Abstract
We compared viability, phenotype, in vitro function and therapeutic efficacy of murine unpulsed-dendritic cells (-DC), DC pulsed with keyhole limpet hemocyanin (KLH-DC) and cryopreserved KLH-DC (C-KLH-DC). Mean viability (%±SE) of unpulsed-DC, KLH-DC and C-KLH-DC was 93.6±0.9, 93.9±0.8 and 87.4±1.6, respectively. Pulsing DC with KLH did not induce maturation or affect in vitro function. Cryopreservation of KLH-DC reduced MHC I, CD80 and CD86 expression, endocytic capacity and allogeneic splenocyte stimulatory capacity. Intratumoral (i.t.) vaccination of mice bearing s.c. D5 melanoma with unpulsed-DC, KLH-DC or C-KLH-DC elicited comparable anti-tumor immune responses and inhibited tumor growth to the same extent. Combining radiotherapy with i.t. unpulsed-DC, KLH-DC or C-KLH-DC administration enhanced induction of anti-tumor immune responses and inhibition of tumor growth to a similar degree. Cryopreservation of KLH-DC slightly reduces viability, expression of co-stimulatory cell surface markers and in vitro function; however, in vivo anti-tumor activity is fully maintained with or without radiotherapy.
Keywords: Cancer vaccines, Cellular immunotherapy, Dendritic cells, Keyhole Limpet Hemocyanin, Cryopreservation
INTRODUCTION
Hemocyanin, a high molecular weight copper containing glycoprotein that reversibly binds oxygen, is the extra-cellular respiratory protein of mollusks (1, 2). Keyhole Limpet Hemocyanin (KLH) is purified from the hemolymph of Megathura Crenulata, also known as Giant Keyhole Limpet, a mollusk related to giant sea snails that is native to the coast of southern California and Mexico. KLH is also a potent and well known immunostimulant in both experimental animals and humans. It has been used as a hapten carrier protein to enhance antigen-specific T cell priming and is known to stimulate a CD4+ T-cell response (3, 4). Complexes of KLH-conjugated F(ab')2 fragments of antibodies targeting various tumor associated antigens (TAA) have been shown to induce antigen-specific humoral responses (5–8). Addition of KLH to tumor lysate- or peptide-pulsed dendritic cell (DC) immunization has been reported to enhance therapeutic efficacy in mice bearing melanoma tumors (9). Furthermore, KLH constitutes a strong foreign neo-antigen and therefore has been shown by us and others to serve as a valuable marker molecule to monitor induction of immunological responses in clinical DC-based cancer vaccine studies (10–15).
In recent years, our attention has focused on intratumoral (i.t.) vaccination utilizing immature unpulsed-DC (16–18). This approach for immunotherapy of cancer is based on in vivo – in situ pulsing of DC and uses the readily available host’s tumor mass as the source of tumor antigens. Advantages of this strategy include eliminating the need to perform in vitro loading of DC with tumor antigens or to induce in vitro DC maturation, ensuring delivery of functional myeloid DC into the desired site for antigen uptake, and eliciting immunity against multiple relevant host-specific tumor antigens without MHC allele restrictions. We have previously shown that i.t. unpulsed-DC vaccination, particularly in combination with local tumor irradiation, induces a systemic anti-tumor immune response leading to regression of established tumors in mice (16, 17). A phase I clinical trial conducted in patients with advanced hepatoma demonstrated that the combination of radiotherapy and i.t. unpulsed-DC injection is feasible, safe, and capable of eliciting tumor-specific immune responses as well as clinical responses (19).
However, monitoring induction of anti-tumor immune responses in cancer patients treated via this approach may prove difficult. Potential obstacles include unavailability of autologous tumor, undefined TAA in some human malignancies, and lack of expression of defined TAA in a substantial number of patients. In vitro pulsing of DC with KLH, prior to i.t. administration, would provide an immunological tracer molecule that could facilitate post-vaccination immune monitoring. Furthermore, use of KLH pulsed-DC may enhance the therapeutic efficacy of i.t. vaccination (9). On the other hand, in vitro pulsing of DC with KLH may induce DC maturation and thereby reduce their capacity for in vivo tumor antigen uptake ultimately resulting in decreased anti-tumor efficacy. As the resolution of this matter may have considerable clinical implications, we undertook this study to examine the effect of KLH pulsing on i.t. DC vaccination in a murine melanoma tumor model.
Preparation of DC vaccines for clinical trials is labor- and cost- intensive each time a vaccine is produced. The need for multiple vaccines per patient makes this task even more challenging. Cryopreserving the final DC vaccine preparation into aliquots for multiple vaccine administrations, rather than generating the DC vaccine from peripheral blood mononuclear cells (PBMC) each time the vaccine is required, would greatly facilitate vaccine production. Implementation of this practice, however, requires that both the viability and the function of frozen DC are well maintained after thawing. Several studies have shown that the viability, phenotype and in vitro functional properties of cryopreserved human DC are comparable to freshly prepared DC (20–28). However, the in vivo anti-tumor efficacy of cryopreserved human or murine DC has not been evaluated so far. Therefore, in this study we also directly compared the therapeutic efficacy of i.t. administration of cryopreserved KLH pulsed-DC, fresh KLH pulsed-DC and fresh unpulsed-DC into established s.c. D5 murine melanoma tumors.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN) and housed in specific pathogen-free conditions at the Animal Maintenance Facility of the University of Michigan Medical Center. The mice were used for experiments at 8 weeks of age or older. The University of Michigan Committee on Use and Care of Animals reviewed and approved all animal protocols.
Tumors
D5 melanoma is a poorly immunogenic subclone of the B16-BL6 tumor of spontaneous origin in the C57BL/6 strain (29). D5 tumors are aggressive and relatively resistant to radiation therapy (16). PAN 02 is a pancreatic adenocarcinoma syngeneic to C57BL/6 mice and was used as a specificity control. Tumor cells were maintained by in vitro culture in complete medium (CM) (16). Cells were harvested using trypsin EDTA (Mediatech, Herndon, VA).
Generation of bone marrow-derived unpulsed-DC, and KLH or lipopolysaccharide (LPS) DC pulsing
Erythrocyte-depleted bone marrow cells from flushed marrow cavities of femurs and tibias of naive syngeneic mice were cultured in CM supplemented with 10 ng/ml granulocyte macrophage colony-stimulating factor (GM-CSF) and 10 ng/ml IL-4 (Pepro Tech, Inc., Rocky Hill, NJ) at 1 × 106 cells/ml. KLH (50 µg/ml; Calbiochem, La Jolla, CA) or LPS (1 µg/ml; Sigma, St. Louis, MO) was added for the final 18 hours of culture. On day 5, DC were harvested and then enriched using 14.5% metrizamide (Sigma)-CM gradients. DC were washed twice with PBS (Mediatech) and enumerated. For i.t. injection, DC were suspended at 1 × 106 cells/0.05ml PBS.
