Abstract
Voltage-dependent potassium (Kv) channels play a pivotal role in the modulation of macrophage physiology. Macrophages are professional antigen-presenting cells and produce inflammatory and immunoactive substances that modulate the immune response. Blockage of Kv channels by specific antagonists decreases macrophage cytokine production and inhibits proliferation. Numerous pharmacological agents exert their effects on specific target cells by modifying the activity of their plasma membrane ion channels. Investigation of the mechanisms involved in the regulation of potassium ion conduction is, therefore, essential to the understanding of potassium channel functions in the immune response to infection and inflammation. Here, we demonstrate that the biophysical properties of voltage-dependent K+ currents are modified upon activation or immunosuppression in macrophages. This regulation is in accordance with changes in the molecular characteristics of the heterotetrameric Kv1.3/Kv1.5 channels, which generate the main Kv in macrophages. An increase in K+ current amplitude in lipopolysaccharide-activated macrophages is characterized by a faster C-type inactivation, a greater percentage of cumulative inactivation, and a more effective margatoxin (MgTx) inhibition than control cells. These biophysical parameters are related to an increase in Kv1.3 subunits in the Kv1.3/Kv1.5 hybrid channel. In contrast, dexamethasone decreased the C-type inactivation, the cumulative inactivation, and the sensitivity to MgTx concomitantly with a decrease in Kv1.3 expression. Neither of these treatments apparently altered the expression of Kv1.5. Our results demonstrate that the immunomodulation of macrophages triggers molecular and biophysical consequences in Kv1.3/Kv1.5 hybrid channels by altering the subunit stoichiometry.
INTRODUCTION
Macrophages play an important role in the inflammatory responses triggered by hormones and cytokines. These cells, which also act as professional antigen-presenting cells, modify the cytokine milieu and the intensity of T cell signaling. Therefore, macrophages may tune the immune response toward inflammation or tolerance. The activation and proliferation of cells in the immune system are modulated by membrane transduction of extracellular signals. Some interactions occur via the regulation of transmembrane ion fluxes, and several studies suggest that some signaling occurs through ion movements in macrophages (Eder, 1998). Thus, macrophages change their membrane electrophysiological properties depending on their state of functional activation (Vicente et al., 2003). Changes in membrane potential are among the earliest events occurring upon stimulation of macrophages, and ion channels underlie the Ca2+ signal involved in the leukocyte activation. In this context, potassium channels indirectly determine the driving force for Ca2+ entry (Cahalan and Chandy, 1997; Panyi et al., 2004).
Voltage-dependent potassium (Kv) channels have the crucial functions in excitable cells of determining the resting membrane potential and controlling action potentials (Hille, 2001). In addition, they are involved in the activation and proliferation of leukocytes (Cahalan and Chandy, 1997; Panyi et al., 2004). Accumulating evidence indicates that Kv channels play a pivotal role in the modulation of macrophage immunomodulatory responses. Kv channels are tightly regulated during proliferation and activation in macrophages, and their functional activity is important for cellular responses (Vicente et al., 2003, 2005, 2006, 2008; Villalonga et al., 2007). Proliferation and activation trigger an induction of the outward K+ current that is under transcriptional, translational, and posttranslational control (Vicente et al., 2003). Assigning specific K+ channel clones to native currents is difficult because this complexity is further enhanced by the heteromultimeric assembly of different Kv subunits (Vicente et al., 2006). Kv1.5 coassociates with Kv1.3 to generate functional Kv1.3/Kv1.5 heterotetrameric channels in macrophages. In response to different physiological stimuli, changes in the oligomeric composition of functional Kv could have a crucial effect on intracellular signals, determining the specific macrophage response (Vicente et al., 2003, 2006, 2008; Villalonga et al., 2007).
Bacterial lipopolysaccharide (LPS) activates macrophages, leading to the secretion of bioactive molecules such as cytokines (e.g., TNF-α) and nitric oxide (NO) (Soler et al., 2001). However, there is tight regulation of signaling events to avoid an exaggerated response by macrophages during infection and injuries. These mechanisms include the release of glucocorticoids (GCs) by the adrenal gland. GCs, which exert their antiinflammatory action, in part, by influencing macrophages, inhibit the expression of inflammatory mediators, and thus are used in the treatment of many inflammatory diseases (Lloberas et al., 1998). GCs may trigger long-term changes in cell excitability by regulating K+ channel gene expression. Thus, while macrophage activation induces Kv1.3, dexamethasone (DEX), a GC receptor agonist, inhibits Kv1.3 in T cells but differentially regulates Kv1.5 in several cells and tissues (Attardi et al., 1993; Takimoto et al., 1993; Takimoto and Levitan, 1994, 1996; Levitan et al., 1996; Lampert et al., 2003). Experimental evidence indicates that in macrophages, the major Kv is mainly a heterotetrameric Kv1.3/Kv1.5 channel (Vicente et al., 2006; Villalonga et al., 2007). Therefore, the analysis of macrophage hybrid channels under immunomodulation has physiological relevance. The aim of the present work was to investigate heteromeric Kv1.3/Kv1.5 channels in macrophages and to analyze the molecular and biophysical consequences upon activation and immunosuppression.
Here, we demonstrate that, in contrast to LPS, DEX inhibits Kv1.3. Neither LPS nor DEX apparently regulated Kv1.5 in macrophages, leading to different oligomeric Kv1.3/Kv1.5 channels. While LPS-induced activation increased, DEX diminished the Kv1.3 ratio in the complex. Protein and mRNA regulation correlated with the electrophysiological and pharmacological properties of the K+ currents upon activation and immunosuppression. Our results demonstrate that different channel compositions change biophysical properties and could physiologically tune the membrane potential to achieve precise immune responses.
MATERIALS AND METHODS
Cell culture
Raw 264.7 macrophages were used. Cells were cultured in RPMI culture media containing 5% FBS supplemented with 2 mM l-glutamine and 10 U/ml penicillin and streptomycin. Cells were grown in 100-mm tissue culture dishes for sample collection and on noncoated glass coverslips for electrophysiology. In some experiments, Raw 264.7 cells were incubated with 100 ng/ml LPS and 1 µM DEX (Sigma-Aldrich) for 24 h.
RNA isolation, reverse transcription (RT)-PCR analysis, and real-time PCR
Total RNA from rat tissues (brain and heart), Jurkat T cells, L6E9 myoblasts, and Raw 264.7 macrophages were isolated using the Tripure reagent (Roche). All animal handling was approved by the ethics committee of the University of Barcelona in accordance with European Union regulations. RNA was treated with DNase I, and PCR controls were performed in the absence of RT. cDNA synthesis was performed using transcriptor RT (Roche) with a random hexanucleotide and oligo dT according to the manufacturer’s instructions. Once cDNA was synthesized, the conditions were set for further PCR: 92°C for 30 s, 55°C for 1 min, and 72°C for 2 min. These settings were applied for 30 cycles. Primer sequences and accession numbers were: Kv1.3 (GenBank accession number M30441), F: 5′-CTCATCTCCATTGTCATCTTCTGA-3′ and R: 5′-TTGAATTGGAAACAATCAC-3′ (base pairs length: 718); Kv1.5 (GenBank accession number AF302768), F: 5′-GGATCACTCCATCACCAG-3′ and R: 5′-GGCTTCCTCCTCTTCCTTG-3′ (base pairs length: 334). 10 µL from the final RT-PCR reactions was electrophoresed in a 1% agarose TBE gel (40 mM Tris, 20 mM acetic acid, and 1 mM EDTA, pH 8.0).
