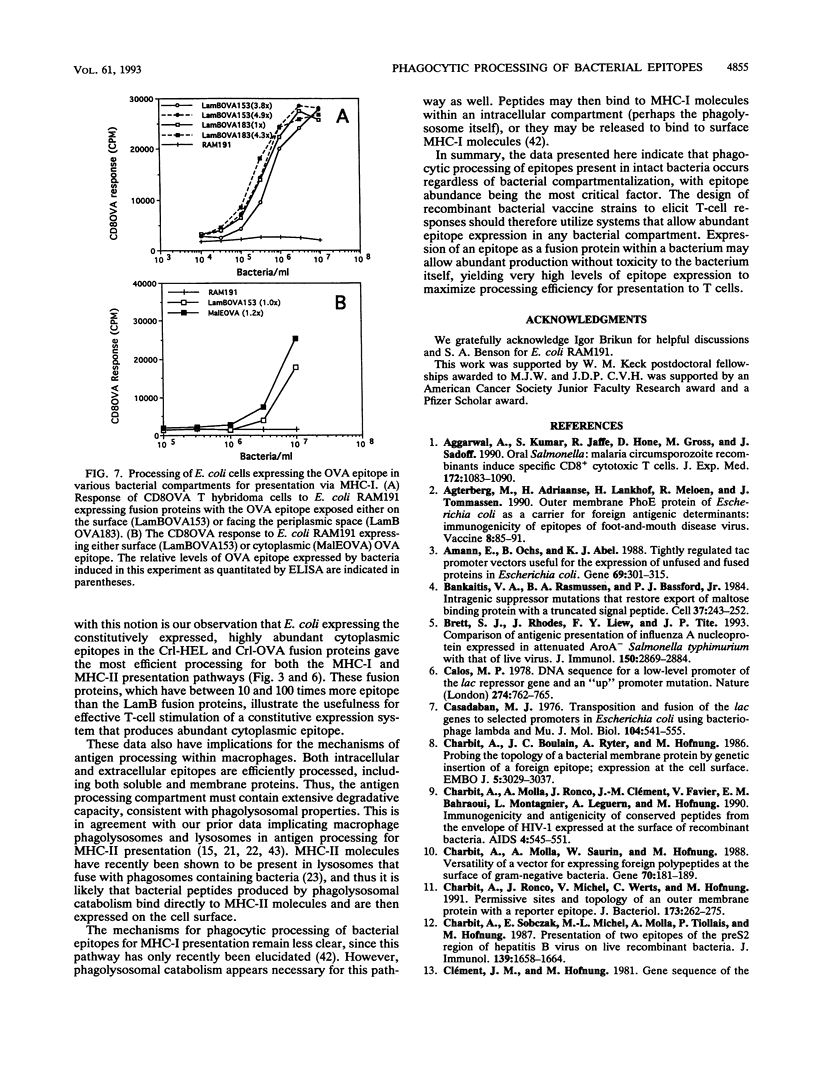
The effect of abundance and compartmentalization of antigenic epitopes expressed in Escherichia coli on phagocytic processing was studied by expressing fusion proteins containing the epitope from positions 52 to 61 of hen egg white lysozyme [HEL(52-61)], which binds the I-Ak murine major histocompatibility complex class II (MHC-II) molecule or the epitope from positions 257 to 264 of chicken egg ovalbumin [OVA(257-264]), which binds the Kb murine MHC-I molecule. Epitopes expressed as fusion proteins in the outer membrane protein LamB allowed exposure of the epitopes either at the bacterial surface, in the periplasmic space, or in the cytoplasm. Regardless of epitope compartmentalization within the bacterium, MHC-II-restricted or MHC-I-restricted presentation to T hybridoma cells occurred after macrophages phagocytosed bacteria producing the HEL(52-61) epitope or the OVA(257-264) epitope, respectively. Increased epitope abundance within a given microbial compartment resulted in increased processing and presentation to epitope-specific T hybridoma cells. Minor differences in the efficiency of epitope processing between the constructs was observed, and the HEL or OVA epitope exposed in the periplasmic space was processed most efficiently compared with the surface- or cytoplasm-localized epitopes. These differences could be overcome by increasing the amount of epitope per bacterium as little as two to five times. The minor differences in processing efficiency may be due to differing protein contexts of the epitope as well as differing epitope compartmentalizations within the bacteria. Thus, production of abundant epitope is the important parameter influencing processing of epitopes expressed in E. coli to induce T-cell responses rather than targeting of an epitope to a specific bacterial compartment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg M., Adriaanse H., Lankhof H., Meloen R., Tommassen J. Outer membrane PhoE protein of Escherichia coli as a carrier for foreign antigenic determinants: immunogenicity of epitopes of foot-and-mouth disease virus. Vaccine. 1990 Feb;8(1):85–91. doi: 10.1016/0264-410x(90)90184-n. [DOI] [PubMed] [Google Scholar]
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Rasmussen B. A., Bassford P. J., Jr Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984 May;37(1):243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Rhodes J., Liew F. Y., Tite J. P. Comparison of antigen presentation of influenza A nucleoprotein expressed in attenuated AroA- Salmonella typhimurium with that of live virus. J Immunol. 1993 Apr 1;150(7):2869–2884. [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Charbit A., Boulain J. C., Ryter A., Hofnung M. Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope; expression at the cell surface. EMBO J. 1986 Nov;5(11):3029–3037. doi: 10.1002/j.1460-2075.1986.tb04602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Molla A., Ronco J., Clément J. M., Favier V., Bahraoui E. M., Montagnier L., Leguern A., Hofnung M. Immunogenicity and antigenicity of conserved peptides from the envelope of HIV-1 expressed at the surface of recombinant bacteria. AIDS. 1990 Jun;4(6):545–551. doi: 10.1097/00002030-199006000-00008. [DOI] [PubMed] [Google Scholar]