Cryopreservation of KLH pulsed-DC
KLH pulsed-DC were suspended in a freezing medium containing 10% dimethyl sulfoxide (Sigma) and 90% heat inactivated fetal bovine serum (Biomeda, Foster City, CA). Cells were slowly frozen to −80°C overnight using a Cryo 1°C Freezing Container (Nalgene Labware, Rochester, NY) filled up with isopropanol (Fisher Scientific, Fair Lawn, NJ). The frozen cells were then transferred to a liquid nitrogen tank and stored for a mean ± SE of 28.2 ± 10.3 days. Cryopreserved KLH pulsed-DC were thawed in a 37°C water bath, washed in CM and immediately used for in vitro or in vivo assays.
Viability assays
DC viability was determined by dye exclusion using 0.4% trypan blue solution (Sigma). The percentage of viable cells was defined as: (number of live cells/number of total cells) × 100. In addition, DC were stained using 7-AAD (BD Pharmingen, San Diego, CA) and analyzed by a FACSCalibur flow cytometer using Cellquest software (Becton Dickinson, Mountain View, CA).
Cell-surface phenotyping
All monoclonal antibodies (mAbs) were purchased from BD Pharmingen. Fc receptors were blocked using purified anti-CD16/CD32 mAb (18). DC were stained using PE-conjugated anti-H-2Kb, anti-I-Ab or anti-CD40, FITC-conjugated anti-CD80 or anti-CD86 and PE-Cy7-conjugated anti-CD11c mAbs (18). Duplicate samples were stained with isotype matched control mAbs as recommended by the manufacturer. Cell samples were analyzed by flow cytometry.
Enzyme-linked immunosorbrent assay (ELISA)
DC (2 × 106 cells in 2 ml of CM supplemented with 10 ng/ml GM-CSF and 10 ng/ml IL-4) were incubated in 24-well culture plates (Becton Dickinson, Franklin Lakes, NJ) at 37°C, 5% CO2 with or without LPS (1 µg/ml) for 18 hours. At the end of culture, supernatants were collected and analyzed for IL-12p70 and IL-10 content using ELISA sets (BD Biosciences) according to the manufacturer’s instructions.
Endocytosis assay
The capacity of DC to acquire antigen was evaluated by a fluorescein isothiocyanate (FITC)-dextran endocytosis assay. DC (1 × 106/250 µl CM) were placed in 5 ml, 12 × 75 mm, polypropylene tubes and incubated in the presence of 40,000 MW FITC-dextran (Sigma) at a final concentration of 1 mg/ml in a 37°C water bath for 2 hours. Incubation of DC with FITC-dextran at 4°C served as a negative control. After incubation, cells were washed twice in ice-cold PBS to quench endocytic activity and remove free FITC-dextran. Cells were fixed in 2% paraformaldehyde (Sigma) in PBS and analyzed by flow cytometry.
Allogeneic mixed lymphocyte reaction
Erythrocyte-depleted BALB/c splenocytes (2 × 105 cells in 50 µl of CM) were cultured in 96-well U-bottom culture plates (Becton Dickinson) at 37°C, 5% CO2 with or without increasing numbers of DC (1 × 102, 1 × 103 or 1 × 104 cells in 50 µl of CM). DC cultured alone served as controls. After 5 days, proliferation was assessed using the Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA) according to the manufacturer’s instructions. Briefly, WST-1 was added to all wells, and cells were cultured for an additional 4 hours. The amount of formazan dye that had formed was quantified using a multi-well spectrophotometer (EL800 Bio-Tek Instruments, Winooski, Vermont).
Ionizing radiation
Mice were placed in individual plastic restraints to ensure immobilization. Radiation was directed to the tumor site located s.c. in the right flank with the rest of the mouse being shielded. Radiation was delivered in 5 consecutive daily fractions using an X-ray unit (Pantak Therapax DXT 300 Model; Pantak, East Haven, CT) at a dose rate of ~3 Gy/min. The total radiation dose administered was 42.5 Gy (8.5 Gy × 5). Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration.
In vivo treatment protocols
Mice were inoculated s.c. in the right flank with 2 × 106 D5 cells on day 0. DC (1 × 106) were administered i.t. on days 6, 11, 14 and 18. Tumors were locally irradiated on days 7 to 11. Tumors were measured three times per week in the largest perpendicular diameters, and the size was recorded in mm2.
IFNγ ELISPOT assay
Erythrocyte-depleted splenocytes (1 × 106 in 100 µl CM), harvested from treated or control mice 21 days after tumor inoculation, were incubated for 24 hours in an IFNγ ELISPOT assay (16) with irradiated (60 Gy) D5 or PAN 02 tumor cells (1 × 105 in 100 µl CM). Tumor specific IFNγ spots were calculated by subtracting the number of spots detected in response to PAN 02 stimulation from the number of spots detected in response to D5 stimulation.
Intracellular IFNγ staining
Blood samples (~100µl) were drawn from treated mice via the lateral saphenous vein 15 days after tumor inoculation. PBMC (1 × 106 in 1 ml CM) were placed into 24-well culture plates and stimulated for 20 hours with irradiated (60 Gy) D5 tumor cells (1 × 105 in 1 ml CM). Brefeldin A (GolgiPlug, 1 µl/ml, BD Pharmingen) was added to all wells, and cells were cultured for an additional 16 hours. Cells were then harvested and stained for surface markers using FITC-conjugated anti-CD4 and PE-Cy5-conjugated anti-CD8 or isotype matched control mAbs (all from BD Pharmingen). Intracellular staining was performed using PE-conjugated anti-IFNγ mAb or an isotype matched control mAb (both from BD Pharmingen) according to the manufacturer’s recommendations. Samples were then analyzed by flow cytometry.
Statistical analysis
Data were evaluated by paired t test (2 cohorts) or one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test for multiple comparisons (>2 cohorts). P values < 0.05 were considered statistically significant.
RESULTS
Pulsing DC with KLH does not affect cell viability or maturation status
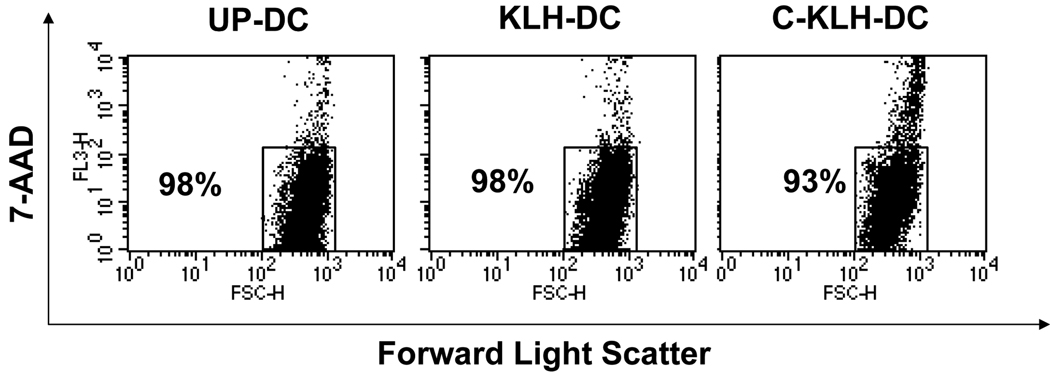
We examined whether pulsing murine DC with KLH during the final 18 hours of cell culture followed by exposure to a freeze-thaw cycle impacts cell viability or state of maturation. Viability of unpulsed-DC and KLH pulsed-DC at the end of a 5-day cell culture was determined using trypan blue dye exclusion. As shown in Table 1, in 14 independent experiments the mean percentage viability of both DC types exceeded 93%. Pulsing DC with KLH had no statistically significant effect on cell viability (p = 0.7 versus unpulsed-DC). After thawing, cryopreserved KLH pulsed-DC that had been frozen for a mean ± SE of 28.2 ± 10.3 days exhibited a mean viability of 87.4%. This small reduction in cell survival rate between cryopreserved KLH pulsed-DC and KLH pulsed-DC was statistically significant (p = 0.005). Analysis of 7-AAD stained DC using flow cytometry yielded slightly higher absolute values of cell viability for all DC types (Fig. 1). However, the above described impact of KLH pulsing and cryopreservation on DC viability was confirmed.