Real-time PCR was performed using a LightCycler machine (Roche) with LightCycler FastStart DNA MasterPLUS SYBR Green I (Roche), according to the manufacturer’s instructions. PCR primers were: Kv1.5, F: 5′-TCCGACGGCTGGACTCAATAA-3′ and R: 5′-CAGATGGCCTTCTAGGCTGTG-3′ (base pairs length: 133); Kv1.3, F: 5′-AGTATATGGTGATCGAAGAGG-3′ and R: 5′-AGTGAATATCTTCTTGATGTT-3′ (base pairs length: 136). The reactions were performed under the following conditions: 95°C for 5 s, 55°C for 8 s, and 72°C for 9 s, preceded by 10 min at 95°C and followed by 10 min at 95°C. Melting curves were performed to verify the specificity of the product, and 18S (GenBank accession number X00686; F: 5′-CGCAGAATTCCCACTCCCGACCC-3′ and R: 5′-CCCAAGCTCCAACTACGAGC-3′; base pairs length: 212) was included as an internal reference, as described previously. Results were analyzed with Light Cycler software 3.5 (Roche). For each primer set, a standard curve was made and the slope factor calculated. Values were normalized to the corresponding 18S. The corresponding real-time PCR efficiency (E) of one cycle in the exponential phase was calculated according to the equation: E = 10(−1/slope). The normalized Kv1.5/Kv1.3 ratio was calculated as follows: Ratio = (1+E)ΔCt(Kv1.5)/(1+E)ΔCt(Kv1.3), where Ct signifies the threshold cycle.
Crude membrane preparations and Western blot
Cells were washed twice in cold PBS and lysed on ice with lysis solution (1% Triton X-100, 10% glycerol, 50 mM HEPES, pH 7.5, and 150 mM NaCl) supplemented with 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin, and 1 ml phenylmethylsulfonyl fluoride as protease inhibitors. To obtain enriched membrane preparations, homogenates were centrifuged at 3,000 g for 10 min, and the supernatant was further centrifuged at ∼150,000 g for 90 min. The pellet was resuspended in 30 mM HEPES, pH 7.4, and protein content was determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories). Samples were aliquoted and stored at −80°C.
Crude membrane protein samples (50 µg) were boiled in Laemmli SDS loading buffer and separated on 10% SDS-PAGE. Next, they were transferred to nitrocellulose membranes (Immobilon-P; Millipore) and blocked in 5% dry milk–supplemented 0.2% Tween 20 PBS before immunoreaction. Filters were immunoblotted with antibodies against Kv1.3 (1/200; Alomone Labs), Kv1.5 (1/500; Alomone Labs), and inducible NO synthase (iNOS; 1/500; Santa Cruz Biotechnology, Inc.). As a loading and transfer control, a monoclonal anti–β actin antibody (1/5,000; Sigma-Aldrich) was used. The specificity of Kv1.3 and Kv1.5 commercial antibodies was tested with control antigen peptides provided by the manufacturer. Densitometric analysis of the filters was performed by Phoretix software (Nonlinear Dynamics). Results are presented as the mean ± SEM of each experimental group.
Electron microscopy
Macrophages were fixed in 4% paraformaldehyde (PFA)/0.2% glutaraldehyde (GA) in PBS for 30 min at room temperature, and then left in 2% PFA/0.1% GA in PBS overnight at 4°C. Fixation was stopped by washing with 20 mM glycine in PBS. Cells were scraped and collected in tubes (Eppendorf) with 12% gelatin. After solidifying on ice, gelatin blocks were infiltrated in 2.3 M sucrose in PBS overnight at 4°C. Blocks were frozen in liquid nitrogen. Ultrathin cryosections were obtained using Leica ULTRACUT EM FCS at −118°C and subjected to immunogold labeling. Cryosections were incubated at room temperature on drops of 2% gelatin in phosphate buffer for 20 min at 37°C, followed by 50 mM glycine in PBS for 15 min and 5% normal goat serum (NGS) in PBS for 10 min. Then they were incubated with anti-Kv1.5 and anti-Kv1.3 polyclonal antibodies in 5% NGS in PBS for 30 min. After three washes with drops of 5% NGS in PBS for 20 min, sections were incubated for 60 min using immunoglobulin G anti–rabbit coupled to 10- or 15-nm diameter colloidal gold particles (Aurion) using a 1:60 dilution in 5% NGS in PBS. This was followed by three washes with drops of PBS for 10 min and two washes with distilled water. As a control for nonspecific binding of the colloidal gold–conjugated antibody, the primary polyclonal antibody was omitted. Finally, the cryosections were contrasted and embedded in a mixture of methylcellulose and uranyl acetate. For the double immunogold, immunolabeling with one of the primary antibodies was done. We used the secondary antibody conjugated with the small gold (10 nm) in the first immunolabeling procedure. Then, the anti–immunoglobulin G binding sites were inactivated using 3% PFA/2% GA in PBS for 2 h. After inactivation, the immunolabeling with the other primary antibody was done using the secondary antibody conjugated to 15-nm gold particles. Primary antibody dilutions were 1:50 in both cases. Samples were viewed with an electron microscope (1010; JEOL).
Electrophysiological recordings
Whole cell currents were measured using the patch clamp technique with an amplifier (Axopatch 1C; Axon Instruments) and stored on a personal computer (TD Systems) with an analogue-to-digital converter (DigiData 1322A; Axon Instruments). pClamp version 9 software (Axon Instruments) was used for data acquisition and analysis. Currents were recorded at room temperature (21–23°C) at a stimulation frequency of 0.1 Hz and sampled at 4 kHz after anti-alias filtering at 2 kHz. Patch electrodes of 2–4 MΩ were fabricated in a puller (P-87; Sutter Instrument Co.) from borosilicate glass capillary tubes (GD-1; Narishige). GigaΩ seal formation was achieved by suction (2–5 GΩ; n = 12). After seal formation, cells were lifted from the bath and the membrane patch was ruptured with a brief additional suction. The capacitive transients elicited by symmetrical 10-mV steps from −80 mV were recorded at 50 kHz and filtered at 10 kHz for subsequent calculations of capacitative surface area, access resistance, and input impedance. Thereafter, capacitance and series resistance compensation were optimized and, usually, 80% compensation of the effective access resistance was obtained. Electrodes were filled with an intracellular pipette solution containing (in mM): 80 K-aspartate, 50 KCl, 3 phosphocreatine, 10 KH2PO4, 3 MgATP, 10 HEPES-K, and 5 EGTA-K, and was adjusted to pH 7.25 with KOH. The bath solution contained (in mM): 130 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES-Na, and 10 glucose, and was adjusted to pH 7.40 with NaOH. Uncompensated series resistances were 4–8 MΩ, as currents evoked were <1 nA; voltage errors from uncompensated series resistance were <2 mV.
Inactivation was fit to an exponential process, using an equation of the form:
where τ is the system time constant, A is the amplitude, and C is the baseline value. To analyze the cumulative inactivation, currents were elicited by a train of eight depolarizing voltage steps of 200 ms to + 60 mV once every 400 ms. The voltage dependence of activation curves were fitted with a Boltzmann equation:
in which k represents the slope factor, V the membrane potential, and Vh the voltage at which 50% of the channels are open (Franqueza et al., 1998; González et al., 2001).
The number of channels per cell and their surface density were calculated from the conductance of Kv1.3 (13 pS) and Kv1.5 (8 pS) following the Kv1.3/ Kv1.5 ratio obtained from real-time PCR results following the equation:
where G represents the total conductance, Imax is the peak amplitude, V is the voltage applied, and VK is the K+ equilibrium potential.
To characterize the K+ current pharmacologically, 750 pM margatoxin (MgTx; Alomone Labs) was added to the external solution (Vicente et al., 2003, 2006). Toxin was reconstituted at 10 µM in Tris buffer (0.1% BSA, 100 mM NaCl, and 10 mM Tris, pH 7.5). Solutions were perfused by using a gravity-flow perfusion setup. The inhibition by MgTx was defined by the remaining current fraction in the presence and absence of the toxin. All recordings were performed at room temperature (20–23°C).
Lentivirus infection
To knock down Kv1.3 in Raw macrophages, Kv1.3 shRNA (mouse) lentiviral (LTV) particles were used (Santa Cruz Biotechnology, Inc.). Cells were cultured in 12-well dishes to get ∼50% confluence and infected with 5,000 infectious units in the presence of 2 µg/ml polybrene (Sigma-Aldrich). 24 h after infection, the medium was replaced by regular culture medium supplemented with 10 µg/ml puromycin (Sigma-Aldrich) for clone selection. Selected clones were maintained with 2 µg/ml puromycin. Specific silenced expression of Kv1.3, but not Kv1.5, was confirmed by Western blot. Control shRNA LTV particles containing a scrambled sequence were also used (not depicted).
