Keeping Wee1B in the nucleus is important to maintain meiotic arrest, but its timely export is also required for meiosis to resume.
Abstract
After a long period of quiescence at dictyate prophase I, termed the germinal vesicle (GV) stage, mammalian oocytes reenter meiosis by activating the Cdc2–cyclin B complex (maturation-promoting factor [MPF]). The activity of MPF is regulated by Wee1/Myt1 kinases and Cdc25 phosphatases. In this study, we demonstrate that the sequestration of components that regulate MPF activity in distinct subcellular compartments is essential for their function during meiosis. Down-regulation of either Wee1B or Myt1 causes partial meiotic resumption, and oocytes reenter the cell cycle only when both proteins are down-regulated. Shortly before GV breakdown (GVBD), Cdc25B is translocated from the cytoplasm to the nucleus, whereas Wee1B is exported from the nucleus to the cytoplasm. These movements are regulated by PKA inactivation and MPF activation, respectively. Mislocalized Wee1B or Myt1 is not able to maintain meiotic arrest. Thus, cooperation of Wee1B, Myt1, and Cdc25 is required to maintain meiotic arrest and relocation of these components before GVBD is necessary for meiotic reentry.
Introduction
In all species studied, the regulation of the meiotic cell cycle is orchestrated by maturation-promoting factor (MPF), a complex of the Cdc2/cdk1 kinase and the regulatory cyclin B. Activation of the Cdc2 kinase requires the association with cyclins as well as the dephosphorylation of Thr 14 and Tyr 15 residues (Nurse, 1990; Morgan, 1995). The phosphorylation state of these two critical residues on Cdc2 is dictated by a balance between the activity of the Wee1 kinases and the Cdc25 phosphatases (Lew and Kornbluth, 1996). In mouse oocytes, genetic studies have established that Cdc25B rather than Cdc25C, as in frogs, is critical for meiotic reentry (Kumagai and Dunphy, 1992; Lincoln et al., 2002); conversely, several Wee kinases, including the somatic Wee1, Myt1, and, more recently, the embryonic Wee1B (Wee2), have been implicated in meiotic arrest (Mitra and Schultz, 1996; Kanatsu-Shinohara et al., 2000; Okumura et al., 2002; Han et al., 2005). In both mitosis and meiosis, after an initial activation in discrete cytoplasmic compartments, the Cdc2–cyclin B complex enters the nucleus to phosphorylate specific targets required for germinal vesicle (GV) breakdown (GVBD), the hallmark of the prophase to metaphase transition (Ookata et al., 1992; Mitra and Schultz, 1996; Marangos and Carroll, 2004). Thus, the local activation and translocation of MPF is an important event for prophase to anaphase transition. Although MPF translocation to the oocyte's nucleus has been described (Mitra and Schultz, 1996; Marangos and Carroll, 2004), the subcellular localization of regulatory kinases and phosphatase has not been extensively explored in mammalian oocytes.
Results and discussion
Cooperative functions of Wee1B with Myt1 in maintaining meiotic arrest
We previously reported that down-regulation of the maternal Wee1B mRNA causes resumption of meiosis of mouse oocytes (Han et al., 2005), implicating this kinase in the maintenance of meiotic arrest. Because only a portion of the oocytes resumed meiosis, we hypothesized that this incomplete phenotype is caused by compensation of the loss of Wee1B activity by Myt1 and/or the somatic Wee1 (termed in this study Wee1A). To investigate the relative contribution of the three kinases in imposing the meiotic arrest, we designed morpholino oligonucleotides (MOs) corresponding to Wee1A, Myt1, and Wee1B by overlapping the initiator ATG of each mRNA. Less than 10% GVBD was induced with the injection of Wee1A MO, a value comparable with scrambled control MO (Fig. 1 A), indicating that this kinase is not involved in enforcing the meiotic arrest. This conclusion is consistent with the low mRNA levels for Wee1A detected in mature oocytes (Han et al., 2005). In contrast, 35% of the oocytes underwent GVBD after injection of Wee1B MO, which is a result consistent with our previous experiments (Han et al., 2005). Down-regulation of Myt1 also induced GVBD by ∼22% (Fig. 1 A). Only combined down-regulation of Wee1B and Myt1 produced GVBD in the majority of the oocytes (Fig. 1 A). Similar results were obtained in pde3a-null oocytes, which are permanently arrested in GV stage because of high cAMP and PKA activity (Fig. 1 B). These results indicate that Wee1B and Myt1 but not Wee1A cooperate in maintaining the meiotic arrest in mouse oocytes. The presence of a fraction of oocytes that do not reenter meiosis after combined MO injection is likely caused by the incomplete knockdown of these proteins. Available antibodies do not detect the endogenous Myt1 and Wee1B in immunocytochemistry, and a large number of oocytes are required to obtain a significant signal in Western blot analysis, rendering technically difficult the detection of endogenous proteins in injected oocytes. Therefore, it has not been possible to directly monitor the efficiency of the MO down-regulation. Nevertheless, the specificity and efficacy of each MO was confirmed by down-regulation of recombinant protein expression in frog and mouse oocytes and by a rescue experiment (Fig. S1).