Table 1.
Viability (%) of unpulsed-DC (UP-DC), KLH pulsed-DC (KLH-DC), and cryopreserved KLH pulsed-DC (C-KLH-DC) by trypan blue dye exclusion.
| Mean Viability (%) | SE | P | |
|---|---|---|---|
| UP-DC | 93.6 | 0.9 | |
| KLH-DC | 93.9 | 0.8 | 0.7 versus UP-DC |
| C-KLH-DCa | 87.4 | 1.6 | 0.005 versus KLH-DC |
Cryopreserved KLH pulsed-DC were frozen for a mean ± SE of 28.2 ± 10.3 days.
Data are reported as mean viability and SE of 14 independent experiments.
FIGURE 1.
Viability (%) of unpulsed-DC (UP-DC), KLH pulsed-DC (KLH-DC) and cryopreserved KLH pulsed-DC (C-KLH-DC) by 7-AAD staining. UP-DC, KLH-DC, and C-KLH-DC were stained with 7-AAD and analyzed by flow cytometry. Numbers in dot plots represent percentage of viable cells. This experiment was repeated three times with similar results.
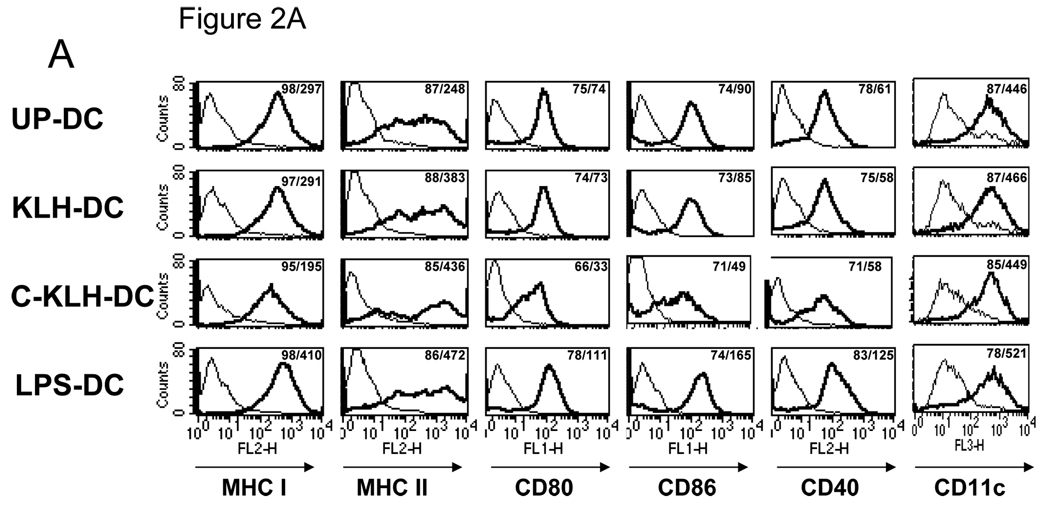
DC surface expression of MHC class I, MHC class II, CD80, CD86, CD40 and CD11c was evaluated using fluorochrome-conjugated monoclonal antibodies and flow cytometry. DC pulsed with LPS served as a positive control for induction of maturation. While pulsing DC with LPS up-regulated all tested cell surface markers, KLH pulsing increased only expression of MHC class II (Fig. 2A). Immediately after thawing, cryopreserved KLH pulsed-DC expressed decreased levels of MHC class I, CD80 and CD86.
FIGURE 2.
A. Phenotype of unpulsed-DC (UP-DC), KLH pulsed-DC (KLH-DC), cryopreserved KLH pulsed-DC (C-KLH-DC) and LPS pulsed-DC (LPS-DC). UP-DC, KLH-DC, C-KLH-DC and LPS-DC were stained using fluorochrome-conjugated monoclonal antibodies (mAbs) for MHC class I, MHC class II, CD80, CD86, CD40 and CD11c and analyzed by flow cytometry. Thin lines represent staining with matched isotype control mAbs, thick lines represent staining with the designated mAbs. Numbers in histogram plots designate percentage of positive cells and geometrical mean of fluorescence intensity of positive cells, respectively. B. Cytokine secretion by UP-DC, KLH-DC, C-KLH-DC and LPS-DC. Supernatants of DC cultures were analyzed for IL-12p70 and IL-10 content using ELISA. These experiments were repeated three times with similar results.
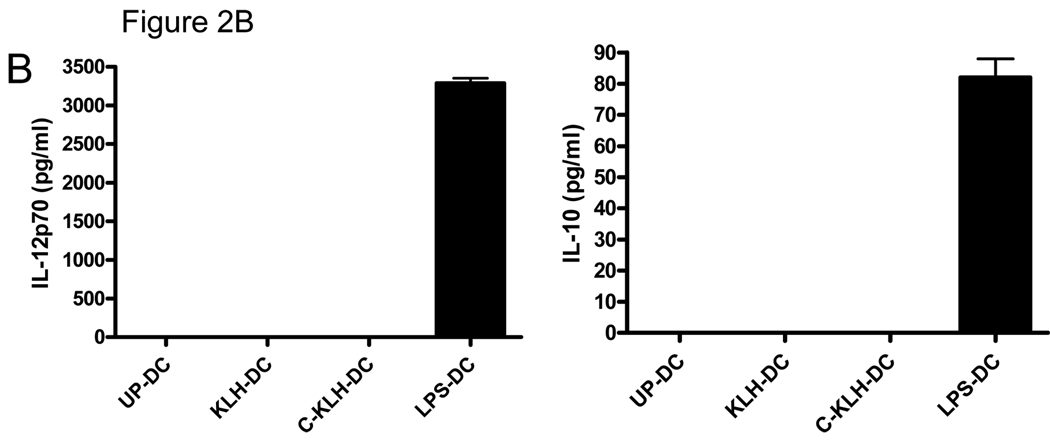
The amount of IL-12p70 and IL-10 in DC culture supernatants was measured by ELISA. As shown in Fig. 2B, supernatants of LPS matured-DC contained both cytokines. On the other hand, undetectable levels of these cytokines were found in supernatants of unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC cultures. These findings indicate that KLH pulsed–DC maintain viability and relative immaturity. Cryopreservation of KLH pulsed-DC may lead to a slight reduction in both parameters.