Statistics
Values are expressed as the mean ± SEM. The significance of differences was established by Student’s t test or Pearson product-moment correlation coefficient with a two-tailed p-value (PRISM 4.0; GraphPad Software, Inc.) when indicated. The curve-fitting procedure used a nonlinear least-squares (Gauss-Newton) algorithm; results were displayed in linear format. Goodness of fit was judged by the χ2 criterion and by inspection for systematic nonrandom trends in the difference plot. A value of P < 0.05 was considered significant.
RESULTS
Kv1.3 and Kv1.5 generate Kv currents in Raw 264.7 macrophages
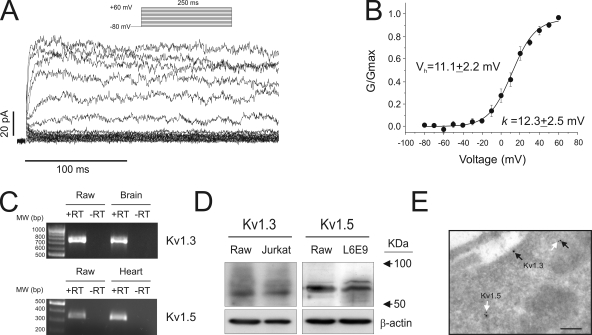
Kv currents were evoked in Raw cells by applying depolarizing pulses from a holding potential of −80 mV to different depolarizing voltages from −80 to +60 mV in 10-mV steps (Fig. 1 A). Fig. 1 B plots normalized conductance against test potential. The threshold for activation was about −20 mV. Vh and k slope were 11.1 ± 2.2 and 12.3 ± 2.5 mV, respectively. Previous work indicates that macrophages exhibit Kv currents mainly generated by Kv1.3 and Kv1.5 channels (Vicente et al., 2003, 2005, 2006, 2008; Villalonga et al., 2007). Therefore, we analyzed the presence of these channels in Raw macrophages. To analyze the K+ channel mRNA expression, we performed RT-PCR analysis. Mouse brain and heart RNAs were used as positive controls for Kv1.3 and Kv1.5, respectively. Fig. 1 C demonstrates that Kv1.3 and Kv1.5 mRNA were detected in Raw macrophages in the presence (+RT), but not in the absence, of the retrotranscriptase (−RT). In addition, a specific Kv1.3 and Kv1.5 signal was obtained by Western blot (Fig. 1 D). As expected, Kv1.3 protein was detected in macrophages and Jurkat T cells, whereas Raw cells shared Kv1.5 expression with L6E9 skeletal muscle myoblasts (Villalonga et al., 2008). We have recently shown that although Kv1.3 may form homotetrameric structures, and may be located at the membrane surface, Kv1.5 mostly coassociates with Kv1.3 to form heterotetrameric channels in macrophages (Vicente et al., 2006; Villalonga et al., 2007). In addition, the presence of Kv1.5 impairs the membrane surface location of the complex (Martínez-Mármol et al., 2008; Vicente et al., 2008). Electron microscopic immunocytochemical detection studies with specific antibodies showed that although Kv1.3 was detected at the membrane surface, many heteromeric structures containing Kv1.5 were located intracellularly (Fig. 1 E). Collectively, these data indicate that Raw 264.7 macrophages have outward delayed rectifier K+ currents that are mainly conducted by Kv1.3 and Kv1.5 channels.
Figure 1.
Macrophages express Kv1.3 and Kv1.5. Cells were held at −80 mV, and pulse potentials were applied as indicated. (A) Representative traces of delayed rectifier K+ currents. (B) Steady-state activation curve of the outward current. Conductance was plotted against test potentials. (C) mRNA expression of Kv1.3 and Kv1.5 in Raw 264.7 cells. Mouse brain and heart RNA were used as positive controls for Kv1.3 and Kv1.5, respectively. PCR reactions were performed in the presence (+) or absence (−) of the retrotranscriptase reaction. (D) Kv1.3 and Kv1.5 protein expression in Raw macrophages. Jurkat T lymphocytes and L6E9 skeletal muscle myoblasts were used as positive controls for Kv1.3 and Kv1.5, respectively. (E) Immunocytochemical electron microscopic detection of Kv1.3 and Kv1.5 proteins. Arrows show specific channel protein localization. Black arrow, Kv1.3; white arrow, Kv1.5; bar, 0.20 µm.
Immunomodulation of macrophages triggers differential biophysical and pharmacological consequences
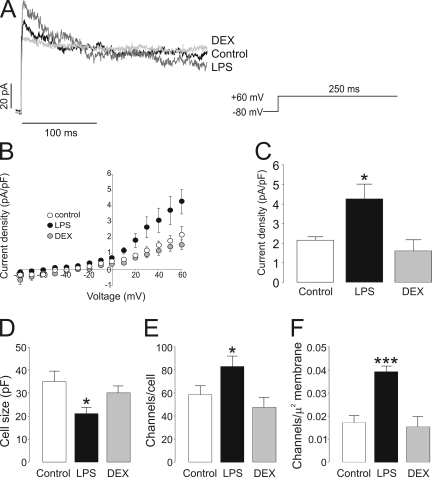
Once Kv currents were characterized and the molecular basis of the responsible channels was identified, we next analyzed their biophysical characteristics upon activation or immunosuppression in macrophages (Fig. 2). Raw cells were either activated with LPS or cultured in the presence of DEX. Fig. 2 A shows representative traces from a single depolarizing 250-ms pulse potential from −80 to +60 mV. Unlike DEX, LPS slightly increased the peak current amplitude. However, the inactivation time course was clearly affected by both treatments. While LPS accelerated, DEX slowed it. The effects of LPS and DEX on Raw cells were analyzed as the changes in current density observed at different test potentials during the application of 250-ms depolarizing pulses from −80 to +60 mV in 10-mV steps. The current density versus voltage relationships are depicted in Fig. 2 B. Once the channels opened (above −20 mV), LPS, but not DEX, increased currents at all depolarizing voltages applied (from −20 to +60 mV). While LPS increased peak currents (at +60 mV) up to twofold, DEX slightly decreased the current amplitude (Fig. 2 C). This apparent discrepancy between current amplitudes and current densities was related to the size of the cell. Raw cells incubated with LPS were ∼40% smaller than control cells (Fig. 2 D). On the other hand, apparently DEX did not modify the cell size.
Figure 2.
Voltage-dependent K+ currents in Raw 264.7 macrophages. (A) Representative traces of delayed rectifier K+ currents in control, LPS-, and DEX-treated cells. Macrophages were treated with 100 ng/ml LPS and 1 µM DEX for 24 h. Cells were held at −80 mV, and currents were elicited by a depolarizing pulse to +60 mV (250-ms duration). Black trace, control; dark gray trace, LPS; light gray trace, DEX. (B) Current density (pA/pF) versus voltage relationship of K+ currents. Current density was calculated as a function of peak amplitude and the cell capacitance of each recorded cell. (C) Peak current density at +60 mV. (D) Cell capacitance (pF) from the same cell population from B. (E) Channel density was calculated from the conductance of Kv1.3 (13 pS) and Kv1.5 (8 pS) following the real-time PCR results in Fig. 4 and the Kv1.3/ Kv1.5 ratio shown in Fig. 8. (F) The results in E as a function of the plasma cell membrane surface. White (bar or circle), control; black (bar or circle), LPS; gray (bar or circle), DEX. *, P < 0.05; ***, P < 0.001 versus control; n > 10 cells per group (Student’s t test).
The number and density of the channels increased during LPS-induced activation (Fig. 2, E and F, respectively). While at +60 mV, resting cells expressed ∼60 channels/cell with a density of ∼0.018 channel/µm2, and activated cells showed ∼82 channels/cell and ∼0.04 channel/µm2 (P < 0.05 and P < 0.001 vs. control for number and density, respectively). On the other hand, DEX did not modify the number or the density of the channels.