Figure 1.
Effect of down-regulation of Wee1 kinases on mouse oocyte meiotic arrest. (A and B) WT (A) or pde3a−/− (B) oocytes were injected with MO corresponding to Wee1A, Wee1B, or Myt1. Scrambled MO (Ctrl) was used as a negative control. Error bars indicate SEM. Numbers above the bars indicate the number of oocytes in the GVBD stage and number of total oocytes, respectively, as well as the number of experiments performed. *, P < 0.005; and **, P < 0.0001 compared with control. ***, P = 0.0192.
Dynamic localization of MPF regulators
Because both Myt1 and Wee1B are required to stabilize the GV state, we next asked why two kinases with overlapping functions are necessary to maintain meiotic arrest. An explanation for this redundancy is that the two kinases are confined to different compartments within the oocyte. To test this possibility, we microinjected Wee1B and Myt1 as well as the phosphatase Cdc25B tagged with GFP, YFP, and RFP, respectively, into GV oocytes (Fig. 2 A). It should be noted that all three proteins were rendered catalytically inactive to avoid confounding effects on oocyte maturation. In GV oocytes, GFP-Wee1B was entirely confined to the nucleus, whereas YFP-Myt1 was excluded from the nucleus and distributed in a punctate pattern in the cytoplasm. This localization was confirmed and further resolved by scanning confocal microscopy (Fig. S2). In contrast, RFP-Cdc25B was localized mainly in the cytoplasm of GV oocytes. Identical localization of the three proteins was observed when these constructs were used in the transfection of somatic cells (unpublished data). Moreover, the localization was independent of the amount of recombinant protein expressed and of the tag used to detect the expressed proteins (unpublished data).
Figure 2.
Localization of Wee1B, Myt1, and Cdc25B during meiotic resumption. (A) Subcellular localization of Wee1B, Myt1, and Cdc25B was monitored by imaging live cells during meiotic resumption. (B) GFP-Wee1B–injected GV oocytes were stained with anti–lamin A antibody during the course of Wee1B export. (C) Oocyte nuclei were injected with 70 kD of Texas red–labeled dextran, and fluorescence was monitored during the export of GFP-Wee1B. DIC, differential interference contrast. Bars: (A and C) 20 µm; (B) 60 µm.
Under conditions that allow meiotic resumption, a significant fraction of Wee1B was translocated to the cytoplasm shortly before GVBD at a time when Cdc25B had entered the nucleus (Fig. 2 A). In contrast, the localization of Myt1 did not change during meiotic resumption. After GVBD, both Wee1B and Cdc25B were distributed throughout the entire oocyte intracellular space. A detailed time course analysis comparing Cdc25B import and Wee1B export from the nucleus showed that Cdc25B entry preceded Wee1B extrusion (Fig. S3 and Videos 1–3). Wee1B export occurs at a time that roughly coincides with the timing of Cdc2–cyclin B translocation to the nucleus (15–20 min before GVBD; Marangos and Carroll, 2004). It has been previously shown that Swe1, a yeast homologue of Wee1, shuttles between the nucleus and cytoplasm during the mitotic cell cycle (Longtine et al., 2000; Keaton et al., 2008) and that the nuclear shuttling of Cdc25B accompanies meiotic resumption (Solc et al., 2008; Zhang et al., 2008; Pirino et al., 2009). Thus, our data are consistent with these previous observations in the mitotic cell cycle.
Because the nuclear export occurred just before GVBD, we investigated whether the accumulation of GFP-Wee1B fluorescence in the cytoplasm is simply the result of passive diffusion during the breakdown of the nuclear envelope. Two experiments reject this possibility. Staining of the nuclear lamina with anti–lamin A antibody showed that the nuclear envelope was still intact when GFP-Wee1B was detected in both the nucleus and cytoplasm (Fig. 2 B). Furthermore, nuclear injected 70-kD dextran, with size comparable with GFP-Wee1B and excluded by the nuclear pores, was still retained in the nucleus at a time when significant amounts of Wee1B accumulated in the cytoplasm (Fig. 2 C). These results strongly suggest that Wee1B is actively exported from the nucleus rather than being subjected to passive redistribution with the breakdown of the nuclear membrane at GVBD.