Pulsing DC with KLH does not affect their in vitro function
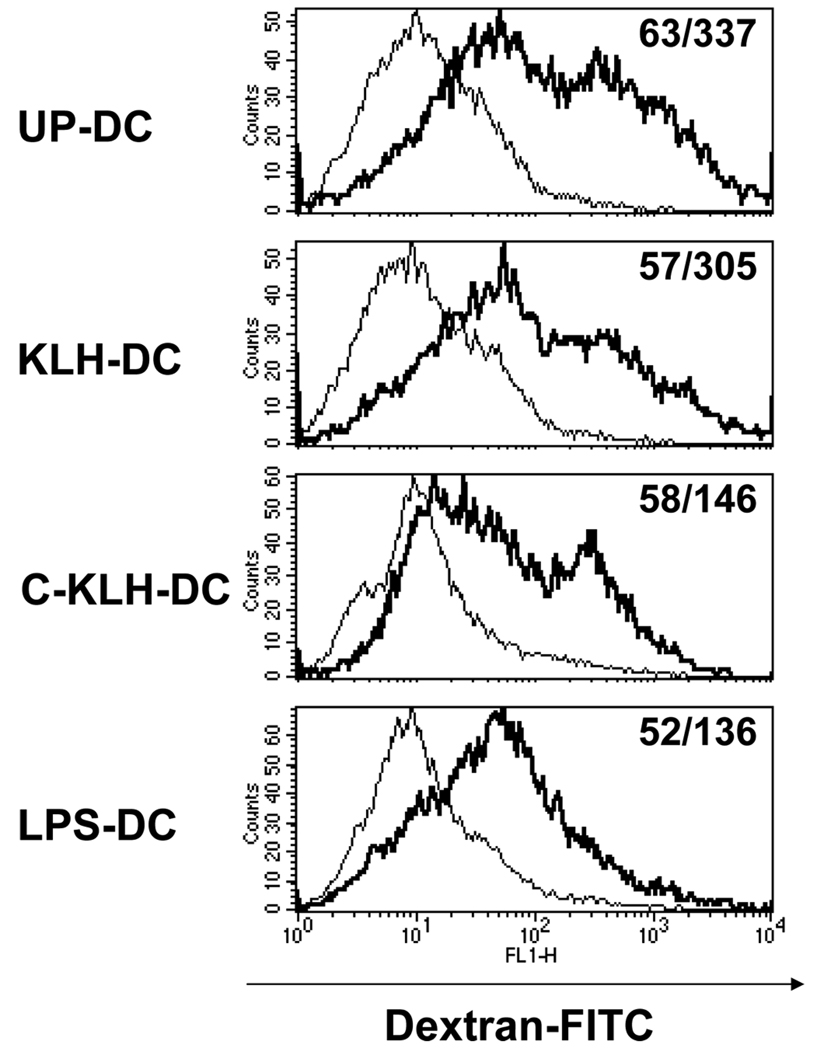
The endocytic capacity of unpulsed-DC, KLH pulsed-DC and cryopreserved KLH pulsed-DC was evaluated by measuring FITC-dextran uptake after two hours of co-incubation at 37°C using flow cytometry. Co-culture of DC with FITC-dextran at 4°C served as a negative control. Since mature DC are known to have a reduced capacity to acquire antigen, LPS pulsed-DC served as an additional negative control as well as a frame of reference. As expected, LPS pulsed-DC demonstrated reduced endocytic capacity compared with unpulsed-DC (Fig. 3). While KLH pulsing appeared to have no substantial effect on DC antigen acquisition, thawed cryopreserved KLH pulsed-DC exhibited reduced endocytic capacity.
FIGURE 3.
Endocytic capacity of unpulsed-DC (UP-DC), KLH pulsed-DC (KLH-DC), cryopreserved KLH pulsed-DC (C-KLH-DC) and LPS pulsed-DC (LPS-DC). UP-DC, KLH-DC, C-KLH-DC and LPS-DC were incubated for 2 hours with fluorescein isothiocyanate-dextran at 37°c (thick line) or 4°C (thin line) and then analyzed by flow cytometry. Numbers in histogram plots designate percentage of positive cells and geometrical mean of fluorescence intensity of positive cells, respectively. This experiment was repeated three times with similar results.
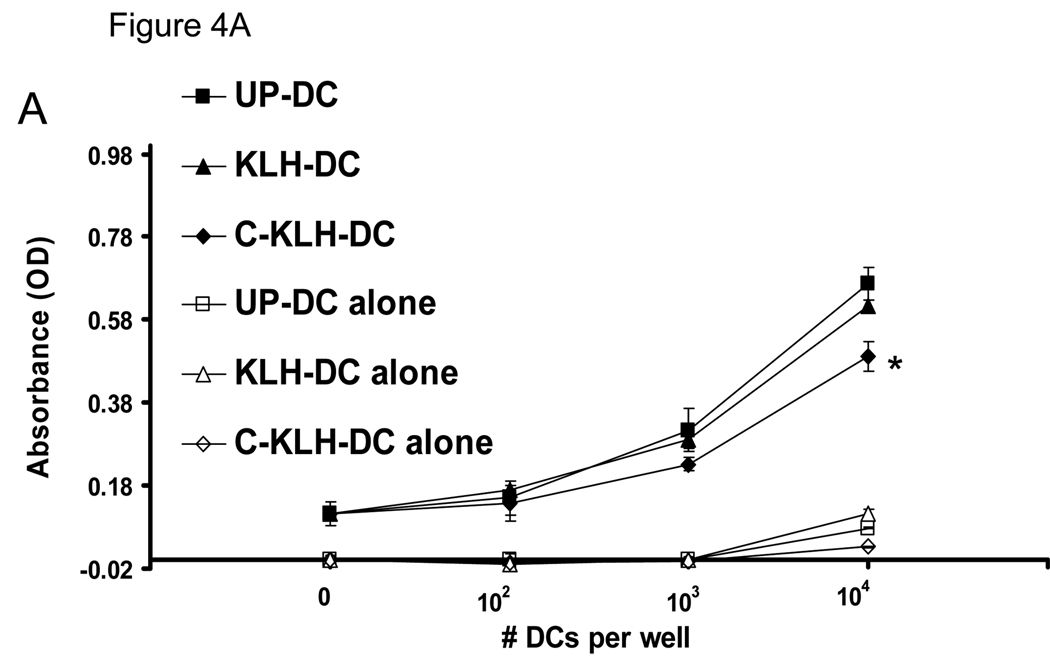
The capacity of unpulsed-DC, KLH pulsed-DC and cryopreserved KLH pulsed-DC to induce proliferation of allogeneic splenocytes was examined in a mixed lymphocyte reaction (MLR). In all splenocytes to DC ratios tested, the stimulatory capacity of KLH pulsed-DC was comparable to that of unpulsed-DC (Fig. 4A). On the other hand, thawed cryopreserved KLH pulsed-DC showed a small but statistically significant reduction in their ability to elicit an allogeneic splenocyte response.
FIGURE 4.
