Abstract
Background
When 2 treatment choices (ie, mastectomy vs. breast conserving therapy) show no difference in a primary clinical outcome (ie, survival), patient satisfaction becomes an important marker of the quality of care received.
Objectives
To assess the impact of physician–patient discussion of primary surgical treatment outcomes on patients’ satisfaction with medical care (MC) among women with incident breast cancer (BC).
Method
We used self-report data of a population-based survey of 495 women ≥50 years of age with stage I–II BC in Los Angeles, California in 2000 conducted a mean of 7.5 and 24 months after diagnosis. Using multivariable analyses, we evaluated the impact of physician–patient outcome discussions (ie, BC recurrence, BC survival, breast appearance, and arm swelling/pain/movement difficulty) on patient satisfaction at baseline and follow-up.
Results
Most women were satisfied with their MC (>65%). More than half reported physician–patient discussions of BC recurrence (54%), breast appearance (50%), and arm pain/swelling/movement difficulty (55%). Thirty-one percent discussed BC survival. Women who discussed arm swelling, pain, movement difficulty were significantly more likely to be satisfied at baseline (odds ratio: 1.8, 95% confidence interval: 1.1–3.0, P < 0.05) and follow-up (odds ratio: 1.9, 95% confidence interval: 1.2–3.0, P > 0.01). The more treatment outcomes patients discussed with their physicians, the higher patient satisfaction ratings were at baseline and follow-up.
Conclusions
Physician–patient discussions of BC treatment outcomes were highly correlated with patients’ satisfaction with overall MC regardless of the procedure received. This suggests that the quality of BC care should include assessments of physician–patient communication.
Background
Effective physician–patient communication is the key to improved clinical outcomes, better quality of life, increased patient satisfaction with medical care, and decreased morbidity and mortality.1–3 Effective physician–patient communication is particularly important in the health care delivery process for women with newly diagnosed breast cancer. Although both breast conserving therapy (BCT) and mastectomy for early stage breast cancer provide women with equivalent cancer survival,4–6 these treatments differ in other important outcomes such as body image and the need for radiation therapy.7,8 Previous literature has shown that physician–patient discussions of specific outcomes for primary surgical treatment of breast cancer can significantly impact the type of surgery patients receive.9,10 When 2 treatment choices (ie, mastectomy vs. BCT) show no difference in a primary clinical outcome (ie, survival), patient satisfaction becomes an important marker of the quality of care they received. However, no study to date has examined the impact of physician–patient discussions of outcomes for primary surgical treatment of breast cancer on patient satisfaction with their overall medical care. Identifying specific aspects of physician–patient discussions of treatment outcomes that significantly impact patient satisfaction can provide the empirical basis for developing intervention strategies to increase patient satisfaction with medical care, and potentially also improve patient quality of life. The purpose of this study is to assess the impact of physician–patient discussion of how primary surgical treatment alternatives influence outcomes on patients’ satisfaction with overall medical care among a population-based sample of women with breast cancer.
METHODS
We used patient self-report data from the Los Angeles Women’s Study, a population-based, longitudinal, telephone health survey of women with breast cancer 50 years and older in Los Angeles County.11 Ninety-minute computer-assisted telephone interviews were conducted in English and Spanish. The sample for the survey was drawn from a census of incident breast cancer cases diagnosed March through November 2000 identified by Rapid Case Ascertainment (RCA) of the Los Angeles County Cancer Surveillance Program (LAC CSP). The LAC CSP staff screen pathology reports in all LAC hospitals at least monthly to identify incident cancers and enter relevant information (eg, type of cancer, surgery, patient contact information) into a central database. Cancer researchers can then petition to use the information collected by RCA for research purposes. To minimize respondent burden, the LAC CSP did not release information pertaining to Asian American women 50–59 years of age and more than 75 years of age for this study because these women had already participated in another study.