Nucleocytoplasmic shuttling of Wee1B is mediated by NLS and nuclear export signal (NES) sequences
Because Wee1B is found in both the cytoplasm and nucleus depending on the stage of maturation, it must encode mechanisms for nucleocytoplasmic shuttling. NLS and NES sequences often mediate protein transport between the nucleus and cytoplasm (Sorokin et al., 2007). Thus, we analyzed the amino acid sequence of Wee1B and identified putative NLS and NES sequences in the regulatory N-terminal domain and catalytic domain of Wee1B, respectively (Fig. 3 A). To confirm the functionality of these putative NLS and NES sequences in nucleocytoplasmic shuttling of Wee1B during meiotic resumption, we generated an NLS deletion mutant (Wee1B-ΔNLS), an NES mutant in which critical hydrophobic amino acid residues were replaced by Ala residues (Wee1B-NESmut), and an NLS and NES double mutant (Wee1B-ΔNLS/NESmut). In GV oocytes, Wee1B wild type (WT; Wee1B-WT) and Wee1B-NESmut were confined to the nucleus, whereas Wee1B-ΔNLS exhibited cytoplasmic localization. Wee1B-ΔNLS/NESmut was found in the nucleus as well as in the cytoplasm (Fig. 3 B).
Figure 3.
Nucleocytoplasmic shuttling of Wee1B is mediated by NLS and NES. (A) Schematic representation of the structure of Wee1B. The catalytic domain is shaded. The potential NLS sequence is indicated by a blue bar and aligned with human homologues. The putative NES sequence is indicated by a red bar and shown with the other known NES sequences. The consensus sequences of NLS and NES are shown in bold. The numbers above the structure refer to the amino acids of Wee1B. (B) Localization of Wee1B-targeting mutants was shown with representative brightfield and fluorescence images. (C) Immunoprecipitation (IP) of Wee1B-targeting mutants and Crm1. WB, Western blot. Bar, 20 µm.
Nuclear export of NES-containing proteins is dependent on interactions with the Crm1 nuclear export receptor, which is located on the nuclear envelope (Sorokin et al., 2007). To determine whether Wee1B binds to Crm1 in vivo, we performed an immunoprecipitation assay in which WT and mutant Wee1B were expressed in a heterologous system. Wee1B-WT and Wee1B-ΔNLS coprecipitated with Crm1, whereas little immunoprecipitate was observed with Wee1B-NESmut and Wee1B-ΔNLS/NESmut (Fig. 3 C). Together with the localization data, these findings document that Wee1B binds to Crm1 through the identified NES sequence and point mutations in this region disrupt this interaction.
Different signaling pathways are involved in Cdc25B and Wee1B translocation
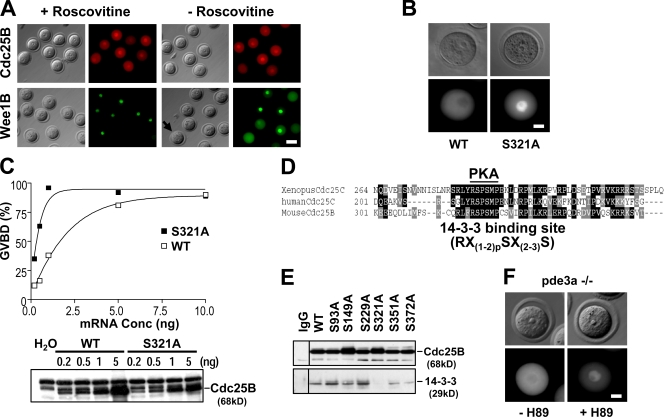
Because MPF regulates the activities of Wee1 and Cdc25 by positive and negative feedback mechanisms (Perry and Kornbluth, 2007; Ferrell, 2008), we investigated whether the nucleocytoplasmic shuttling of Wee1B or Cdc25B is under the control of this cell cycle regulator. GFP-Wee1B or RFP-Cdc25B was microinjected into GV oocytes and incubated with or without the Cdc2 inhibitor roscovitine. This inhibition of MPF completely blocked Wee1B export but had no effect on the nuclear import of Cdc25B (Fig. 4 A).
Figure 4.
Regulation of subcellular localization of Wee1B and Cdc25B. (A) RFP-Cdc25B– or GFP-Wee1B mRNA–injected oocytes were incubated with or without 50 µM roscovitine for 1 h. The arrow indicates a GV oocyte in which Wee1B is exported from the nucleus. (B) Subcellular localization of Ser 321 mutants of Cdc25B. (C) WT or S321A mutant Cdc25B mRNAs were injected into Xenopus oocytes, and the percentage of GVBD was determined after 6 h. The amounts of injected mRNAs were compared by immunoblot analysis. (D) The Ser 321 PKA phosphorylation site is overlapped with the 14-3-3 binding site. Identical and conserved residues are shaded in black and gray, respectively. (E) Immunoprecipitation of Cdc25B mutants and 14-3-3. Black lines indicate that intervening lanes have been spliced out. (F) RFP-Cdc25B was injected into pde3a−/− oocytes and incubated with or without 50 µM H89 for 2 h, and localization of fusion proteins was shown. Bars: (A) 60 µm; (B and F) 20 µm.