- Charbit A., Molla A., Saurin W., Hofnung M. Versatility of a vector for expressing foreign polypeptides at the surface of gram-negative bacteria. Gene. 1988 Oct 15;70(1):181–189. doi: 10.1016/0378-1119(88)90116-3. [DOI] [PubMed] [Google Scholar]
- Charbit A., Ronco J., Michel V., Werts C., Hofnung M. Permissive sites and topology of an outer membrane protein with a reporter epitope. J Bacteriol. 1991 Jan;173(1):262–275. doi: 10.1128/jb.173.1.262-275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Sobczak E., Michel M. L., Molla A., Tiollais P., Hofnung M. Presentation of two epitopes of the preS2 region of hepatitis B virus on live recombinant bacteria. J Immunol. 1987 Sep 1;139(5):1658–1664. [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. S., Unanue E. R., Harding C. V. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991 Dec 15;147(12):4054–4059. [PubMed] [Google Scholar]
- Dougan G., Sellwood R., Maskell D., Sweeney K., Liew F. Y., Beesley J., Hormaeche C. In vivo properties of a cloned K88 adherence antigen determinant. Infect Immun. 1986 Apr;52(1):344–347. doi: 10.1128/iai.52.1.344-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Emr S. D., Hedgpeth J., Clément J. M., Silhavy T. J., Hofnung M. Sequence analysis of mutations that prevent export of lambda receptor, an Escherichia coli outer membrane protein. Nature. 1980 May 8;285(5760):82–85. doi: 10.1038/285082a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gao X. M., Tite J. P., Lipscombe M., Rowland-Jones S., Ferguson D. J., McMichael A. J. Recombinant Salmonella typhimurium strains that invade nonphagocytic cells are resistant to recognition by antigen-specific cytotoxic T lymphocytes. Infect Immun. 1992 Sep;60(9):3780–3789. doi: 10.1128/iai.60.9.3780-3789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Kanagawa O., Unanue E. R. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991 Nov 1;147(9):2860–2863. [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Slot J. W., Geuze H. J., Unanue E. R. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991 Jan 25;64(2):393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J Cell Biol. 1992 Nov;119(3):531–542. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Unanue E. R. Turnover of Ia-peptide complexes is facilitated in viable antigen-presenting cells: biosynthetic turnover of Ia vs. peptide exchange. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4230–4234. doi: 10.1073/pnas.86.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard L., Klemm P. Type 1 fimbriae of Escherichia coli as carriers of heterologous antigenic sequences. Gene. 1989 Dec 21;85(1):115–124. doi: 10.1016/0378-1119(89)90471-x. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., Leitner M., Zamir A., Salomon D., Arnon R. Priming immunization against cholera toxin and E. coli heat-labile toxin by a cholera toxin short peptide-beta-galactosidase hybrid synthesized in E. coli. EMBO J. 1985 Dec 1;4(12):3339–3343. doi: 10.1002/j.1460-2075.1985.tb04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzer M., Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc C., Martineau P., Van der Werf S., Deriaud E., Duplay P., Hofnung M. Induction of virus-neutralizing antibodies by bacteria expressing the C3 poliovirus epitope in the periplasm. The route of immunization influences the isotypic distribution and the biologic activity of the antipoliovirus antibodies. J Immunol. 1990 Apr 15;144(8):3174–3182. [PubMed] [Google Scholar]
- Leclerc C., Charbit A., Molla A., Hofnung M. Antibody response to a foreign epitope expressed at the surface of recombinant bacteria: importance of the route of immunization. Vaccine. 1989 Jun;7(3):242–248. doi: 10.1016/0264-410x(89)90237-5. [DOI] [PubMed] [Google Scholar]
- Maskell D. J., Sweeney K. J., O'Callaghan D., Hormaeche C. E., Liew F. Y., Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987 Mar;2(3):211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Misra R., Benson S. A. Isolation and characterization of OmpC porin mutants with altered pore properties. J Bacteriol. 1988 Feb;170(2):528–533. doi: 10.1128/jb.170.2.528-533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Charbit A., Le Guern A., Ryter A., Hofnung M. Antibodies against synthetic peptides and the topology of LamB, an outer membrane protein from Escherichia coli K12. Biochemistry. 1989 Oct 3;28(20):8234–8241. doi: 10.1021/bi00446a040. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Charbit A., Martineau P., Leclerc C., van der Werf S., Nauciel C., Hofnung M. Immunogenicity of foreign peptide epitopes expressed in bacterial envelope proteins. Res Microbiol. 1990 Sep-Oct;141(7-8):963–969. doi: 10.1016/0923-2508(90)90136-e. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Russell D. G., Normark S. J., Harding C. V. Recombinant Escherichia coli express a defined, cytoplasmic epitope that is efficiently processed in macrophage phagolysosomes for class II MHC presentation to T lymphocytes. J Immunol. 1992 Oct 15;149(8):2576–2584. [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICKENBERG H. V., LESTER G. The preferential synthesis of beta-galactosidase in Escherichia coli. J Gen Microbiol. 1955 Oct;13(2):279–284. doi: 10.1099/00221287-13-2-279. [DOI] [PubMed] [Google Scholar]
- Van Die I., Wauben M., Van Megen I., Bergmans H., Riegman N., Hoekstra W., Pouwels P., Enger-Valk B. Genetic manipulation of major P-fimbrial subunits and consequences for formation of fimbriae. J Bacteriol. 1988 Dec;170(12):5870–5876. doi: 10.1128/jb.170.12.5870-5876.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf S., Charbit A., Leclerc C., Mimic V., Ronco J., Girard M., Hofnung M. Critical role of neighbouring sequences on the immunogenicity of the C3 poliovirus neutralization epitope expressed at the surface of recombinant bacteria. Vaccine. 1990 Jun;8(3):269–277. doi: 10.1016/0264-410x(90)90057-s. [DOI] [PubMed] [Google Scholar]