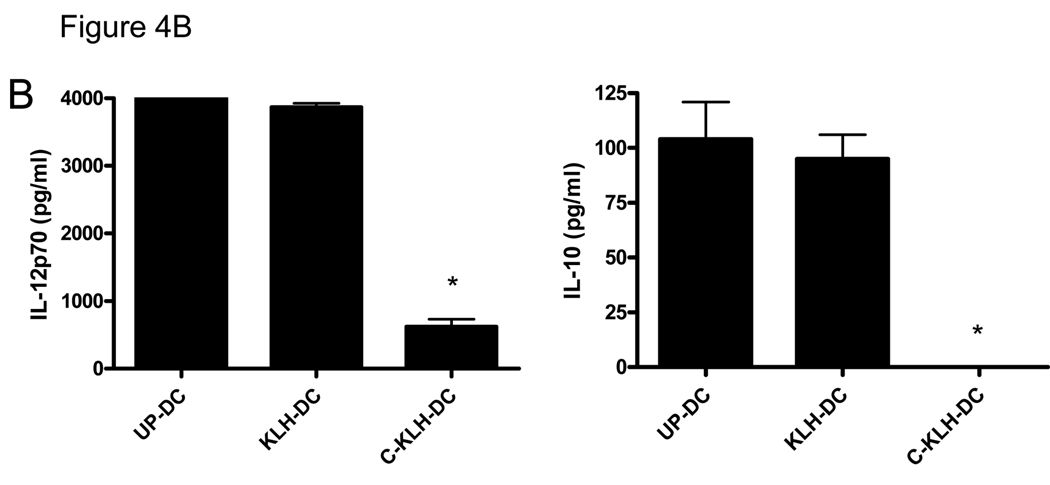
A. Allogeneic splenocyte stimulatory capacity of unpulsed-DC (UP-DC), KLH pulsed-DC (KLH-DC) and cryopreserved KLH pulsed-DC (C-KLH-DC). Allogeneic splenocytes (2 × 105 per well), as responders, were incubated with or without increasing numbers of each type of DC as stimulators. Proliferation was measured after 5 days of co-culture using a WST-1-based assay as described in the Materials and Methods. Data are reported as the average absorbance ± SD of triplicate samples. * P < 0.001 versus UP-DC and KLH-DC. B. Cytokine release capacity of UP-DC, KLH-DC and C-KLH-DC upon induction of maturation. Supernatants of DC cultured in the presence of LPS were analyzed for IL-12p70 and IL-10 content using ELISA. * P < 0.05 versus UP-DC and KLH-DC. These experiments were repeated at least two times with similar results.
The capacity of unpulsed-DC, KLH pulsed-DC and cryopreserved KLH pulsed-DC to secrete cytokines upon induction of maturation was tested by measuring the amount of IL-12p70 and IL-10 in the supernatants of DC cultured in the presence of LPS. As shown in Fig. 4B, unpulsed-DC and KLH pulsed-DC secreted comparable amounts of both cytokines. However, cryopreserved KLH pulsed-DC released significantly less IL-12p70 and undetectable levels of IL-10. Altogether, these results suggest that the in vitro function of KLH pulsed-DC is completely preserved. However, cryopreservation of KLH pulsed-DC appears to compromise their in vitro function.
I.t. KLH pulsed-DC or cryopreserved KLH pulsed-DC vaccination is as effective as unpulsed-DC vaccination in eliciting anti-tumor immune responses and inhibiting tumor growth
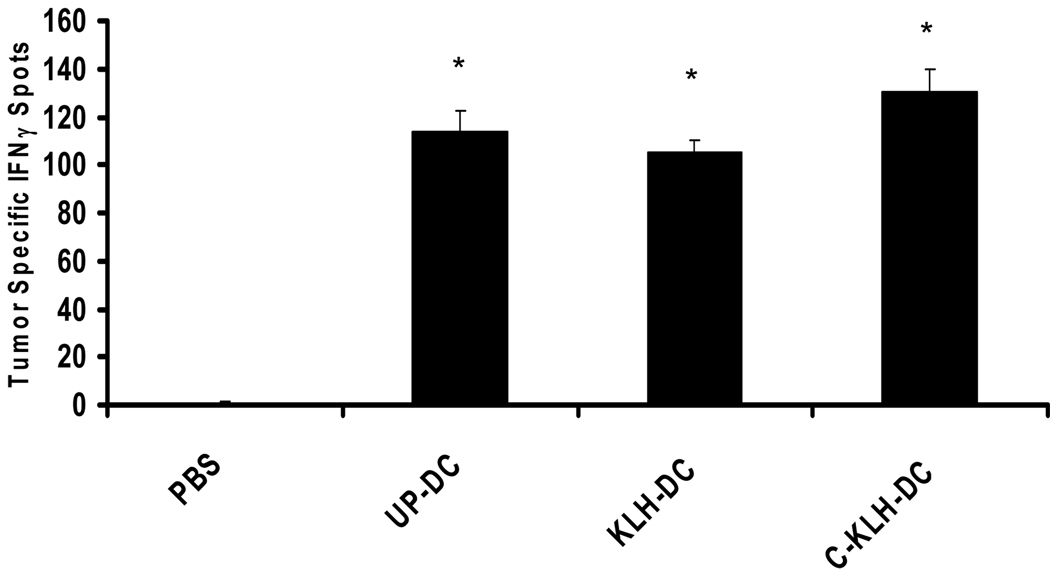
We examined the capacity of unpulsed-DC, KLH pulsed-DC and cryopreserved KLH pulsed-DC to induce tumor-specific immune responses in vivo using the murine D5 melanoma tumor model. Mice bearing s.c. D5 melanoma tumors received four i.t. injections of either unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC on days 6, 11, 14 and 18 after tumor inoculation. On day 21, splenocytes from treated and control mice were incubated with irradiated D5 or PAN 02 tumor cells in an IFNγ ELISPOT assay. PAN 02 is a non-relevant tumor syngeneic to C57BL/6 mice that served as a specificity control. As shown in Fig. 5, i.t. vaccination with unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC induced comparable numbers of tumor-specific IFNγ-secreting cells in the spleens of treated mice. In contrast, splenocytes from untreated mice contained significantly less IFNγ-secreting cells (P < 0.0001 versus all other groups).
FIGURE 5.
Induction of tumor-specific IFNγ-secreting splenocytes by i.t. vaccination with unpulsed-DC (UP-DC), KLH pulsed-DC (KLH-DC) or cryopreserved KLH pulsed-DC (C-KLH-DC). Mice were inoculated s.c. with 2 × 106 D5 tumor cells on day 0. UP-DC, KLH-DC or C-KLH-DC (1 × 106) were administered i.t. on days 6, 11, 14 and 18. A control group received no treatment (i.t. PBS). Twenty one days after tumor inoculation, splenocytes from treated and control mice were incubated with specific (D5) or irrelevant (PAN 02) irradiated tumor cells in an IFNγ ELISPOT assay. Six spleens were harvested from each group and pooled together. Data are reported as the average number of tumor-specific spots per 1 × 106 responders ± SE of triplicate samples after subtracting non-specific spots as detailed in the Materials and Methods section. *, P < 0.0001 versus PBS.