Given that Wee1B and Cdc25B are both substrates of PKA (Han et al., 2005; Han and Conti, 2006; Zhang et al., 2008; Pirino et al., 2009; Schultz, 2009), it is possible that Wee1B or Cdc25B phosphorylation by PKA controls their subcellular localization. Whereas point mutations of a putative PKA site on Wee1B (Ser 15 and Thr 170 residues) did not affect its subcellular localization (not depicted), a PKA regulatory role in the localization of Cdc25B was supported by the mutation of Ser 321 residue, which promoted the nuclear import of Cdc25B (Fig. 4 B). Moreover, this Cdc25B Ser 321 mutant was more effective in inducing GVBD than WT control (Fig. 4 C), and this mutation affected Cdc25B association with a 14-3-3 (Fig. 4, D and E). Finally, using pde3a−/− oocytes, we show that PKA inhibition caused prompt nuclear translocation of the injected RFP-Cdc25B (Fig. 4 F). These findings are consistent with data published while this paper was in preparation demonstrating that Cdc25B Ser 321 phosphorylation by PKA negatively regulates Cdc25B by preventing its nuclear accumulation (Zhang et al., 2008; Pirino et al., 2009; Schultz, 2009). These results also indicate that the mechanisms activating nuclear import of Cdc25B precede and are upstream of the control of Wee1B export. Indeed, nuclear import of Cdc25B occurs before Wee1B export during meiotic resumption (Fig. S3 and Videos 1–3). Collectively, these results suggest that this reciprocal translocation of the enzymes is an important event for the regulation of nuclear MPF and for meiotic resumption.
Correct localization is necessary for the proper function of Wee1B and Myt1
To investigate the functional significance of Wee1B export from the nucleus, we determined the biological activity of a cytoplasmic Wee1B. Active Wee1B-targeting mutants were microinjected into GV-arrested oocytes, and meiotic resumption was determined by scoring GVBD. Microinjection of nuclear Wee1B proteins (Wee1B-WT and Wee1B-NESmut) led to an efficient and complete block of meiotic resumption, whereas cytoplasmic Wee1B mutants (Wee1B-ΔNLS and Wee1B-ΔNLS/NESmut) failed to block maturation (Fig. 5 A). It is noteworthy that the level of protein expression was comparable for the different Wee1B mutants and that the activity of different Wee1B constructs measured by their effects on Cdc2-dependent histone H1 phosphorylation was not significantly different (Fig. 5 B). These results confirm that the inability of cytoplasmic Wee1B to block MPF activity is not simply caused by decreased activity associated with the disruption of the NLS or NES sequences but rather results from mislocalization of the mutant protein. Proper localization of Myt1 is also important for its function. Indeed, Myt1 chimeric proteins containing a C-terminal NLS sequence from SV40 large T antigen (Myt1-NLS) were designed, and appropriate nuclear expression of this Myt1 mutant by microinjection in GV oocytes was verified (Fig. 5 C). Microinjection of Myt1-NLS blocked GVBD less efficiently than WT (Myt1-WT; Fig. 5 D).
Figure 5.
Localization and function of Wee1 kinases in meiotic arrest. (A and D) Oocytes were injected with the indicated Wee1B (A) or Myt1 (D) mRNAs, washed out of the inhibitor, and monitored for the absence of a GV (GVBD). These experiments were repeated three times, and the error bars indicate SEM. The expression levels of injected mRNAs were compared by immunoblotting with the GFP antibody. (B) The activity of Wee1B-targeting mutants was measured by H1 kinase assay. The data are representative of three independent experiments. The ratio of 32P incorporation into histone H1 and the amount of immunocomplexes were densitometrically measured and represented as a mean percentage ± SEM of control (Mock). (C) Localization of WT and NLS mutants of Myt1 was shown with brightfield and fluorescence images. Bar, 20 µm.
Because Wee1B is a Cdc2 inhibitor, it must be inactivated during M-phase progression. Several mechanisms of Wee1 inactivation have been identified, including phosphorylation and degradation as well as binding to other regulatory proteins (Rothblum-Oviatt et al., 2001; Katayama et al., 2005; Kim et al., 2005; Okamoto and Sagata, 2007). In the same vein, it has been previously shown that nuclear export of Swe1 is required for its destruction (Keaton et al., 2008). However, the mouse Wee1B protein is resistant to degradation in frog oocytes even when Cdc2 is maximally activated, rendering it less likely that degradation is a mechanism of Wee1B inactivation during mammalian prophase to metaphase transition. Thus, one can hypothesize that the nuclear export of Wee1B, likely dependent on nuclear Cdc2 activity, may either promote the inactivation of this kinase or prevent its access to substrate MPF. The data showing greatly reduced ability of cytoplasmic Wee1B to block maturation are consistent with this hypothesis. At variance with most proteins containing NES sequences, we found that Wee1B has an NES sequence within the catalytic domain. This raises the interesting possibility that the binding of nuclear export proteins to the NES may have a role not only in nuclear export but also in the modulation of the kinase activity by masking the Wee1B catalytic domain. Alternatively and after the nuclear import of Cdc25B, Cdc2 activation may cause phosphorylation and inactivation of Wee1B, and the associated conformational change allows Crm1 access to the Wee1B NES and export of the protein to the cytoplasm.