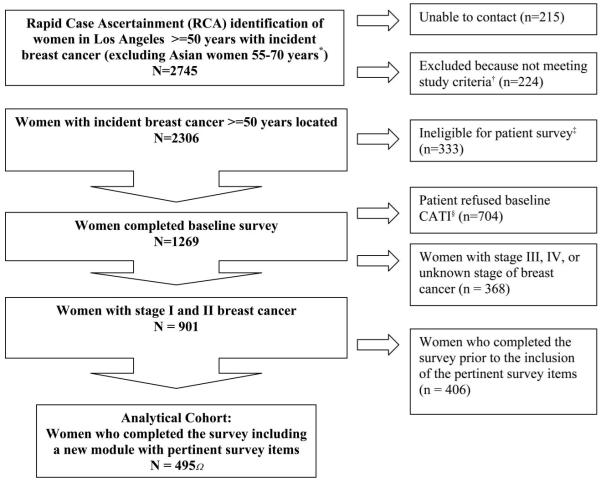
A total of 2745 patients were initially identified by RCA from 103 hospitals. Of these women, 1269 completed the baseline telephone interview for a response rate of 64% (Fig. 1). The baseline survey was conducted a mean of 223 days after diagnosis (median 185 days, interquartile range, 159–255). Follow-up surveys were conducted 24 months later. The response rate for the follow-up survey was 79%. Institutional Review Board approval for the study was obtained from University of California and Los Angeles and RAND.
FIGURE 1.
Flow chart of analytic sample at baseline.
We restricted this analysis to women with stages I and II breast cancer to assure patients were somewhat homogeneous with respect to their tumor burdens (n = 901). Three months into the 9-month ascertainment window, the survey was enriched with a new set of items pertinent to physician–patient discussions about the effects of surgical treatment options on outcomes. We restricted our analysis cohort to the subset of 495 patients whose survey included these items. Patients who were initially approached for study enrollment after these new items were included in the survey and those who delayed responding to the computer-assisted telephone interview survey until the items were available were eligible for completing the new questions pertinent to the effects of surgical treatment options on outcomes. Compared with the respondents who were not asked the outcome questions, the subsample who answered the outcome questions were more likely to have lower income. Otherwise there was no difference in sociodemographic characteristics between patients who were and were not asked the outcome questions.
Analytical Variables
Dependent Variables
Respondents were asked to rate their overall medical care from 0 (poor) to 10 (best) on both the baseline and follow-up survey.12 Because the responses were skewed to the right, we dichotomized the patient satisfaction variable as satisfied (9 and 10) versus not satisfied (1–8).
Main Independent Variables
The main independent study variables assess physician–patient discussions of how primary surgical treatment alternatives influence outcomes (Table 1). First, we constructed 4 dichotomous variables indicating physician–patient discussions of (1) breast cancer recurrence, (2) survival from breast cancer, (3) breast or chest wall appearance, and (4) arm swelling, pain, or difficulty with movement. Lastly we constructed a count variable of treatment outcome discussions (range, 0–4).
TABLE 1.
Survey Questions From the Los Angeles Women Studies Assessing Physician–Patient Discussions of Possible of Treatment Outcomes
| When your doctors discussed with you their recommendations for the best type of surgery for you, did they discuss with you the likely effect of the surgical procedures on: |
|
Covariates
The covariates we assessed included age, self-reported race/ethnicity (white, African American, Latina who completed a survey in Spanish, Latina who completed the survey in English, and other), education, income, health insurance status, employment, married, or living with a companion (y/n) and social support. We separated Latinas into 2 groups by language to account for English fluency, which we hypothesize to be a predictor of physician–patient interactions mediated directly through communication issues and indirectly through acculturation and health literacy. We assessed patient’s social support by calculating the mean of responses to 8 questions which ask, how often do you have someone to help you if confined to bed, take you to the doctor if you needed it, have a good time with, prepare meals if you are unable to, help with daily chores if sick, turn to for suggestions or deal with problems, who understands your problems, and love and make you feel wanted? Individuals can choose from 5 response categories: all of the time (score = 100), most of the time (75), some of the time (50), a little of the time (25), and none of the time (0).13 We hypothesize that patients with greater social support would have more help engaging their physicians and thus be more satisfied with their medical care.