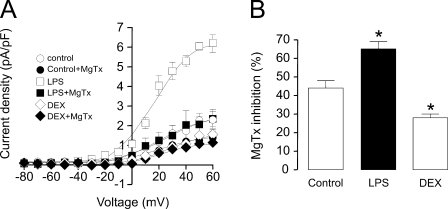
Different Kv1.3/Kv1.5 hetero-oliogomeric structures lead to distinct pharmacological properties of the channel (Vicente et al., 2006). Although Kv1.3 and Kv1.3/Kv1.5 heteromeric channels are sensitive to MgTx, a highly specific Kv1.3 blocker, Kv1.5 homotetrameric structures are resistant (Vicente et al., 2006). As described previously (Villalonga et al., 2007), pharmacological analysis of the Kv currents in the presence of MgTx demonstrated that K+ currents were fully sensitive to MgTx in Raw macrophages (not depicted). When K+ currents were analyzed in the presence of 750 pM MgTx, a concentration close to the IC50 of the toxin (Villalonga et al., 2007), K+ currents were inhibited (Fig. 3 A). The efficiency of MgTx in LPS-treated macrophages was noticeable, as the majority of LPS-dependent, induced K+ current was blocked (Fig. 3 A). The percentage of inhibition of the peak current density at +60 mV in the presence of MgTx (Fig. 3 B) indicated that while in control cells the current was blocked 44 ± 3%, this inhibition was 65 ± 4 and 28 ± 2% in the presence of LPS and DEX, respectively (n = 4–6; P < 0.05 vs. control). These results further confirm that although Kv1.3 could form homomeric channels in macrophages, Kv1.5 mostly coassociates with Kv1.3 to generate multiple heteromeric structures with distinct pharmacological properties.
Figure 3.
MgTx inhibits K+ currents in macrophages. Cells were held at −80 mV, and pulse potentials were applied as indicated in Fig. 1 in the presence or absence of 750 pM MgTx. (A) Current density (pA/pF) versus voltage relationship of K+ currents. Current density was calculated as a function of the peak amplitude and the capacitance for each recorded cell in the presence (black symbols) and absence (white symbols) of MgTx. (B) Percentage of MgTx inhibition at the peak current density (+60 mV). White bar, control; black bar, LPS; gray bar, DEX. *, P < 0.05 versus control; n = 4–6 cells per group (Student’s t test).
Differential expression of Kv1.3 and Kv1.5 upon activation and immunosuppression
Fig. 4 A illustrates the Kv1.3 and Kv1.5 gene expression after the addition of LPS and DEX. Although Kv1.3 is differentially regulated by LPS and DEX, Kv1.5 remained almost unchanged. However, although the expression of Kv1.3 increased up to 2.5-fold in LPS-treated macrophages, its expression decreased by 50% in the presence of DEX. Similar changes were obtained for the Kv1.3 and Kv1.5 protein abundance in macrophages incubated for 24 h in the presence of LPS and DEX (Fig. 4 B). No differences were observed with Kv1.5 throughout the study. The expression of the iNOS was used as a marker of macrophage activation (Soler et al., 2001). As expected, iNOS was induced in the presence of LPS, but not DEX. Fig. 4 (C and D) represents the quantification of at least three independent blots. Collectively, these data indicate that LPS-induced activation led to long-term increases in current densities of outward K+ currents in accordance with an induction in Kv1.3 mRNA and protein expression. On the contrary, DEX, which slightly decreased current densities, decreased Kv1.3 expression. Because neither LPS nor DEX incubation produced K+ currents resistant to MgTx (not depicted), our results are consistent with an increased or decreased number of Kv1.3 subunits, either as homomeric Kv1.3 or hybrid Kv1.3/Kv1.5 channels upon activation or immunosuppression.
Figure 4.
Activation and immunosuppression differentially regulates Kv1.3 and Kv1.5 in macrophages. Control cells were incubated for 24 h in the presence of LPS and DEX. Samples were collected after the addition of agents, and Kv1.3 and Kv1.5 expression was analyzed. (A) Kv1.3 (white bar) and Kv1.5 (black bar) relative mRNA expression by real-time PCR. Values are the mean ± SEM (n = 4). Significant differences were only found with Kv1.3 (*, P < 0.05; **, P < 0.01 vs. control; Student’s t test). Using standard curves, fold variation in arbitrary units (A.U.) was normalized to 18S relative quantity (RQ) as follows: (Kv1.5 or Kv1.3 RQ (LPS or DEX)/18S RQ (LPS or DEX))/(Kv1.5 or Kv1.3 RQ (control)/18S RQ (control)). (B) Kv1.3, Kv1.5, and iNOS protein expression in macrophages. Representative blots are shown. (C and D) Kv1.3 (C) and Kv1.5 (D) quantification of data in B. Values were normalized to β actin. In all cases, arbitrary units are standardized to the control value in the absence of treatment. White bars, control; black bars, LPS; gray bars, DEX. Values are the mean ± SEM of at least three independent experiments. *, P < 0.05; **, P < 0.01 versus control (Student’s t test).
Inactivation properties are differentially regulated during immunomodulation
As demonstrated (Fig. 2 A), Kv currents recorded from Raw cells inactivate with a different time course in the absence or presence of LPS or DEX. Inactivation parameters are useful to evaluate possible differences in the tetrameric K+ channel phenotype. Kv1.3 is well characterized by its C-type inactivation, which is absent in Kv1.5 (Po et al., 1992; Gutman et al., 2005). Because C-type inactivation involves the cooperative interaction of all four subunits, this parameter could be used to determine heterotetramers (Panyi et al., 1995). Fig. 5 shows the time course of K+ current inactivation after applying a depolarizing pulse from −80 to +60 mV for 5 s (Fig. 5 A). Although K+ currents inactivated with a time constant of inactivation of 1,630 ± 40 ms (n = 5) in control macrophages, in LPS-treated cells, it was 885 ± 35 ms (n = 6; P < 0.05 vs. control). However, this process became much slower in Raw cells treated with DEX (2,940 ± 70 ms; n = 5; P < 0.05 vs. control) (Fig. 5 B). Our results further support the notion that Kv currents generated upon depolarization in macrophages are generated by Kv channels with different subunit stoichiometry upon activation or immunosuppression.
Figure 5.
Biophysical properties of the C-type inactivation of K+ currents in Raw macrophages. (A) Representative traces of C-type inactivation. Cells were held at −80 mV, and pulse potentials were applied from −80 to +60 mV for 5 s. For comparison, the intensity in each group has been normalized. (B) Time constant of inactivation (τ). Values are the mean ± SEM of at least 10 independent cells. *, P < 0.05 versus control (Student’s t test).
Cumulative inactivation of certain K+ channels is characterized by an inactivation that accumulates during repetitive depolarizing pulses because recovery during the interpulse interval is incomplete (Grissmer et al., 1994). Fig. 6 (A–C) depicts representative traces of K+ currents in macrophages in the presence and absence of LPS or DEX during the application of a train of repetitive pulses, each of 200 ms at a frequency of 4 Hz. The standardized peak current amplitude rapidly diminished in control Raw cells (Fig. 6 A). In LPS-activated macrophages, the accumulation of inactivation increased (Fig. 6 B). However, this was much less apparent in Raw cells treated with DEX (Fig. 6 C). Although the cumulative inactivation was 12 ± 3% (n = 7) in control cells, in LPS-induced cells, activation rose to 31 ± 6% (n = 7; P < 0.01 vs. control) (Fig. 6 D). In contrast, incubation with DEX led to a marked decrease, and cumulative inactivation was almost absent (0.5 ± 3; n = 7; P < 0.01 vs. control). The normalized area of the K+ currents demonstrated that, contrary to DEX, cumulative inactivation was noticeable in control and LPS-activated macrophages from the first pulse (Fig. 6 E). Once again, these results indicate that Kv1.5 associates with Kv1.3, forming different heteromeric structures, and this stoichiometry may be changed by different stimuli.
Figure 6.