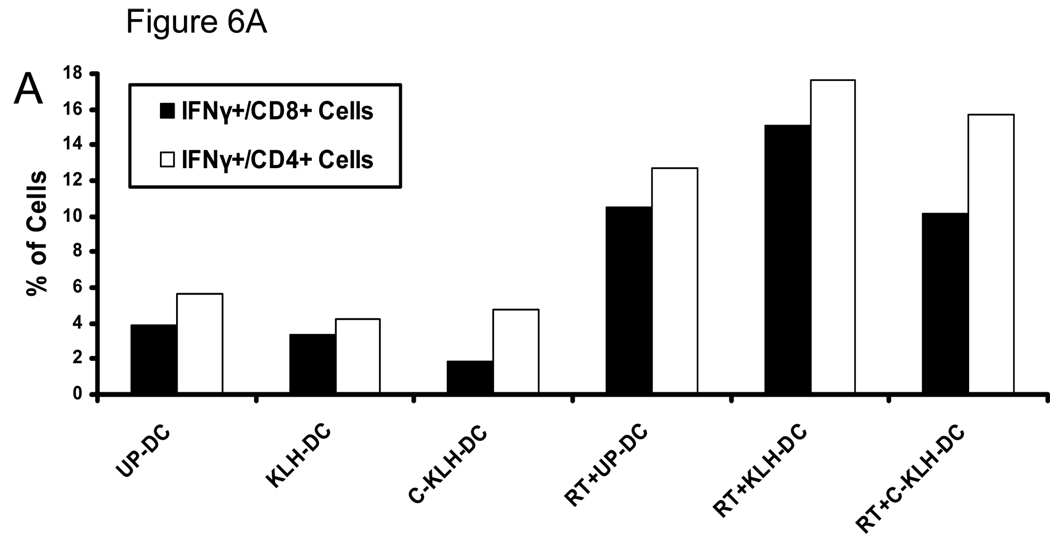
We have previously reported that combining local tumor irradiation with i.t. unpulsed-DC therapy enhances induction of anti-tumor immune responses and inhibition of tumor growth (16, 17). Therefore, we tested whether a combination of radiotherapy and i.t. vaccination with KLH pulsed-DC or cryopreserved KLH pulsed-DC could elicit augmented anti-tumor immune responses as well. To this end, mice bearing s.c. D5 tumors received four i.t. injections of either unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC as described above. In addition, tumors were locally irradiated with 5 consecutive daily fractions of 8.5 Gy on days 7–11. Fifteen days after tumor inoculation, PBMC were collected from treated and control mice and incubated with irradiated D5 tumor cells. Intracellular IFNγ production and cell surface expression of CD4 and CD8 were analyzed using flow cytometry. As shown in Fig. 6A, combining radiotherapy with i.t. administration of either unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC enhanced induction of circulating tumor-reactive IFNγ-producing CD8+ and CD4+ cells to a similar degree.
FIGURE 6.
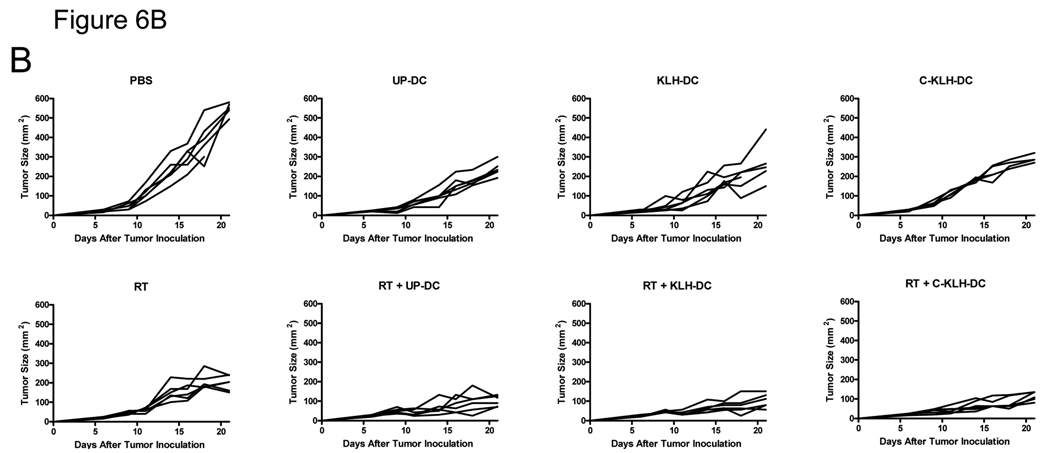
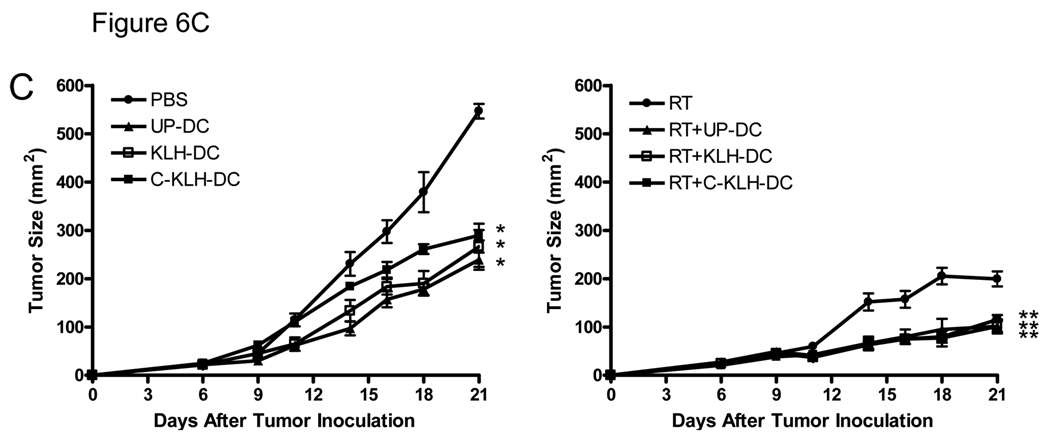
I.t. KLH pulsed-DC (KLH-DC) or cryopreserved KLH pulsed-DC (C-KLH-DC) vaccination is as effective as unpulsed-DC (UP-DC) vaccination in eliciting anti-tumor immune responses (A) and inhibiting D5 tumor growth (B, C). Mice were inoculated s.c. with 2 × 106 D5 tumor cells on day 0. UP-DC, KLH-DC or C-KLH-DC (1 × 106) were administered i.t. on days 6, 11, 14 and 18. Tumors were locally irradiated on days 7–11 (8.5 Gy×5). Control groups received either no treatment (i.t. PBS), i.t. UP-DC alone, i.t. KLH-DC alone, i.t. C-KLH-DC alone or radiotherapy (RT) alone. A. Induction of circulating tumor-reactive IFNγ-producing CD8+ and CD4+ cells by i.t. vaccination with UP-DC, KLH-DC or C-KLH-DC combined with radiotherapy. Fifteen days after tumor inoculation, peripheral blood mononuclear cells were collected from treated mice and incubated with irradiated D5 tumor cells. Intracellular IFNγ production along with cell surface expression of CD4 and CD8 were analyzed using flow cytometry. Specificity of staining was confirmed using isotype matched control monoclonal antibodies. Blood samples (100 µl) were drawn from 6 mice per group and pooled together in order to obtain sufficient numbers of cells for analysis. B. Tumor growth curves of individual mice are shown. Each group consisted of 6 mice. C. Data are reported as the average tumor size ± SE of six mice per group. All eight groups of mice in the left and right panel were treated simultaneously in a single experiment. Data are presented in two separate graphs to enhance clarity. *, P < 0.0001 versus PBS. **, P < 0.004 versus all mono-therapies. This experiment was repeated two times with similar results.