We assessed clinical characteristics including the number of self-reported comorbidities (0, 1–2, >3) from a list of 14 items (Table 2),14,15 body mass index, Short Form-12 (SF-12) physical and mental component scores,16 stage of breast cancer (I vs. II), surgical procedure received (lumpectomy vs. mastectomy), and adjuvant therapy received (ie, chemotherapy, tamoxifen, and radiation therapy). We hypothesize that healthier patients are more likely to be satisfied with their medical care than sicker patients. Likewise, we included receipt of adjuvant therapies because of their side-effects and potential to affect quality of life and satisfaction with medical care.17 All covariates were obtained from the baseline survey except for SF-12 physical and mental component scores, which we extracted from both baseline and follow-up surveys.
TABLE 2.
Descriptive Statistics of Cohort With Stage I or II Breast Cancer at Baseline
| (n = 495) | |
|---|---|
| Age, yr | |
| 50–59 | 36% |
| 60–69 | 32% |
| >70 | 32% |
| Ethnicity | |
| White | 71% |
| English-speaking Latina | 8% |
| Spanish-speaking Latina | 7% |
| African American | 7% |
| Other | 5% |
| Education | |
| High school graduate or less | 35% |
| Some college | 31% |
| College graduate or more | 33% |
| Income | |
| <$20,000 | 20% |
| $20,001–$40,000 | 23% |
| >$40,000 | 57% |
| Health insurance (yes) | 97% |
| Employment (yes) | 38% |
| Physical SF-12,† mean (SD) | 50.2 (9.2) |
| Mental SF-12,† mean (SD) | 53.3 (9.5) |
| No. comorbidities‡ | |
| None | 19% |
| 1–2 | 49% |
| >3 | 32% |
| Body mass index, mean (SD) | 26.0 (5.9) |
| Stage§ | |
| 1 | 55% |
| 2 | 45% |
| Mastectomy | 30% |
| Lumpectomy | 74% |
| Radiation therapy | 59% |
| Chemotherapy | 40% |
| Tamoxifen | 59% |
| Social support score,¶ mean (SD) | 82.2 (20.9) |
| Married or living with a companion (yes) | 56% |
For the general population, the SF-12 physical component and mental component scores have a mean of 50 and a SD of 10 (Ware et al 1996,17).
The comorbidity number is a sum of self-reported diagnoses the following list: hypertension, coronary artery disease, congestive heart failure, myocardium infarction, other heart problems, stroke, emphysema/asthma, Crohns disease, arthritis hip/knee, arthritis of hand, sciatic leg pain, diabetes, other cancer, osteoporosis, scleroderma, and ever had previous radiation therapy to chest.
The stage of breast cancer was obtained by matching data with SEER.
The social support score (range, 0–100) was calculated as the mean of responses to 8 questions.
SEER indicates Surveillance, Epidemiology, and End Results.
Statistical Analysis
We first performed univariate descriptive statistics of our cohort. Second, we checked for correlation of the independent variables and found no evidence of multicollinearity (r < 0.4). Third, we used 4 separate multivariate logistic regression models to evaluate the impact of physician–patient discussions of treatment outcomes on patient satisfaction with overall medical care at baseline and at follow-up. In model 1, the dependent variable was patient satisfaction (y/n) at base-line and the main independent variables were dummy variables representing patient–physician discussions of each of 4 treatment outcomes: breast cancer recurrence; survival from breast cancer; breast or chest wall appearance; and arm swelling, pain, or difficulty with movement. Other covariates in the model include baseline age, ethnicity, education, income, health insurance status, employment, mental and physical SF-12, comorbidity count, body mass index, stage, procedure received, receipt of adjuvant therapy, social support, and marital status. The variables in model 2 are the same as model 1 except the main dependent variable is replaced with the count of physician–patient discussions of treatment outcome items (range, 0–4) at baseline. In model 3, the dependent variable was patient satisfaction (y/n) at follow-up and the main independent variables were patient–physician discussions of treatment outcomes at baseline. Other covariates in model 3 are the same as model 1 except we used follow-up mental and physical SF-12 scores instead of baseline scores. Lastly, model 4 is the same as model 3, except the main dependent variable is replaced with the count of physician–patient discussions of treatment outcome items (range, 0–4) (Table 4). For all 4 models, the P values are <0.0001 and Hosmer and Lemeshow goodness of fit tests (C-statistics = 0.8 and P = 0.2 or 0.3) indicates good fit.18 For all multivariable analyses, we controlled for the intracluster correlation among women diagnosed in the same hospital.