Biophysical properties of the cumulative inactivation of K+ currents in Raw macrophages. (A–C) Representative traces showing cumulative inactivation of K+ currents. Cells were held at −80 mV, and currents were elicited by a train of eight depolarizing voltage steps (200-ms duration) to +60 mV once every 400 ms. (A) Control. (B) LPS. (C) DEX. (D) Percentage of cumulative inactivation at the peak current. The percentage was calculated as a result of the difference between the peak current at the first pulse and the remaining current at the last. (E) Plot of normalized current area versus pulse. The current amplitude became progressively smaller from the first trace to the last in control and LPS, but not in DEX-treated macrophages. Inset represents K+ current area decay from control and DEX-treated macrophages for magnification. Values are the mean ± SEM of at least 10 independent cells. **, P < 0.01 versus control (Student’s t test).
Kv1.3 gene silencing in Raw 264.7 macrophages counteracts LPS-dependent biophysical changes
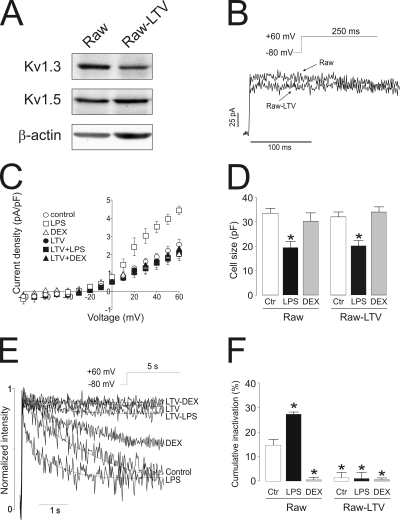
LPS and DEX regulated Kv1.3 expression in agreement with changes in electrophysiological properties, which suggest changes of Kv1.3 subunits either as Kv1.3 homotetrameric or Kv1.3/Kv1.5 heterotetrameric channels. Therefore, we next analyzed whether Kv1.3 gene silencing impairs LPS and DEX modulation. LTV shRNA Kv1.3 infection in macrophages led to an ∼65% decrease in Kv1.3 protein expression (Fig. 7 A). Although macroscopic outward K+ currents were similar between control (Raw) and LTV-infected cells (Raw-LTV), K+ currents in LTV cells were slightly lower and inactivate less than control (Fig. 7 B). Fig. 7 C demonstrates that neither LPS nor DEX modulated the current density in LTV cells. However, LPS still triggered changes in the cell size (Fig. 7 D). Unlike in Raw macrophages, 5-s pulses did not inactivate currents in Raw-LTV cells (Fig. 7 E). In addition, LPS and DEX did not significant modify the time constant for inactivation in LTV cells. Cumulative inactivation was also affected in LTV cells (Fig. 7 F). Although LPS triggered an increase in Raw macrophages, DEX, LTV, and LTV-treated cells showed no inactivation. These results show that (a) K+ currents in LTV cells did not recapitulate Kv1.3 characteristics, and (b) LPS and DEX incubation did not alter the electrophysiological phenotype.
Figure 7.
Kv1.3 gene silencing abolishes LPS- and DEX-dependent biophysical changes in macrophages. LTV shRNA (mouse) Kv1.3 was used to silence the Kv1.3 gene in Raw 264.7 macrophages. (A) A representative Western blot. Note that although Kv1.3 expression is lower in LTV, relative Kv1.5 abundance was similar in both groups. Raw, control macrophages; Raw-LTV, LTV-silenced Kv1.3 macrophages. (B) Representative traces of K+ currents in macrophages. Cells were held at −80 mV, and currents were elicited by a depolarizing pulse to +60 mV (250-ms duration). (C) Current density (pA/pF) versus voltage relationship of K+ currents. Current density was calculated as a function of peak amplitude and the capacitance of each recorded cell in the presence or absence of LPS and DEX. (D) Cell capacitance (pF) from the same cell population from B. (E) Representative traces of C-type inactivation. Cells were held at −80 mV, and pulse potentials were applied from −80 to +60 mV for 5 s. For comparison, the intensity in each group has been normalized. (F) Percentage of cumulative inactivation at the peak current. Cells were held at −80 mV, and currents were elicited by a train of eight depolarizing voltage steps (200-ms duration) to +60 mV once every 400 ms. The percentage was calculated as a result of the difference between the peak current at the first pulse and the remaining current at the last. (D and F) Raw, no infected Raw macrophages; Raw-LTV, lentivirus-infected Raw cells; white bars, control (no treatment); black bars, LPS; gray bars, DEX. Values are the mean ± SEM of six to eight independent cells. *, P < 0.001 versus control (Student’s t test).
Our results suggest that LPS and DEX regulated Kv1.3, leading to pharmacologically distinct channels. MgTx differentially inhibited K+ currents upon activation and immunosuppression in Raw macrophages (Fig. 3). Therefore, we analyzed Kv currents in Raw-LTV cells in the presence of 750 pM MgTx. Unlike Raw control cells, Kv currents in Raw-LTV cells were highly resistant to the toxin. Thus, peak current densities (+60 mV) were 2.22 ± 0.02 versus 2.19 ± 0.12 pA/pF (control), 2.31 ± 0.02 versus 2.25 ± 0.03 pA/pF (LPS), and 2.33 ± 0.04 versus 2.28 ± 0.33 pA/pF (DEX) in the absence or presence of MgTx, respectively (n = 4–6).
DISCUSSION
As regulation of macrophage function involves ion channels, this study was performed to elucidate the influence of proinflammatory and antiinflammatory agents on macrophage Kv channel activity. Here, we describe, for the first time, that Kv1.3/Kv1.5 heteromeric channel expression and their biophysical and pharmacological properties change differentially upon activation and immunosuppression. Our results have physiological relevance indicating that Kv heteromeric expression is important as an early regulatory step, and that fine-tuning modulation of their expression is crucial to the specific membrane signaling that triggers the appropriate immune response. Activated macrophages secrete proinflammatory substances like cytokines (e.g., TNF-α) or NO with potential cellular toxicity (Xaus et al., 2001). This release activity is triggered by LPS (Soler et al., 2001). In previous work, we showed that LPS and TNF-α induce Kv1.3 in macrophages (Vicente et al., 2003, 2005, 2006; Villalonga et al., 2007). Those experiments were designed to characterize the regulation of individual channels. However, accumulating evidence indicates that Kv1.3 and Kv1.5 associate in mononuclear phagocytes to generate distinct hybrid channels (Villalonga et al., 2007; Martínez-Mármol et al., 2008; Vicente et al., 2008). In the present work, we address this issue, characterizing the effects of LPS on the Kv1.3/Kv1.5 heterotetrameric channel and analyzing biophysical and pharmacological consequences. Unlike Kv1.5, Kv1.3 was induced; therefore, heteromeric channels contain more Kv1.3 units in LPS-activated macrophages than in control ones. Because C-type and cumulative inactivation, which are Kv1.3 hallmarks, need the cooperative interaction of the four subunits (Panyi et al., 1995), the altered subunit stoichiometry was in accordance with these biophysical changes. The presence of Kv1.3 at the membrane surface assures the specific activation, and the presence of Kv1.5 in the hybrid channel shifts the activation curve toward depolarizing potentials (Vicente et al., 2006). Indeed, a +25-mV change modifies interleukin 2 production, thus reducing the activation and the antibody production by lymphocytes (Freedman et al., 1992). In addition, as we have suggested previously and further confirmed here, Kv1.3/ Kv1.5 heteromers tend to be more intracellular, fine-tuning the response (Vicente et al., 2008). Thus, Kv1.5 impairs the location of Kv1.3 in lipid raft microdomains, but this is partially reversed by incubation with LPS (Martínez-Mármol et al., 2008; Vicente et al., 2008). In fact, lipid rafts play a role in LPS-induced signaling in macrophages (Olsson and Sundler, 2006).