We observed that overexpressed Myt1-WT inhibited GVBD less effectively than Wee1B (Fig. 5, A and D; and Fig. S1, A and E), even though Myt1 was previously identified as a Cdc2 inhibitor (Mueller et al., 1995; Fattaey and Booher, 1997). In agreement with our results, it has been reported that the inhibitory activity of XeMyt1 is also weaker than that of Wee1 in Xenopus laevis oocytes (Okamoto et al., 2002). It is likely that small amounts of MPF in immature oocytes continuously shuttle between the nucleus and cytoplasm. In this case, overexpression of Wee1B would completely prevent the activation of MPF in the nucleus, which is a prerequisite for the induction of meiotic resumption, whereas Myt1, although able to inactivate MPF in the cytoplasm, cannot block the activation of the MPF localized in the nucleus. It is well documented that MPF relocation to the nucleus is closely related to GVBD during meiotic maturation of the oocytes (Ookata et al., 1992; Mitra and Schultz, 1996). Collectively, these results provide evidence that the efficient maintenance of meiotic arrest requires the localization of Wee1B in the nucleus and Myt1 in the cytoplasm.
Our data support the concept that the compartmentalization of kinases and phosphatases regulating MPF is critical for the control of mouse oocyte meiotic arrest and reentry into the cell cycle. Wee1B and Myt1 cooperate to maintain meiotic arrest in mouse oocytes, whereas the somatic Wee1 is not involved. The two kinases act in two distinct subcellular compartments of the oocyte. The subcellular localization of Wee1B is dependent on the presence of NLS and NES motifs, implying active processes of sequestration. Targeting of the enzyme to the cytosol precludes its cytostatic function. Different signaling pathways determine the localization of Cdc25B and Wee1B in the nuclear and cytoplasmic compartments. Whereas the Cdc25B cytoplasmic localization is dependent on PKA phosphorylation, the nuclear localization of Wee1B does not require PKA regulation. Myt1 localization is static, but Cdc25 and Wee1B undergo reciprocal translocation before GVBD. Cdc25 entry into the nucleus is dependent on a decrease in cAMP and PKA, whereas Wee1B export is dependent on Cdc2 activation. It is at present unknown whether Myt1 activity is constitutive or regulated in a cAMP-dependent manner in the quiescent state or before the reentry into the cell cycle, a possibility which needs to be clarified by further experimentation.
Collectively, the aforementioned findings can be accommodated in the following working model. A decrease in oocyte cAMP and inactivation of PKA causes a rapid and progressive Cdc25B translocation to the nucleus, at least initially in the absence of changes in MPF activity. The accumulation of the phosphatase in the nucleus causes dephosphorylation and activation of the fraction of MPF that is shuttling into the nucleus. In this compartment, the activated MPF promotes the export of Wee1B to the cytoplasm through indirect or direct mechanisms. This Wee1B translocation functions as an amplification step to promote further activation of the nuclear MPF until a threshold of activity sufficient for nuclear envelope breakdown is reached. Thus, our findings provide a novel mode of Wee1B regulation that involves its subcellular relocation at the prophase to metaphase transition. The most straightforward and simple consequence of this transfer is that Wee1B no longer has access to its MPF substrate and that this is sufficient to cause MPF activation. However, it is also possible that Wee1B becomes inactivated during the export process or during its accumulation in the cytoplasm, further suppressing its cytostatic activity.
Materials and methods
Collection and culture of mouse oocytes and H1 kinase assay
GV oocytes were recovered from the ovaries of 21–24-d-old mice that had been administered 5 IU pregnant mare's serum gonadotropin (PMSG) 46–48 h earlier. Ovaries were punctured with a fine needle to release oocytes into M2 medium supplemented with 4 mg/ml BSA and 5 µM cilostamide to prevent GVBD. Only oocytes with an intact layer of cumulus cells were recovered, and cumulus cells were subsequently removed by repeated pipetting with a mouth-operated micropipette. Denuded oocytes were then placed in drops of media under oil and cultured at 37°C in 5% CO2 atmosphere.
Metaphase II–arrested oocytes were recovered from mice superovulated by i.p. injections of PMSG and human chorionic gonadotrophin 48 h apart. Ovulated oocytes were released from the ampullae of oviducts 16 h after human chorionic gonadotrophin. The cumulus cells were dispersed by brief exposure to 0.1 M hyaluronidase.
