Abstract
Two methods commonly used for slime detection in coagulase-negative staphylococci (tube biofilm formation and colony morphology in Congo red agar) were used to study 144 ruminant mastitis Staphylococcus aureus strains. Slime production was detected in 21 strains. A majority of cells (85%) in slime-producing (SP) strains and a minority of cells (5%) in non-slime-producing (NSP) strains showed a condensed exopolysaccharide matrix (slime) surrounding the bacterial cell wall, as revealed by electron microscopy and immunofluorescence. In vivo slime production was also detected immunohistochemically after experimental infection of the mammary gland in sheep. Upon repeated subcultures in Congo red agar, NSP variants were obtained from four ovine and four bovine SP strains at a frequency ranging from 0.5 x 10(-4) to 10(-4). Because SP variants could not be obtained from NSP strains within this range or at a higher frequency, they were obtained by the tube biofilm formation (requiring repeated subculturing of NSP strains in tryptic soy broth containing 2% glucose for subsequent recovery of colonies adherent to the walls of the culture tubes). In experimental challenge, the SP variant showed a significantly higher colonization capacity than did the NSP variant of the same strain used (P < 0.001). However, the NSP variant had a higher virulence than did the SP variant (P < 0.001). These results may help to explain the different roles of S. aureus slime production cell types (SP and NSP) coexisting in disease.
Full text
PDF


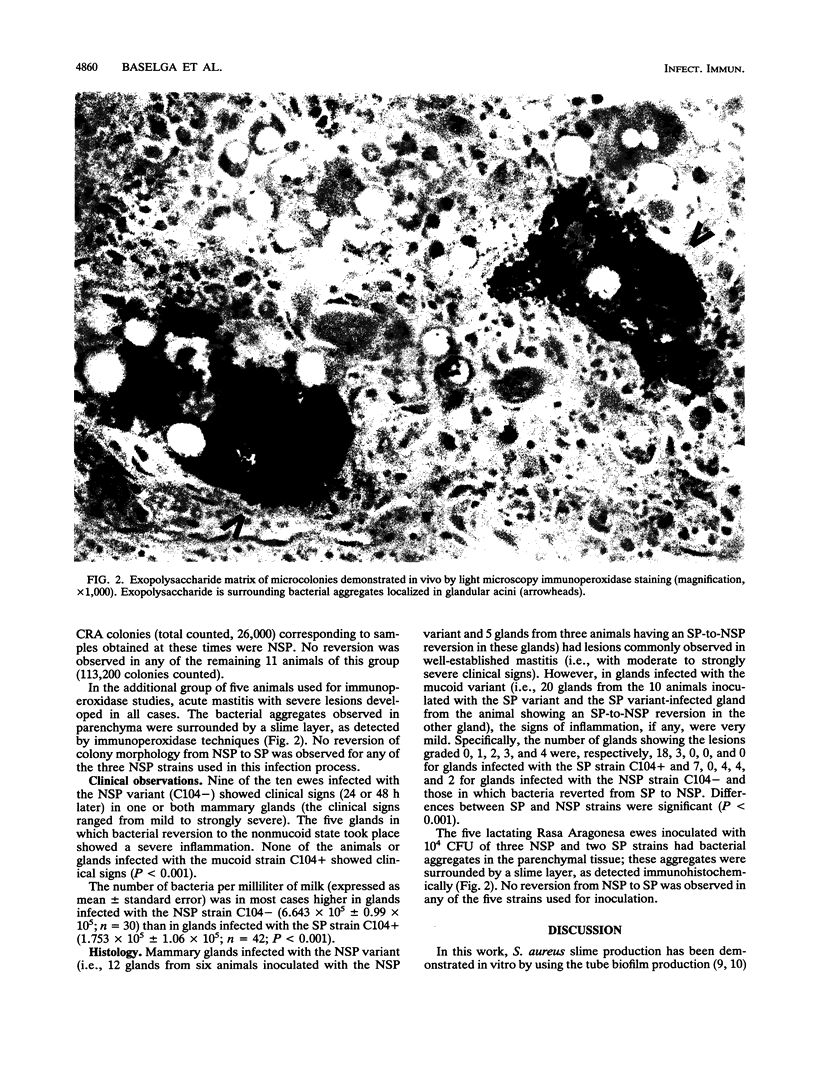


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar B., Iturralde M., Baselga R., Amorena B. An efficient microtest to study adherence of bacteria to mammalian cells. FEMS Microbiol Lett. 1992 Jan 1;69(2):161–164. doi: 10.1016/0378-1097(92)90621-t. [DOI] [PubMed] [Google Scholar]
- Amorena B., Baselga R., Aguilar B. Factors influencing the degree of in vitro bacterial adhesion to ovine mammary gland epithelial cells. Vet Microbiol. 1990 Jul;24(1):43–53. doi: 10.1016/0378-1135(90)90049-2. [DOI] [PubMed] [Google Scholar]
- Amorena B., García de Jalón J. A., Baselga R., Ducha J., Latre M. V., Ferrer L. M., Sancho F., Månsson I., Krovacek K., Faris A. Infection of rabbit mammary glands with ovine mastitis bacterial strains. J Comp Pathol. 1991 Apr;104(3):289–302. doi: 10.1016/s0021-9975(08)80041-2. [DOI] [PubMed] [Google Scholar]
- Anastassiou E. D., Mintzas A. C., Kounavis C., Dimitracopoulos G. Alginate production by clinical nonmucoid Pseudomonas aeruginosa strains. J Clin Microbiol. 1987 Apr;25(4):656–659. doi: 10.1128/jcm.25.4.656-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chan R., Acres S. D., Costerton J. W. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun. 1982 Sep;37(3):1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Madison B. M., Parisi J. T., Abraham S. N., Hasty D. L., Lowrance J. H., Josephs J. A., Simpson W. A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta-lactam antibiotics, and virulence. J Infect Dis. 1990 Jun;161(6):1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. M., Hannon K., Moreau M., Karakawa W. W., Vann W. F. Isolation of type 5 capsular polysaccharide from Staphylococcus aureus. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):561–567. doi: 10.1016/0769-2609(87)90041-x. [DOI] [PubMed] [Google Scholar]