Finally, we compared the therapeutic efficacy of i.t. vaccination with unpulsed-DC, KLH pulsed-DC and cryopreserved KLH pulsed-DC administered with or without radiotherapy. As shown in Fig. 6B and 6C, s.c. D5 tumors in untreated mice rapidly progressed. Unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC administration alone significantly inhibited D5 tumor growth compared with untreated mice (p < 0.0001). No statistically significant differences between the 3 groups were observed. Local tumor irradiation alone caused inhibition of tumor growth as well (p < 0.0001 versus untreated mice). I.t. injection of unpulsed-DC, KLH pulsed-DC or cryopreserved KLH pulsed-DC combined with radiotherapy resulted in an additive inhibition of D5 tumor growth (p < 0.004 versus all mono-therapies). In all 3 types of DC vaccination, radiation enhanced anti-tumor efficacy to the same extent. Collectively, these results demonstrate that KLH pulsed-DC and cryopreserved KLH pulsed-DC retain full in vivo function.
DISCUSSION
Clinical trials of DC-based tumor vaccines have shown limited therapeutic efficacy to date (30, 31). Evaluating novel strategies designed to enhance the potency of DC-based immunotherapy of cancer may enable future development of effective treatment protocols for combating human malignancies. Pre-clinical studies performed by us and others (16, 17, 32–34) and a phase I clinical trial conducted in advanced hepatoma patients (19) suggest that the combination of radiotherapy and i.t. unpulsed-DC vaccination constitutes an attractive approach that warrants further investigation.
Radiotherapy is already applied in the treatment of a wide array of human cancers due to its direct cytotoxic effect. However, local tumor irradiation can also augment DC-based immunotherapy by various pathways. These include enhancing the capacity of DC to capture tumor antigens, home to the draining lymph node, and mediate cross-priming of T cells (18). Furthermore, radiation may modulate the immunosuppressive microenvironment within solid tumors (35) leading to increased accumulation (36, 37) and improved function (38) of DC-elicited effector T cells. Molecular mechanisms by which exposure of tumor cells to ionizing radiation augment DC function have recently been the focus of intensive studies. In addition to providing an excellent source of tumor antigens (39), irradiated dying tumor cells have been shown to release the high-mobility-group box 1 (HMGB1) alarmin (40). Extracellular HMGB1 has been reported to act as a chemoattractant and activator of DC (41). Binding of HMGB1 to Toll-like receptor 4 (TLR4) expressed on DC was found to induce efficient processing and cross-presentation of tumor-derived antigens (40). Moreover, tumor irradiation has been shown to down-regulate CCL21 gene expression (18) which may explain the facilitated migration of exogenously i.t. administered DC to the draining lymph nodes. Together these findings form a reasonably sound scientific basis for combining radiotherapy with i.t. DC therapy.
Further clinical testing of this promising approach would be facilitated by adding a reliable well-accepted marker to the vaccination protocol. This will allow monitoring of induction of immune responses to the same antigen in all treated patients. As most patients currently eligible for cancer immunotherapy clinical trials have refractory disseminated advanced stage disease, the ability to assess induction of vaccine-elicited immune responses is of utmost importance. In this study, we show that murine bone marrow-derived DC pulsed with KLH are comparable to unpulsed-DC regarding viability, phenotype, in vitro function and most importantly in vivo anti-tumor activity upon i.t. vaccination administered with or without radiotherapy. Hence, KLH pulsed-DC appear suitable for use in clinical i.t. vaccination trials and offer the advantage of providing an immunogenic tracer molecule that enables monitoring of induced immune responses (10–15).
DC are now widely used in experimental animal studies and in clinical trials of cancer immunotherapy. In both applications, the ability to use aliquots of cryopreserved DC vaccine preparations immediately after thawing would substantially reduce effort, time, expense and variability inherent in repeatedly culturing fresh DC from precursor cells. Sai et al (42) have reported that after being thawed and cultured for 3 days, cryopreserved murine bone marrow-derived DC retained their phenotype, in vitro function, trafficking capacity, and ability to elicit T cell responses in vivo. Lewalle et al (20) have shown that frozen immature human DC generated from PBMC maintain their capacity to acquire and present antigens in vitro as well as to undergo CD40 ligand and IFNγ-induced maturation. Furthermore, antigen-loaded or unloaded matured human DC that were exposed to a freeze-thaw cycle exhibited a similar phenotype and in vitro functional properties as freshly prepared DC (21, 24, 26–28). Human DC transfected with cDNA of a tumor antigen maintained similar levels of antigen cell surface expression before and after freezing (23). In contrast, John et al (25) have reported that cryopreservation of immature monocyte-derived human DC results in enhanced cell maturation, reduced endocytic activity and decreased efficiency of adenoviral transduction. Garderet et al (22) have demonstrated that cryopreservation of human DC in a −80°C mechanical freezer or using a liquid nitrogen controlled-rate freezer at -1°C/min yields similar results regarding viability, phenotype and allogeneic lymphocyte stimulatory capacity of the thawed cells.
In this study, we report that cryopreservation of murine bone marrow-derived DC pulsed with KLH slightly reduces viability, expression of co-stimulatory cell surface markers and in vitro function. Immediate post-thawing evaluation performed in the present study, rather than assessment after a recovery period in culture as was performed in most previous studies, may account for these results. Nevertheless, cryopreserved KLH pulsed-DC maintained full in vivo function upon immediate post-thawing i.t. vaccination. This finding suggests that complete recovery of function may occur in vivo or that the slightly reduced function detected in vitro has no significant translational implications. Either way, this is the first report to show that vaccination using cryopreserved DC is as effective as fresh DC in inducing anti-tumor immune responses and inhibiting tumor growth. All the data presented in this report are based on animal studies. Nevertheless, our results strongly support the use of cryopreserved DC for both research and clinical applications.