The activity of Wee1B kinase was determined with five randomly selected metaphase II–arrested oocytes. H1 kinase assay was performed as previously described (Svoboda et al., 2001). In brief, various GFP-Wee1B–targeting mutants were transiently transfected into HEK293 cells, and overexpressed Wee1B proteins were immunoprecipitated with V5 antibodies (Invitrogen). Oocytes were lysed in 5 µl of lysis buffer (10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 mM p-nitrophenyl phosphate, 20 mM β-glycerophosphate, 0.1 mM Na orthovanadate, and 5 mM EGTA) and preincubated with Wee1B immunoprecipitates for 20 min. The kinase reaction was initiated by the addition of 5 µl of kinase buffer (24 mM p-nitrophenyl phosphate, 90 mM β-glycerophosphate, 24 mM MgCl2, 24 mM EGTA, 0.2 mM EDTA, 4.6 mM Na orthovanadate, 4 mM NaF, 1.6 mM DTT, 60 µg/ml aprotinin, 60 µg/ml leupeptin, 2.2 µM cAMP-dependent protein kinase inhibitor, 0.6 mM ATP, and 2 mg/ml histone [type III-S; Sigma-Aldrich] with 500 µCi/ml γ-[32P]ATP [3000 Ci/mmol; GE Healthcare]). The reaction was conducted for 30 min at 30°C and terminated by the addition of 10 µl of 2× SDS sample buffer and boiling for 3 min. After SDS-PAGE, the gel was transferred to a polyvinylidene fluoride membrane and analyzed by autoradiography. The membrane was immunoblotted with V5 antibodies (Invitrogen).
Plasmids and site-directed mutagenesis
The full-length cDNAs encoding the mouse Wee1B, Myt1, and Cdc25B were subcloned into the pcDNA3.1/V5-His-TOPO vector (Invitrogen) as previously described (Han et al., 2005). The coding sequences for GFP, YFP, and RFP were excised from the vector pEGFP-N1, pYFP-N1, and pDsRed-N1 (Takara Bio Inc.), respectively, and inserted in frame at the 3′ end of the Wee1B, Myt1, and Cdc25B sequences to produce the GFP-Wee1B, YFP-Myt1, and RFP-Cdc25B, respectively. The catalytically inactive mutants of Wee1B (K237M), Myt1 (N229A), and Cdc25B (C483S) were generated using site-directed mutagenesis. Wee1B mutants were generated using the QuikChange Site-Directed Mutagenesis kit (Agilent Technologies) using the following primers: Wee1B-ΔNLS, 5′-CCTTCAATCAAAGGGCACCAGAGACTATCTTG-3′; and Wee1B-NESmut, 5′-CTGCAGATTTCCGCCGGAGCTAAGTACATCCAC-3′. To generate Myt1-NLSmutant, SV40 large T antigen NLS sequence was inserted between Myt1 and YFP sequence using PCR-based site-directed mutagenesis with the primer 5′-GGACTCCCTAGACCCACCAAAAAAGAAGAGAAAGGTAGCCTGGGCACAGGCC-3′. The Crm1 cDNA in pCMV-SPORT6 vector was obtained from Thermo Fisher Scientific. To create HA-tagged Crm1, the sequence encoding the HA tag was introduced immediately upstream of the initiation start codon of Crm1 cDNA. All constructs were sequenced to verify the correct insertion and mutation.
In vitro mRNA production for microinjection
mRNAs for microinjection were produced by in vitro transcription using mMESSAGE mMACHINE (Applied Biosystems). After in vitro transcription, mRNAs were immediately polyadenylated using Poly(A) Tailing kit (Applied Biosystems) and purified with RNeasy kit (QIAGEN). The concentration of mRNAs was determined by OD260 and agarose gel electrophoresis.
Microinjection and imaging
In vitro transcribed mRNAs were microinjected into the cytoplasm of GV oocytes in M2 medium with 4 mg/ml BSA and 5 µM cilostamide using a micropipette and Eppendorf manipulators mounted on an inverted microscope (DMI 4000B; Leica). Approximately 10 pl of the mRNA solution containing 250 ng/µl was injected per oocyte. For dextran, oocytes were microinjected with Texas red–labeled 70-kD dextran (Invitrogen) dissolved at 2.5 µg/ml in PBS into the nucleus. After microinjection, oocytes were further incubated at 37°C in 5% CO2 until distinct fluorescent signal was detected. For meiotic resumption, oocytes were washed extensively in cilostamide-free medium and cultured at 37°C in 5% CO2, and GVBD was scored every 30 min for up to 5 h. The microinjected oocytes were observed and imaged live on the DMI 4000B inverted microscope. Subgroups of microinjected oocytes were used for immunoblotting with anti-GFP antibody (Abcam) to confirm the expression of protein. All images were acquired using a DMI 4000B inverted microscope, a charge-coupled device camera (Retiga 2000R; QImaging) and a 20× NA 0.4 objective lens (Leica). All live cell imaging was performed in M2 medium at 37°C. Confocal sections of DAPI, GFP, and YFP staining were performed on a confocal microscope (SP5; Leica). Laser beams of wavelengths 360/40, 480/40, and 546/12 nm were used to excite DAPI, GFP, and RFP or Texas red, respectively. Emission was collected at 470/40 nm for DAPI, 527/30 nm for GFP, and 600/40 nm for RFP or Texas red. Image analysis was performed using either QCapture Pro imaging software (QImaging) or Photoshop (Adobe). Time-lapse images were acquired by using an inverted microscope (DMI 6000B; Leica) and a charge-coupled device camera (DFC360FX; Leica) with a temperature-controlled stage.