Kv1.3 is crucial to the immune response. However, the role of Kv1.5 in the myeloid lineage is under debate. Pyo et al. (1997) demonstrated that outward K+ channels induced by LPS are immunologically related to Kv1.5. Furthermore, although some studies support the unique expression of Kv1.3 without an apparent function, others claim a pivotal role for Kv1.5 (Mackenzie et al., 2003; Park et al., 2006). However, most of the literature describes Kv1.3 and Kv1.5 in the myeloid lineage, and an association of the two proteins has been demonstrated (Kotecha and Schlichter, 1999; Vicente et al., 2003, 2005, 2006, 2008; Fordyce et al., 2005; Judge et al., 2006; Mullen et al., 2006; Pannasch et al., 2006; Villalonga et al., 2007). Indeed, microglia activation by LPS involves the activation of Kv1.5 and Kv1.3 (Pannasch et al., 2006). In Kv1.5−/− mice, the LPS-induced NO production is completely absent, indicating that Kv1.5 expression must be necessary for NO production (Pannasch et al., 2006). Just as in myoblasts, Kv1.5 could have a key role when predominant (Villalonga et al., 2008). Although Kv1.3 may form homotetramers, Kv1.5 seems to be mostly involved in heteromers and could be essential for the function of the complex (Pannasch et al., 2006; Villalonga et al., 2007). Similar to what we found in macrophages, DEX inhibits Kv1.3 in Jurkat T cells, thereby avoiding Ca2+ entry through Ca2+ release–activated Ca2+ channels (Lampert et al., 2003). Because an increase in Ca2+ ions is essential to leukocyte activation (Cahalan and Chandy, 1997), DEX exerts antiinflammatory effects. In addition, down-regulation of Kv1.3 impairs T cell volume regulatory mechanisms, which are reversed by transfection with Kv1.3 (Lampert et al., 2003). However, this is not the case in macrophages. Cell volume regulatory mechanisms are important during the macrophage immune response. The Kv1.3/Kv1.5 composition of the channel assures cell volume control. We found that DEX did not exert changes in the cell size. However, unlike inactivating channels such as Kv1.4, Kv1.5 is involved in regulatory volume control (Felipe et al., 1993). In this context, DEX up-regulates aquaporins and amiloride-sensitive osmotic-activated Na+ channels in A549 lung cells and U937 macrophages, respectively; these two types of channels are also involved in the osmotic control (Gamper et al., 2000; Ben et al., 2008).
Unlike LPS, which activates macrophages secreting proinflammatory substances, GCs, used in the treatment of immune-mediated inflammatory diseases, inhibit the expression of many inflammatory mediators. GCs, probably the most powerful endogenous inhibitors of the innate immune reaction across the organism, are mainly synthesized in the adrenal medulla and regulate many cellular functions with both genomic and nongenomic effects. We found that DEX decreased Kv1.3 expression without affecting Kv1.5. There is a large body of evidence describing discordant DEX effects on channels. Thus, DEX increases Kv1.5, but not Kv1.4 and Kv2.1, expression in pituitary cells in vivo and in vitro (Attardi et al., 1993). In contrast, Kv3.2 is rapidly down-regulated in vivo after corticosterone administration in the hippocampus that probably results in enhanced neuroexcitability (Morsink et al., 2007). GCs, also synthesized locally in cardiac cells, induce Kv1.5 in the rat heart ventricle and skeletal muscle, but not in the atrium, lung, or hypothalamus (Levitan et al., 1996). We found that DEX did not regulate Kv1.5 in macrophages. These results indicate that tissue- and Kv isoform–specific mechanisms may be underlying the corticosteroid endocrine response. For instance, Takimoto and Levitan (1994) found that GCs caused an induction of Kv1.5 gene expression in ventricles of adrenalectomized adult rat. Kv1.5 contributes to Islow in mouse ventricular myocytes. Contrary to Islow, DEX decreases the density of Ito but maintains the biophysical properties and contributes to GC-induced action potential prolongation (Wang et al., 1999). However, differential regulation of Kv1.3 and Kv1.5 in macrophages leads to molecularly distinct complexes with differentiated biophysical properties. Therefore, changes in the myeloid lineage, mainly due to the heteromeric composition, fine-tune the membrane potential upon immunomodulation.
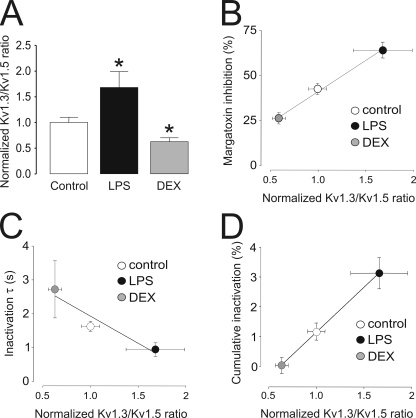
The relationship between Kv expression and biophysical and pharmacological parameters clearly indicates a trend that fits fairly well with changes in the Kv1.3/Kv1.5 heterotetrameric ratio. In addition, our findings suggest that in macrophages, this is mostly generated by Kv1.3 regulation rather than a change in Kv1.5 abundance. Thus, although LPS-induced activation increased the Kv1.3/Kv1.5 ratio for ∼70% of cells, DEX decreased this value in 40% (Fig. 8 A). The biophysical and pharmacological properties were altered in a similar way. The percentage of inhibition, calculated as a function of the remaining fraction in the presence of 750 pM MgTx, the inactivation time constant, and the percentage of cumulative inactivation plotted against the normalized Kv1.3/Kv1.5 ratio gave Pearson correlation coefficients of r = 0.998, r = 0.965, and r = 0.999, respectively (Fig. 8, B–D). Although this pattern is similar to what was observed in heterologous expression systems, some differences between models may exist (Vicente et al., 2006). Many heterologous systems do not express the complete machinery to recapitulate the native Kv complex. For instance, HEK-293 cells lack the expression of Kvβ and KCNE regulatory subunits that are present in native cells (Uebele et al., 1996; Vicente et al., 2005; Arias et al., 2007; Roura-Ferrer et al., 2009; Solé et al., 2009). Diverse Kv channels are produced by tetramerization of channel α subunits that can be encoded by multiple genes. In GH3 pituitary cells, almost all detectable Kv1.4 is associated with Kv1.5, whereas only ∼30% of Kv1.5 protein is present as Kv1.4/Kv1.5 heteromeric channels. DEX specifically increases Kv1.5 homomeric channels, but not Kv1.4/Kv1.5 heteromeric complexes (Takimoto and Levitan, 1996). Thus, hormone induction of one K+ channel subunit gene differentially influences expression of cell surface homomeric and heteromeric channels, leading to specific alteration of excitability. In macrophages, however, pharmacological studies demonstrated that, contrary to pituitary cells, homomeric Kv1.5 channels are not present (Vicente et al., 2006, 2008; Villalonga et al., 2007). We therefore assume that Kv1.5 form heteromers with Kv1.3 in macrophages with properties distinct from homomeric complexes. Similar conclusions have been made in blood-derived dendritic cells in which pharmacological blockade of Kv1.3 and Kv1.5, and with stable expression of an adenoviral Kv1.X dominant negative, suggests a functional role for both proteins (Judge et al., 2006; Mullen et al., 2006). However, by silencing Kv1.3, we demonstrated that an increase of Kv1.3 subunits, either in homotetrameric or heterotetrameric structures, were mainly responsible for the biophysical changes observed during the LPS-induced activation.
Figure 8.
Variations in the Kv1.3/Kv1.5 ratio correlate with changes in biophysical properties of K+ currents in macrophages. (A) Kv1.3/Kv1.5 ratios in Raw cells cultured in the presence or absence of LPS and DEX were calculated from real-time PCR experiments in Fig. 4 and standardized to the value of control cells. Values are the mean ± SEM of at least three independent experiments. *, P < 0.05 versus control (Student’s t test). (B) Percentage of MgTx inhibition plotted against the Kv1.3/Kv1.5 ratio. (C) Time constant of inactivation from Fig. 4 (τ) plotted against the Kv1.3/Kv1.5 ratio. (D) Percentage of cumulative inactivation plotted against the Kv1.3/Kv1.5 ratio. The Pearson product-moment correlation coefficient using a two-tailed p-value was calculated (P < 0.0393, P < 0.0355, and P < 0.0116 for A, B, and C, respectively). White (bar or circle), control; black (bar or circle), LPS; gray (bar or circle), DEX.