Acknowledgments
This work was supported by NIH grant CA59327, the Gillson Longenbaugh Foundation, and a gift from the Danto Family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron. 1999;30:597–623. doi: 10.1016/s0968-4328(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 2.Harris JR, Markl J. Keyhole limpet hemocyanin: molecular structure of a potent marine immunoactivator. A review. Eur. Urol. (Suppl 3) 2000;37:24–33. doi: 10.1159/000052389. [DOI] [PubMed] [Google Scholar]
- 3.Schlienger K, Craighead N, Lee KP, Levine BL, June CH. Efficient priming of protein antigen-specific human CD4(+) T cells by monocyte-derived dendritic cells. Blood. 2000;96:3490–3498. [PubMed] [Google Scholar]
- 4.Mehta-Damani A, Markowicz S, Engleman EG. Generation of antigen-specific CD4+ T cell lines from naive precursors. Eur. J. Immunol. 1995;25:1206–1211. doi: 10.1002/eji.1830250511. [DOI] [PubMed] [Google Scholar]
- 5.Mittelman A, Chen ZJ, Liu CC, Hirai S, Ferrone S. Kinetics of the immune response and regression of metastatic lesions following development of humoral anti-high molecular weight-melanoma associated antigen immunity in three patients with advanced malignant melanoma immunized with mouse antiidiotypic monoclonal antibody MK2-23. Cancer Res. 1994;54:415–421. [PubMed] [Google Scholar]
- 6.Foon KA, John WJ, Chakraborty M, Sherratt A, Garrison J, Flett M, Bhattacharya-Chatterjee M. Clinical and immune responses in advanced colorectal cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. Clin. Cancer Res. 1997;3:1267–1276. [PubMed] [Google Scholar]
- 7.Birebent B, Koido T, Mitchell E, Li W, Somasundaram R, Purev E, Hoey D, Mastrangelo M, Maguire H, Harris DT, Nair S, Cai E, Herlyn D. Anti-idiotypic antibody (ab2) vaccines: coupling of Ab2 BR3E4 to KLH increases humoral and/or cellular immune responses in animals and colorectal cancer patients. J. Cancer Res. Clin. Oncol. 2001;127 Suppl 2:R27–R33. doi: 10.1007/BF01470996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman JM, Levy R. Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J. Immunol. 2000;164:4797–4803. doi: 10.4049/jimmunol.164.9.4797. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Thomas EK, Giedlin M, Mule JJ. Enhancement of tumor lysate-and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001;61:2618–2624. [PubMed] [Google Scholar]
- 10.Redman BG, Chang AE, Whitfield J, Esper P, Jiang G, Braun T, Roessler B, Mule JJ. Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma. J. Immunother. doi: 10.1097/CJI.0b013e31817fd90b. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mule JJ. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin. Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 12.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 13.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 15.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat. Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 16.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, Chang AE. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 17.Huang J, Wang Y, Guo J, Lu H, Lin X, Ma L, Teitz-Tennenbaum S, Chang AE, Li Q. Radiation-induced apoptosis along with local and systemic cytokine elaboration is associated with DC plus radiotherapy-mediated renal cell tumor regression. Clin. Immunol. 2007;123:298–310. doi: 10.1016/j.clim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, Knibbs RN, Stoolman LM, Chang AE. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J. Immunother. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 20.Lewalle P, Rouas R, Lehmann F, Martiat P. Freezing of dendritic cells, generated from cryopreserved leukaphereses, does not influence their ability to induce antigen-specific immune responses or functionally react to maturation stimuli. J. Immunol. Methods. 2000;240:69–78. doi: 10.1016/s0022-1759(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 21.Feuerstein B, Berger TG, Maczek C, Roder C, Schreiner D, Hirsch U, Haendle I, Leisgang W, Glaser A, Kuss O, Diepgen TL, Schuler G, Schuler-Thurner B. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J. Immunol. Methods. 2000;245:15–29. doi: 10.1016/s0022-1759(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 22.Garderet L, Cao H, Salamero J, Verge V, Tisserand E, Scholl S, Gorin NC, Lopez M. In vitro production of dendritic cells from human blood monocytes for therapeutic use. J. Hematother. Stem Cell Res. 2001;10:553–567. doi: 10.1089/15258160152509163. [DOI] [PubMed] [Google Scholar]
- 23.Pecher G, Schirrmann T, Kaiser L, Schenk JA. Efficient cryopreservation of dendritic cells transfected with cDNA of a tumour antigen for clinical application. Biotechnol. Appl. Biochem. 2001;34:161–166. doi: 10.1042/ba20010034. [DOI] [PubMed] [Google Scholar]
- 24.Tuyaerts S, Noppe SM, Corthals J, Breckpot K, Heirman C, De Greef C, Van Riet I, Thielemans K. Generation of large numbers of dendritic cells in a closed system using Cell Factories. J. Immunol. Methods. 2002;264:135–151. doi: 10.1016/s0022-1759(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 25.John J, Hutchinson J, Dalgleish A, Pandha H. Cryopreservation of immature monocyte-derived dendritic cells results in enhanced cell maturation but reduced endocytic activity and efficiency of adenoviral transduction. J. Immunol. Methods. 2003;272:35–48. doi: 10.1016/s0022-1759(02)00430-1. [DOI] [PubMed] [Google Scholar]
- 26.Westermann J, Korner IJ, Kopp J, Kurz S, Zenke M, Dorken B, Pezzutto A. Cryopreservation of mature monocyte-derived human dendritic cells for vaccination: influence on phenotype and functional properties. Cancer Immunol. Immunother. 2003;52:194–198. doi: 10.1007/s00262-002-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celluzzi CM, Welbon C. A simple cryopreservation method for dendritic cells and cells used in their derivation and functional assessment. Transfusion. 2003;43:488–494. doi: 10.1046/j.1537-2995.2003.00359.x. [DOI] [PubMed] [Google Scholar]
- 28.Hori S, Heike Y, Takei M, Maruyama M, Inoue Y, Lee JJ, Kim HJ, Harada Y, Kawai H, Shimosaka A, Kami M, Tanosaki MD, Wakasugi H, Saito S, Takaue Y, Kakizoe T. Freeze-thawing procedures have no influence on the phenotypic and functional development of dendritic cells generated from peripheral blood CD14+ monocytes. J. Immunother. 2004;27:27–35. doi: 10.1097/00002371-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 30.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr. Opin. Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Gilboa E. DC-based cancer vaccines. J. Clin. Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KW, Kim SH, Shin JG, Kim GS, Son YO, Park SW, Kwon BH, Kim DW, Lee CH, Sol MY, Jeong MH, Chung BS, Kang CD. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int. J. Cancer. 2004;109:685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Xia D, Bi X, Saxena A, Sidhu N, El-Gayed A, Xiang J. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J. Gene Med. 2005;7:506–517. doi: 10.1002/jgm.692. [DOI] [PubMed] [Google Scholar]
- 34.Akutsu Y, Matsubara H, Urashima T, Komatsu A, Sakata H, Nishimori T, Yoneyama Y, Hoshino I, Murakami K, Usui A, Kano M, Ochiai T. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int. J. Oncol. 2007;31:509–515. [PubMed] [Google Scholar]
- 35.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 36.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 37.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 39.Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D, Chapoval A, Lu J, Kasperbauer JL, Padley D, Vile R, Gastineau D, Wettstein P, Chen L. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62:1884–1889. [PubMed] [Google Scholar]
- 40.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 42.Sai T, Milling SW, Mintz B. Freezing and thawing of bone marrow-derived murine dendritic cells with subsequent retention of immunophenotype and of antigen processing and presentation characteristics. J. Immunol. Methods. 2002;264:153–162. doi: 10.1016/s0022-1759(02)00100-x. [DOI] [PubMed] [Google Scholar]