TABLE 4.
Odds Ratio for Patient Satisfaction with Care at Baseline and Follow-Up Surveys by Types and Number of Discussions of Treatment Options†
| OR (95%) CI for Patient Report of Satisfaction With Care (n = 495) |
||
|---|---|---|
| Baseline Survey‡ | Follow-up Survey§ | |
| Model 1 | Model 3 | |
| Physician–patient discussions of likely treatment outcomes¶ | ||
| Breast cancer recurrence (y/n) | 1.5 (0.7, 2.9) | 1.0 (0.6, 1.6) |
| Survival from breast cancer (y/n) |
1.0 (0.5, 2.0) | 0.9 (0.5, 1.4) |
| Breast and chest wall appearance (y/n) |
1.1 (0.7, 1.7) | 1.0 (0.6, 1.4) |
| Arm pain, swelling, or difficulty with movement after surgery (y/n) |
1.8 (1.1, 3.0)* | 1.9 (1.2, 3.0)** |
| Model 2 | Model 4 | |
| Count of physician–patient discussions (range, 0–4)∥ |
1.4 (1.1, 1.6)*** | 1.3 (1.04, 1.6)* |
P < 0.05
P < 0.01
P < 0.001.
All 4 models controlled for age, ethnicity, education, income, health insurance status, employment, health status (mental and physical SF-12), comorbidity count, body mass index, stage, procedure received (lumpectomy vs. mastectomy), receipt of adjuvant therapy (ie, radiation, chemotherapy, and tamoxifen), social support, marital status, and clustering within hospitals.
Models 1 and 2 used baseline mental and physical SF-12 scores.
Models 3 and 4 used follow-up mental and physical SF-12 scores.
The reference group for these variables is no discussion of the respective treatment outcomes (eg, no discussion of breast cancer recurrence, no discussion of survival from breast cancer, etc).
We treated this as a linear variable.
We compared the results of our multivariate models with results from propensity models. We used sociodemographic and clinical characteristics as covariates to predict physician–patient discussion of surgical outcomes. We then included indicator variables for the quintiles of the predicted probabilities of physician–patient discussion as well as sociodemographic and clinical characteristics as covariates in to predict patient satisfaction with overall medical care. Because the results of the propensity models were consistent with our multivariate models, we elect to present the results of our multivariate models for ease of comprehension.
We used survey nonresponse weights developed using logistic regression of patients with incident breast cancer noted in the RCA file as a function of age, race, stage at diagnosis, and dummy variables for hospital associated with diagnosis. Comparison of respondents (n = 1269) and non-respondents (n = 704) showed that women who were non-white (P < 0.0001), older (P < 0.0001), and with stage III or IV breast cancer (P < 0.0001) were more likely to be survey nonresponders (Fig. 1). STATA version 9.2 (StatCorp, College Station, TX) was used for all analyses.
RESULTS
Table 2 summarizes the baseline descriptive statistics of the analytical cohort of women with breast cancer in our study. The majority were white (71%), had some form of health insurance (97%), and had annual household incomes of more than $40,000 (57%). One-fifth had no comorbidities (19%), 55% had stage I, and 45% had stage II breast cancer. The majority had lumpectomy (74%). Overall, 59% had radiation therapy, including radiation therapy for 94% of women with lumpectomy. Over half reported receiving adjuvant tamoxifen therapy (59%). Although 40% reported having chemotherapy, we were unable to determine from the data the appropriateness of having chemotherapy.
Approximately half the women reported discussing with their physician the possible impact of surgery on breast cancer recurrence (54%), chest wall or breast appearance (50%), and arm pain, swelling or difficulty with movement (55%) (Table 3). Fewer than a third (31%) reported discussing the possible impact of surgery on breast cancer survival. The mean number of physician–patient treatment outcome discussions was 1.9 (SD 1.4) of 4 possible discussions. Overall, the majority of women reported satisfaction with their medical care at both the baseline (79%) and 2-year follow-up (65%) surveys.
TABLE 3.