For injection into Xenopus oocyte, ovary fragments were surgically removed from PMSG-primed Xenopus, and defolliculated oocytes were isolated after treatment with 2.5 mg/ml collagenase in MBS buffer (10 mM Hepes, pH 7.4, 88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, and 2.4 mM Na2 HCO3) for 1–1.5 h. Stage VI oocytes were selected for all experiments. The indicated amounts of mRNAs, MO, or H2O were injected with a micromanipulator (Narishige USA) into defolliculated stage VI Xenopus oocytes. 16 h after the injections, oocyte maturation was induced by stimulation with 500 nM progesterone, and maturation was scored by the appearance of a white spot on the animal pole.
Immunofluorescence analysis
Oocytes were fixed in 4% paraformaldehyde in PBS for 1 h at room temperature and permeabilized for 15 min in 0.1% Triton X-100 in PBS at room temperature. Oocytes were stained with anti–mouse lamin A antibody (Abcam) overnight at 4°C. After washing three times with PBS containing 1 mg/ml BSA, the oocytes were incubated for 1 h with Alexa Fluor 594 (Invitrogen). Oocytes were observed and imaged on the DMI 4000B inverted microscope.
Injection of MOs in mouse oocytes
For targeted knockdown of Wee1A, Wee1B, and Myt1, antisense MOs were purchased from Gene Tools, LLC. Sequences were as follows; Wee1A MO, 5′-GCTGTCGGCTCAGGAAGCTCATTTC-3′; Wee1B MO, 5′-CTGGTCAGTCTCTGTGTCGGCCATC-3′; and Myt1 MO, 5′-CCTCGGTGGGCATGGTCATGGCAAC-3′. The standard control MO with the sequence 5′-CCTCTTACCTCAGTTACAATTTATA-3′ (Gene Tools, LLC) was used for control injections. All MOs were dissolved in nuclease-free water at a concentration of 1 mM and heated at 65°C for 5 min before injection. Oocytes were microinjected with 10 pl of the MO solutions. After injection, oocytes were transferred to M16 medium supplemented with 4 mg/ml BSA and 1 µM cilostamide and incubated at 37°C in 5% CO2 for 22 h, and GVBD was scored with a DMI 4000B inverted microscope fitted with a Hoffman contrast lens.
Immunoprecipitation
Transient transfection with HA-Crm1 and various GFP-Wee1B mutants was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 48 h, cells were lysed in lysis buffer and homogenized. Cell lysates were incubated with anti-HA antibody and protein A–Sepharose beads (GE Healthcare). The precipitates were then washed with lysis buffer. The proteins were separated by SDS-PAGE, transferred to a polyvinylidene fluoride membrane, and analyzed by immunoblotting with V5 or HA antibodies.
Statistical analysis
Data are representative of at least three independent experiments, unless otherwise specified. Values were analyzed by one-way analysis of variance or Student's t test, and P < 0.05 was considered statistically significant.
Online supplemental material
Fig. S1 shows the specificity and efficacy of MOs. Fig. S2 shows the subcellular localization of Wee1B and Myt1. Fig. S3 shows the time course of nucleocytoplasmic shuttling of Wee1B and Cdc25B. Video 1 shows a time-lapse image of RFP-Cdc25B– and GFP-Wee1B–coinjected oocytes during meiotic resumption. Videos 2 and 3 show time-lapse images of RFP-Cdc25B and GFP-Wee1B, respectively, during meiotic resumption. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200907161/DC1.
Acknowledgments
We thank Sergio Vaccari for contributing a preliminary experiment; Matthew Gormley for the time-lapse experiments; and Laurinda Jaffe, Mark Terasaki, Keith Jones, Diana Laird, and Miguel Ramalho Santos for helpful comments.
This work was supported by National Institutes of Health grant GM 080527 and a grant from Organon. J.S. Oh was supported by a fellowship from the Korea Research Foundation grant funded by the Korean Government (Ministry of Education and Human Resources Development; KRF-2007-357-C00099).