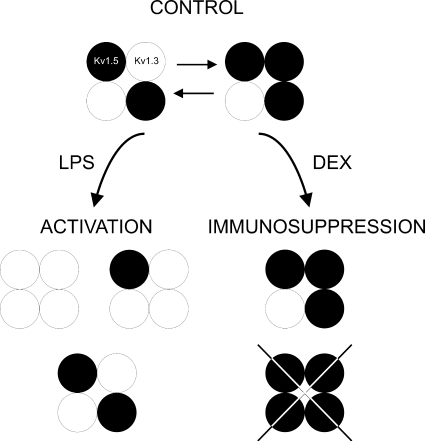
Fig. 9 illustrates several possible heterotetrameric structures in macrophages upon activation and immunosuppression. Macrophages express Kv1.3 and Kv1.5. Because MgTx abolished Kv currents regardless of the immunomodulatory agent, we can conclude that, similar to human dendritic cells (Zsiros et al., 2009), Kv1.5 apparently does not form homomeric complexes. However, the presence of structures solely formed by Kv1.3 cannot be ruled out. We have previously shown that, based on biophysical, molecular, and pharmacological studies, the major Kv channel in Raw macrophages is generated by structures where the predominant subunit is Kv1.5 (Villalonga et al., 2007). Different Kv1.3/Kv1.5 ratios are responsible for the biophysical consequences, fine-tuning the immune response after GC exposure or during an insult.
Figure 9.
Diagram of major heterotetrameric structures in Raw macrophages upon immunomodulation. Raw cells express Kv1.3 and Kv1.5. Because MgTx abolished Kv currents, Kv1.5 does not form homomeric complexes. However, molecular, pharmacological, and biophysical data indicate that Raw cells express more Kv1.5 than Kv1.3. LPS-induced macrophages increased the number of Kv1.3 subunits at the complex. However, the immunosuppressant DEX decreases Kv1.3, which generates Kv1.5-predominant heteromeric channels. Different Kv1.3/Kv1.5 molecular ratios are responsible for the biophysical properties that lead to functional consequences during activation and immunosuppression. White circles, Kv1.3; black circles, Kv1.5.
GCs are secreted in stress conditions. Therefore, they not only affect the cardiac workload, but also the immune response because they are effective repressors of the immune system. Activated GCs are able to interfere with several proinflammatory signaling cascades at different levels. Exogenous GCs are used to treat numerous inflammatory disorders; however, the mechanisms underlying their actions are incompletely understood. Our results could bring some light to this issue because the beneficial effects of GCs in antiinflammatory therapies are linked to Kv channel–mediated immunosuppression. Therefore, the efficacy of GCs in the treatment of inflammatory disorders could be due, at least in part, to their effects on the expression of Kv1.3 and Kv1.5 channels.
Acknowledgments
We thank C. López-Iglesias (Serveis Cientificotècnics, Universitat de Barcelona) for the electron microscopy. We also thank the editorial assistance of American Journal Experts.
This work was supported by the Ministerio de Ciencia e Innovación (MICINN), Spain (grants BFU2005-00695, BFU2008-00431, and CSD2008-00005 to A. Felipe, and grants SAF2007-65868 and FIS D06/0014/0006 to C. Valenzuela). N. Villalonga and J. Bielanska held fellowships from the MICINN. M. David held an FIS RD06/0014/0006 contract. N. Comes is supported by the Juan de la Cierva program (MICINN).
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper:
- DEX
- dexamethasone
- GC
- glucocorticoid
- iNOS
- inducible nitric oxide synthase
- Kv
- voltage-dependent potassium
- LPS
- lipopolysaccharide
- LTV
- lentiviral
- MgTx
- margatoxin
- NO
- nitric oxide
- RT
- reverse transcription
References
- Arias C., Guizy M., David M., Marzian S., González T., Decher N., Valenzuela C. 2007. Kvbeta1.3 reduces the degree of stereoselective bupivacaine block of Kv1.5 channels. Anesthesiology. 107:641–651 10.1097/01.anes.0000282100.32923.5c [DOI] [PubMed] [Google Scholar]
- Attardi B., Takimoto K., Gealy R., Severns C., Levitan E.S. 1993. Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Receptors Channels. 1:287–293 [PubMed] [Google Scholar]
- Ben Y., Chen J., Zhu R., Gao L., Bai C. 2008. Upregulation of AQP3 and AQP5 induced by dexamethasone and ambroxol in A549 cells. Respir. Physiol. Neurobiol. 161:111–118 10.1016/j.resp.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Cahalan M.D., Chandy K.G. 1997. Ion channels in the immune system as targets for immunosuppression. Curr. Opin. Biotechnol. 8:749–756 10.1016/S0958-1669(97)80130-9 [DOI] [PubMed] [Google Scholar]
- Eder C. 1998. Ion channels in microglia (brain macrophages). Am. J. Physiol. 275:C327–C342 [DOI] [PubMed] [Google Scholar]
- Felipe A., Snyders D.J., Deal K.K., Tamkun M.M. 1993. Influence of cloned voltage-gated K+ channel expression on alanine transport, Rb+ uptake, and cell volume. Am. J. Physiol. 265:C1230–C1238 [DOI] [PubMed] [Google Scholar]
- Fordyce C.B., Jagasia R., Zhu X., Schlichter L.C. 2005. Microglia Kv1.3 channels contribute to their ability to kill neurons. J. Neurosci. 25:7139–7149 10.1523/JNEUROSCI.1251-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franqueza L., Valenzuela C., Delpón E., Longobardo M., Caballero R., Tamargo J. 1998. Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br. J. Pharmacol. 125:969–978 10.1038/sj.bjp.0702129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B.D., Price M.A., Deutsch C.J. 1992. Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J. Immunol. 149:3784–3794 [PubMed] [Google Scholar]
- Gamper N., Huber S.M., Badawi K., Lang F. 2000. Cell volume-sensitive sodium channels upregulated by glucocorticoids in U937 macrophages. Pflugers Arch. 441:281–286 10.1007/s004240000410 [DOI] [PubMed] [Google Scholar]
- González T., Longobardo M., Caballero R., Delpón E., Tamargo J., Valenzuela C. 2001. Effects of bupivacaine and a novel local anesthetic, IQB-9302, on human cardiac K+ channels. J. Pharmacol. Exp. Ther. 296:573–583 [PubMed] [Google Scholar]
- Grissmer S., Nguyen A.N., Aiyar J., Hanson D.C., Mather R.J., Gutman G.A., Karmilowicz M.J., Auperin D.D., Chandy K.G. 1994. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 45:1227–1234 [PubMed] [Google Scholar]
- Gutman G.A., Chandy K.G., Grissmer S., Lazdunski M., McKinnon D., Pardo L.A., Robertson G.A., Rudy B., Sanguinetti M.C., Stühmer W., Wang X. 2005. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 57:473–508 10.1124/pr.57.4.10 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. 3rd ed Sinauer Associates, Inc., Sunderland, MA: 814 pp [Google Scholar]
- Judge S.I., Lee J.M., Bever C.T., Jr., Hoffman P.M. 2006. Voltage-gated potassium channels in multiple sclerosis: overview and new implications for treatment of central nervous system inflammation and degeneration. J. Rehabil. Res. Dev. 43:111–122 10.1682/JRRD.2004.09.0116 [DOI] [PubMed] [Google Scholar]
- Kotecha S.A., Schlichter L.C. 1999. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J. Neurosci. 19:10680–10693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert A., Müller M.M., Berchtold S., Lang K.S., Palmada M., Dobrovinskaya O., Lang F. 2003. Effect of dexamethasone on voltage-gated K+ channels in Jurkat T-lymphocytes. Pflugers Arch. 447:168–174 10.1007/s00424-003-1148-2 [DOI] [PubMed] [Google Scholar]
- Levitan E.S., Hershman K.M., Sherman T.G., Takimoto K. 1996. Dexamethasone and stress upregulate Kv1.5 K+ channel gene expression in rat ventricular myocytes. Neuropharmacology. 35:1001–1006 10.1016/0028-3908(96)00095-0 [DOI] [PubMed] [Google Scholar]