Self-Reported Physician–Patient Discussions of Alternative Treatments, Likely Treatment Outcomes, and Overall Satisfaction With Medical Care
| (n = 495) | |
|---|---|
| % Patients Reporting Discussion |
|
| Physician–patient discussion of likely treatment outcomes |
|
| Breast cancer recurrence | 54% |
| Survival from breast cancer | 31% |
| Breast or chest wall appearance | 50% |
| Arm swelling, pain, or difficulty with movement after surgery |
55% |
| Mean number of physician–patient treatment outcome discussions (SD) |
1.9 (1.4)* |
| % Patients Satisfied With Medical Care† |
|
| Overall patient satisfaction with medical care |
|
| Satisfied (baseline survey) | 79% |
| Satisfied (follow-up survey) | 65% |
Mean number of discussions for a total of 4 possible discussions.
Respondents were asked to rate their overall medical care from 1 (poor) to 10 (best) on the baseline survey and on the follow-up survey. We dichotomized the patient satisfaction variable at baseline and follow-up as satisfied (9 and 10) and not satisfied (1–8).
In the multivariate analysis, women who reported discussing the possibility of arm swelling, arm pain, or difficulty with arm movement with their physician were significantly more likely to be satisfied with their medical care than women who did not report discussions of arm symptoms at the baseline survey [odds ratio (OR): 1.8, 95% confidence interval (CI): 1.1–3.0, P < 0.05], or follow-up survey (OR: 1.9, 95% CI: 1.2–3.0, P < 0.01) (Table 4). The more treatment outcome items that patients discussed with their physicians, the higher patient satisfaction ratings were at baseline (OR: 1.4, 95% CI: 1.1–1.6, P < 0.001) and at follow-up (OR: 1.3, 95% CI: 1.04–1.6, P < 0.05). According to the multivariate model, the percentage of respondents who reported satisfaction with their medical care increased approximately 4% per count of each outcome discussion both at baseline and follow-up.
DISCUSSION
Since the publication of the 1990 National Institutes of Health Consensus Statement recommending BCT as an appropriate and preferable treatment for most women with early stage breast cancer,19 some clinicians have suggested that the rate of BCT represents an appropriate measure of quality of care. However, increasingly the literature has shown that the type of surgery (mastectomy vs. lumpectomy) not only has no significant impact of breast cancer survival, it is also not a significant predictor of patient satisfaction or quality of life.20–23 It is rather the nuances of physician–patient interaction, such as clarity of physician–patient communication, and engagement of patients in the decision making process for breast cancer treatment that has been the important predictor of patient outcomes and satisfaction.3,7,23,24
In this study, we found that the more treatment outcomes physicians discussed with their patients in advance of decision-making, the more satisfied patients reported being with their overall medical care. In addition, we found that physician–patient discussion of the likely effect of the surgical procedures on the possibility of arm swelling, arm pain, or difficulty with arm movement was the most significant piece of the outcome discussions. Previous studies have shown that arm pain is significantly associated with worse quality of life,25 and effective physician–patient communication can positively influence patient health outcomes including pain control.24 One hypothesis is that the physician–patient discussions of arm pain, swelling, and difficulty with movement prepared the patient for the experience of pain and modulated their perception and sensation of pain. Further research is needed to explore why the discussion of this specific outcomes has such a significant impact on patient satisfaction with overall medical care at baseline survey and even in the 2-year follow-up survey.
These results should be considered in light of several limitations. This study was limited to women from a single county, though it is a population-based study from the largest and most diverse county in the United States. Some patients may not have recalled the discussion that they had with their physicians about breast cancer treatment outcomes, although surveying women soon after diagnosis may have mitigated this problem. Although our survey was clinically detailed, we were not able to examine important unmeasured factors such as the quality or length of the physician–patient discussions, or patient’s desired level of involvement in the discussions or decision-making process. However, our findings of consistent results between propensity score and multivariable models predicting patient reports of satisfaction, suggest that physician–patient discussions of outcomes are important predictors of satisfaction.
In conclusion, we found that rates of physician–patient discussions of breast cancer treatment outcomes, and especially discussions of arm swelling, pain or difficulty with movement, were highly correlated with patients’ satisfaction with overall medical care regardless of the procedure the women received. This suggests that the quality of breast cancer care should include assessments of verbal physician–patient communication as a supplement to measures of rates of use of procedures.