Footnotes
Abbreviations used in this paper:
- GV
- germinal vesicle
- GVBD
- GV breakdown
- MO
- morpholino oligonucleotide
- MPF
- maturation-promoting factor
- NES
- nuclear export signal
- PMSG
- pregnant mare's serum gonadotropin
- WT
- wild type
References
- Fattaey A., Booher R.N. 1997. Myt1: a Wee1-type kinase that phosphorylates Cdc2 on residue Thr14. Prog. Cell Cycle Res. 3:233–240 [DOI] [PubMed] [Google Scholar]
- Ferrell J.E., Jr. 2008. Feedback regulation of opposing enzymes generates robust, all-or-none bistable responses. Curr. Biol. 18:R244–R245 10.1016/j.cub.2008.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.J., Conti M. 2006. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle. 5:227–231 [DOI] [PubMed] [Google Scholar]
- Han S.J., Chen R., Paronetto M.P., Conti M. 2005. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr. Biol. 15:1670–1676 10.1016/j.cub.2005.07.056 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Schultz R.M., Kopf G.S. 2000. Acquisition of meiotic competence in mouse oocytes: absolute amounts of p34(cdc2), cyclin B1, cdc25C, and wee1 in meiotically incompetent and competent oocytes. Biol. Reprod. 63:1610–1616 10.1095/biolreprod63.6.1610 [DOI] [PubMed] [Google Scholar]
- Katayama K., Fujita N., Tsuruo T. 2005. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol. Cell. Biol. 25:5725–5737 10.1128/MCB.25.13.5725-5737.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaton M.A., Szkotnicki L., Marquitz A.R., Harrison J., Zyla T.R., Lew D.J. 2008. Nucleocytoplasmic trafficking of G2/M regulators in yeast. Mol. Biol. Cell. 19:4006–4018 10.1091/mbc.E08-03-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Song E.J., Lee K.J., Ferrell J.E., Jr 2005. Multisite M-phase phosphorylation of Xenopus Wee1A. Mol. Cell. Biol. 25:10580–10590 10.1128/MCB.25.23.10580-10590.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W.G. 1992. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 70:139–151 10.1016/0092-8674(92)90540-S [DOI] [PubMed] [Google Scholar]
- Lew D.J., Kornbluth S. 1996. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8:795–804 10.1016/S0955-0674(96)80080-9 [DOI] [PubMed] [Google Scholar]
- Lincoln A.J., Wickramasinghe D., Stein P., Schultz R.M., Palko M.E., De Miguel M.P., Tessarollo L., Donovan P.J. 2002. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat. Genet. 30:446–449 10.1038/ng856 [DOI] [PubMed] [Google Scholar]
- Longtine M.S., Theesfeld C.L., McMillan J.N., Weaver E., Pringle J.R., Lew D.J. 2000. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:4049–4061 10.1128/MCB.20.11.4049-4061.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos P., Carroll J. 2004. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction. 128:153–162 10.1530/rep.1.00192 [DOI] [PubMed] [Google Scholar]
- Mitra J., Schultz R.M. 1996. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J. Cell Sci. 109:2407–2415 [DOI] [PubMed] [Google Scholar]
- Morgan D.O. 1995. Principles of CDK regulation. Nature. 374:131–134 10.1038/374131a0 [DOI] [PubMed] [Google Scholar]
- Mueller P.R., Coleman T.R., Kumagai A., Dunphy W.G. 1995. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 270:86–90 10.1126/science.270.5233.86 [DOI] [PubMed] [Google Scholar]
- Nurse P. 1990. Universal control mechanism regulating onset of M-phase. Nature. 344:503–508 10.1038/344503a0 [DOI] [PubMed] [Google Scholar]
- Okamoto K., Sagata N. 2007. Mechanism for inactivation of the mitotic inhibitory kinase Wee1 at M phase. Proc. Natl. Acad. Sci. USA. 104:3753–3758 10.1073/pnas.0607357104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Nakajo N., Sagata N. 2002. The existence of two distinct Wee1 isoforms in Xenopus: implications for the developmental regulation of the cell cycle. EMBO J. 21:2472–2484 10.1093/emboj/21.10.2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura E., Fukuhara T., Yoshida H., Hanada Si S., Kozutsumi R., Mori M., Tachibana K., Kishimoto T. 2002. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol. 4:111–116 10.1038/ncb741 [DOI] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Okano T., Tachibana K., Kishimoto T. 1992. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 11:1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J.A., Kornbluth S. 2007. Cdc25 and Wee1: analogous opposites? Cell Div. 2:12 10.1186/1747-1028-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirino G., Wescott M.P., Donovan P.J. 2009. Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle. 8:665–670 [DOI] [PubMed] [Google Scholar]
- Rothblum-Oviatt C.J., Ryan C.E., Piwnica-Worms H. 2001. 14-3-3 binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ. 12:581–589 [PubMed] [Google Scholar]
- Schultz R. 2009. PKA and CDC25B: at last connected. Cell Cycle. 8:516–517 [PubMed] [Google Scholar]
- Solc P., Saskova A., Baran V., Kubelka M., Schultz R.M., Motlik J. 2008. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev. Biol. 317:260–269 10.1016/j.ydbio.2008.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin A.V., Kim E.R., Ovchinnikov L.P. 2007. Nucleocytoplasmic transport of proteins. Biochemistry (Mosc.). 72:1439–1457 10.1134/S0006297907130032 [DOI] [PubMed] [Google Scholar]
- Svoboda P., Stein P., Schultz R.M. 2001. RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem. Biophys. Res. Commun. 287:1099–1104 10.1006/bbrc.2001.5707 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Z., Xu X.Y., Li X.S., Yu M., Yu A.M., Zong Z.H., Yu B.Z. 2008. Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev. Dyn. 237:3777–3786 10.1002/dvdy.21799 [DOI] [PubMed] [Google Scholar]