- Lloberas J., Soler C., Celada A. 1998. Dexamethasone enhances macrophage colony stimulating factor- and granulocyte macrophage colony stimulating factor-stimulated proliferation of bone marrow-derived macrophages. Int. Immunol. 10:593–599 10.1093/intimm/10.5.593 [DOI] [PubMed] [Google Scholar]
- Mackenzie A.B., Chirakkal H., North R.A. 2003. Kv1.3 potassium channels in human alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L862–L868 [DOI] [PubMed] [Google Scholar]
- Martínez-Mármol R., Villalonga N., Solé L., Vicente R., Tamkun M.M., Soler C., Felipe A. 2008. Multiple Kv1.5 targeting to membrane surface microdomains. J. Cell. Physiol. 217:667–673 10.1002/jcp.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink M.C., Van Gemert N.G., Steenbergen P.J., Joëls M., De Kloet E.R., Datson N.A. 2007. Rapid glucocorticoid effects on the expression of hippocampal neurotransmission-related genes. Brain Res. 1150:14–20 10.1016/j.brainres.2007.02.083 [DOI] [PubMed] [Google Scholar]
- Mullen K.M., Rozycka M., Rus H., Hu L., Cudrici C., Zafranskaia E., Pennington M.W., Johns D.C., Judge S.I., Calabresi P.A. 2006. Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann. Neurol. 60:118–127 10.1002/ana.20884 [DOI] [PubMed] [Google Scholar]
- Olsson S., Sundler R. 2006. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 43:607–612 10.1016/j.molimm.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Pannasch U., Färber K., Nolte C., Blonski M., Yan Chiu S., Messing A., Kettenmann H. 2006. The potassium channels Kv1.5 and Kv1.3 modulate distinct functions of microglia. Mol. Cell. Neurosci. 33:401–411 10.1016/j.mcn.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Panyi G., Sheng Z., Deutsch C. 1995. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys. J. 69:896–903 10.1016/S0006-3495(95)79963-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G., Varga Z., Gáspár R. 2004. Ion channels and lymphocyte activation. Immunol. Lett. 92:55–66 10.1016/j.imlet.2003.11.020 [DOI] [PubMed] [Google Scholar]
- Park S.A., Lee Y.C., Ma T.Z., Park J.A., Han M.K., Lee H.H., Kim H.G., Kwak Y.G. 2006. hKv1.5 channels play a pivotal role in the functions of human alveolar macrophages. Biochem. Biophys. Res. Commun. 346:567–571 10.1016/j.bbrc.2006.05.149 [DOI] [PubMed] [Google Scholar]
- Po S., Snyders D.J., Baker R., Tamkun M.M., Bennett P.B. 1992. Functional expression of an inactivating potassium channel cloned from human heart. Circ. Res. 71:732–736 [DOI] [PubMed] [Google Scholar]
- Pyo H., Chung S., Jou I., Gwag B., Joe E.H. 1997. Expression and function of outward K+ channels induced by lipopolysaccharide in microglia. Mol. Cells. 7:610–614 [PubMed] [Google Scholar]
- Roura-Ferrer M., Etxebarria A., Solé L., Oliveras A., Comes N., Villarroel A., Felipe A. 2009. Functional implications of KCNE subunit expression for the Kv7.5 (KCNQ5) channel. Cell. Physiol. Biochem. 24:325–334 10.1159/000257425 [DOI] [PubMed] [Google Scholar]
- Solé L., Roura-Ferrer M., Pérez-Verdaguer M., Oliveras A., Calvo M., Fernández-Fernández J.M., Felipe A. 2009. KCNE4 suppresses Kv1.3 currents by modulating trafficking, surface expression and channel gating. J. Cell Sci. 122:3738–3748 10.1242/jcs.056689 [DOI] [PubMed] [Google Scholar]
- Soler C., Valdés R., Garcia-Manteiga J., Xaus J., Comalada M., Casado F.J., Modolell M., Nicholson B., MacLeod C., Felipe A., et al. 2001. Lipopolysaccharide-induced apoptosis of macrophages determines the up-regulation of concentrative nucleoside transporters Cnt1 and Cnt2 through tumor necrosis factor-alpha-dependent and -independent mechanisms. J. Biol. Chem. 276:30043–30049 10.1074/jbc.M101807200 [DOI] [PubMed] [Google Scholar]
- Takimoto K., Levitan E.S. 1994. Glucocorticoid induction of Kv1.5 K+ channel gene expression in ventricle of rat heart. Circ. Res. 75:1006–1013 [DOI] [PubMed] [Google Scholar]
- Takimoto K., Levitan E.S. 1996. Altered K+ channel subunit composition following hormone induction of Kv1.5 gene expression. Biochemistry. 35:14149–14156 10.1021/bi961290s [DOI] [PubMed] [Google Scholar]
- Takimoto K., Fomina A.F., Gealy R., Trimmer J.S., Levitan E.S. 1993. Dexamethasone rapidly induces Kv1.5 K+ channel gene transcription and expression in clonal pituitary cells. Neuron. 11:359–369 10.1016/0896-6273(93)90191-S [DOI] [PubMed] [Google Scholar]
- Uebele V.N., England S.K., Chaudhary A., Tamkun M.M., Snyders D.J. 1996. Functional differences in Kv1.5 currents expressed in mammalian cell lines are due to the presence of endogenous Kv beta 2.1 subunits. J. Biol. Chem. 271:2406–2412 10.1074/jbc.271.5.2406 [DOI] [PubMed] [Google Scholar]
- Vicente R., Escalada A., Coma M., Fuster G., Sánchez-Tilló E., López-Iglesias C., Soler C., Solsona C., Celada A., Felipe A. 2003. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J. Biol. Chem. 278:46307–46320 10.1074/jbc.M304388200 [DOI] [PubMed] [Google Scholar]
- Vicente R., Escalada A., Soler C., Grande M., Celada A., Tamkun M.M., Solsona C., Felipe A. 2005. Pattern of Kv beta subunit expression in macrophages depends upon proliferation and the mode of activation. J. Immunol. 174:4736–4744 [DOI] [PubMed] [Google Scholar]
- Vicente R., Escalada A., Villalonga N., Texidó L., Roura-Ferrer M., Martín-Satué M., López-Iglesias C., Soler C., Solsona C., Tamkun M.M., Felipe A. 2006. Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages. J. Biol. Chem. 281:37675–37685 10.1074/jbc.M605617200 [DOI] [PubMed] [Google Scholar]
- Vicente R., Villalonga N., Calvo M., Escalada A., Solsona C., Soler C., Tamkun M.M., Felipe A. 2008. Kv1.5 association modifies Kv1.3 traffic and membrane localization. J. Biol. Chem. 283:8756–8764 10.1074/jbc.M708223200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalonga N., Escalada A., Vicente R., Sánchez-Tilló E., Celada A., Solsona C., Felipe A. 2007. Kv1.3/Kv1.5 heteromeric channels compromise pharmacological responses in macrophages. Biochem. Biophys. Res. Commun. 352:913–918 10.1016/j.bbrc.2006.11.120 [DOI] [PubMed] [Google Scholar]
- Villalonga N., Martínez-Mármol R., Roura-Ferrer M., David M., Valenzuela C., Soler C., Felipe A. 2008. Cell cycle-dependent expression of Kv1.5 is involved in myoblast proliferation. Biochim. Biophys. Acta. 1783:728–736 10.1016/j.bbamcr.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Wang L., Feng Z.P., Duff H.J. 1999. Glucocorticoid regulation of cardiac K+ currents and L-type Ca2+ current in neonatal mice. Circ. Res. 85:168–173 [DOI] [PubMed] [Google Scholar]
- Xaus J., Comalada M., Valledor A.F., Cardó M., Herrero C., Soler C., Lloberas J., Celada A. 2001. Molecular mechanisms involved in macrophage survival, proliferation, activation or apoptosis. Immunobiology. 204:543–550 10.1078/0171-2985-00091 [DOI] [PubMed] [Google Scholar]
- Zsiros E., Kis-Toth K., Hajdu P., Gaspar R., Bielanska J., Felipe A., Rajnavolgyi E., Panyi G. 2009. Developmental switch of the expression of ion channels in human dendritic cells. J. Immunol. 183:4483–4492 10.4049/jimmunol.0803003 [DOI] [PubMed] [Google Scholar]