ACKNOWLEDGMENTS
The authors thank Dennis Deapen of Los Angeles County Registry for allowing access to the Rapid Case Ascertainment program and to Scott Hickey for statistical programing.
Supported by the National Cancer Institute (NCI) Grant R01 CA81338-01A1.
REFERENCES
- 1.Braddock CH, III, Edwards KA, Hasenberg NM, et al. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282:2313–2320. doi: 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- 2.Maly RC, Leake B, Silliman RA. Health care disparities in older patients with breast carcinoma: informational support from physicians. Cancer. 2003;97:1517–1527. doi: 10.1002/cncr.11211. [DOI] [PubMed] [Google Scholar]
- 3.Roter D, Hall J. Patterns of Talk in the Medical Visit in Doctors Talking with Patients/Patients Talking with Doctors: Improving Communication in Medical Visits. Auborn House; Westport, CT: 1993. [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 7.Moyer A. Psychosocial outcomes of breast-conserving surgery versus mastectomy: a meta-analytic review. Health Psychol. 1997;16:284–298. doi: 10.1037//0278-6133.16.3.284. [DOI] [PubMed] [Google Scholar]
- 8.Lantz PV, Zemencuk JK, Katz SJ. Is mastectomy overused? A call for an expanded research agenda. Health Serv Res. 2002;37:417–431. doi: 10.1111/1475-6773.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 10.Katz SJ, Lantz PM, Zemencuk JK. Correlates of surgical treatment type for women with noninvasive and invasive breast cancer. J Womens Health Gend Based Med. 2001;10:659–670. doi: 10.1089/15246090152563533. [DOI] [PubMed] [Google Scholar]
- 11.Los Angeles Women’s Health Care Study RAND. Available at: http://www.rand.org/health/lawhcs.
- 12.Marshall GN, Morales LS, Elliott M, et al. Confirmatory factor analysis of the Consumer Assessment of Health Plans Study (CAHPS) 1.0 Core Survey. Psychol Assess. 2001;13:216–229. doi: 10.1037//1040-3590.13.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meenan RF, Mason JH, Anderson JJ, et al. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 14.Kahn KL, Tisnado DM, Adams JL, et al. Does ambulatory process of care predict health-related quality of life outcomes for patients with chronic disease? Health Serv Res. 2007;42:63–83. doi: 10.1111/j.1475-6773.2006.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn KL, Maclean CH, Wong AL, et al. Assessment of American College of Rheumatology quality criteria for rheumatoid arthritis in a pre-quality criteria patient cohort. Arthritis Rheum. 2007;57:707–715. doi: 10.1002/art.22781. [DOI] [PubMed] [Google Scholar]
- 16.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Ward S, Simpson E, Davis S, et al. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2007;11:1–144. doi: 10.3310/hta11400. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Wiley; New York, NY: 2000. [Google Scholar]
- 19.Treatment of early-stage breast cancer. NIH Consens Statement Online 1990. 2007;8:1–19. [Google Scholar]
- 20.Hopwood P, Haviland J, Mills J, et al. The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2208 women recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial) Breast. 2007;16:241–251. doi: 10.1016/j.breast.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Janni W, Rjosk D, Dimpfl TH, et al. Quality of life influenced by primary surgical treatment for stage I-III breast cancer-long-term follow-up of a matched-pair analysis. Ann Surg Oncol. 2001;8:542–548. doi: 10.1007/s10434-001-0542-2. [DOI] [PubMed] [Google Scholar]
- 22.Moyer A, Salovey P. Patient participation in treatment decision making and the psychological consequences of breast cancer surgery. Womens Health. 1998;4:103–116. [PubMed] [Google Scholar]
- 23.Street RL, Jr, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making. 1997;17:298–306. doi: 10.1177/0272989X9701700306. [DOI] [PubMed] [Google Scholar]
- 24.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 25.Caffo O, Amichetti M, Ferro A, et al. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48. doi: 10.1023/A:1024435101619. [DOI] [PubMed] [Google Scholar]