- Freeman D. J., Falkiner F. R., Keane C. T. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989 Aug;42(8):872–874. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johne B., Jarp J., Haaheim L. R. Staphylococcus aureus exopolysaccharide in vivo demonstrated by immunomagnetic separation and electron microscopy. J Clin Microbiol. 1989 Jul;27(7):1631–1635. doi: 10.1128/jcm.27.7.1631-1635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Fournier J. M., Vann W. F., Arbeit R., Schneerson R. S., Robbins J. B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985 Sep;22(3):445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo W., Fröman G., Wadström T. Interaction of sub-epithelial connective tissue components with Staphylococcus aureus and coagulase-negative staphylococci from bovine mastitis. Vet Microbiol. 1988 Oct;18(2):163–176. doi: 10.1016/0378-1135(88)90062-4. [DOI] [PubMed] [Google Scholar]
- Opdebeeck J. P., Frost A. J., O'Boyle D., Norcross N. L. The expression of capsule in serum-soft agar by Staphylococcus aureus isolated from bovine mastitis. Vet Microbiol. 1987 Mar;13(3):225–234. doi: 10.1016/0378-1135(87)90085-x. [DOI] [PubMed] [Google Scholar]
- Pincus S. H., Cole R. L., Wessels M. R., Corwin M. D., Kamanga-Sollo E., Hayes S. F., Cieplak W., Jr, Swanson J. Group B streptococcal opacity variants. J Bacteriol. 1992 Jun;174(11):3739–3749. doi: 10.1128/jb.174.11.3739-3749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutrel B., Boutonnier A., Sutra L., Fournier J. M. Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J Clin Microbiol. 1988 Jan;26(1):38–40. doi: 10.1128/jcm.26.1.38-40.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., Belani K. K. Coagulase-negative staphylococcal adherence and persistence. J Infect Dis. 1987 Oct;156(4):543–547. doi: 10.1093/infdis/156.4.543. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K. M., Bowen W. H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992 Jan;60(1):284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif G., Sulavik M. C., Jones G. W., Clewell D. B. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect Immun. 1989 Dec;57(12):3945–3948. doi: 10.1128/iai.57.12.3945-3948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry J. M., Piña S. E., Mattingly S. J. Environmental conditions which influence mucoid conversion Pseudomonas aeruginosa PAO1. Infect Immun. 1991 Feb;59(2):471–477. doi: 10.1128/iai.59.2.471-477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman M. M., Clewell D. B., Jones G. W. Ecological implications of glucosyltransferase phase variation in Streptococcus gordonii. Appl Environ Microbiol. 1991 Dec;57(12):3648–3651. doi: 10.1128/aem.57.12.3648-3651.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman M. M., Clewell D. B., Jones G. W. Glucosyltransferase phase variation in Streptococcus gordonii modifies adhesion to saliva-coated hydroxyapatite surfaces in a sucrose-independent manner. Oral Microbiol Immunol. 1992 Apr;7(2):118–120. doi: 10.1111/j.1399-302x.1992.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Watson D. L., Watson N. A. Expression of a pseudocapsule by Staphylococcus aureus: influence of cultural conditions and relevance to mastitis. Res Vet Sci. 1989 Sep;47(2):152–157. [PubMed] [Google Scholar]
- Weiser J. N., Love J. M., Moxon E. R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989 Nov 17;59(4):657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Takahashi M., Ohtomo T., Minegishi Y., Ichiman Y., Haga K., Kono E., San Clemente C. L. Application of fluorescent antibody for detecting capsular substances in Staphylococcus aureus. J Appl Bacteriol. 1979 Feb;46(1):147–152. doi: 10.1111/j.1365-2672.1979.tb02592.x. [DOI] [PubMed] [Google Scholar]





