Abstract
Pericentrin is an integral component of the centrosome that serves as a multifunctional scaffold for anchoring numerous proteins and protein complexes. Through these interactions, pericentrin contributes to a diversity of fundamental cellular processes. Recent studies link pericentrin to a growing list of human disorders. Studies on pericentrin at the cellular, molecular, and, more recently, organismal level, provide a platform for generating models to elucidate the etiology of these disorders. Although the complexity of phenotypes associated with pericentrin-mediated disorders is somewhat daunting, insights into the cellular basis of disease are beginning to come into focus. In this review, we focus on human conditions associated with loss or elevation of pericentrin and propose cellular and molecular models that might explain them.
Introduction
The centrosome is a cellular organelle composed of centrioles and pericentriolar material (PCM). The PCM is a protein matrix surrounding the centrioles that contains protein complexes required for centrosome-associated functions. A key component of the PCM and centrioles is pericentrin, a large conserved coiled-coil protein (Doxsey et al., 1994; Flory and Davis, 2003; Jurczyk et al., 2004; Martinez-Campos et al., 2004; Miyoshi et al., 2006a).
The centrosome has numerous and complex functions that center primarily around cell cycle regulation and microtubule organization. For example, centrosomes act as platforms for assembling regulatory proteins and pathways (Doxsey et al., 2005) such as progression from G1 to S and from G2 to M, events that require centrosome binding of cyclin E and Chk1, respectively (Krämer et al., 2004; Matsumoto and Maller, 2004; Doxsey et al., 2005). In mitotic cells, centrosomes and associated astral microtubules contribute to mitotic spindle organization (Lüders and Stearns, 2007; O'Connell and Khodjakov, 2007) and mitotic spindle orientation (Rebollo et al., 2007; Rusan and Peifer, 2007; Toyoshima and Nishida, 2007; Yamashita et al., 2007). The latter controls orientation of the cell division plane, which, in turn, controls cell fate determination in many stem cell niches (Rebollo et al., 2007; Rusan and Peifer, 2007; Yamashita et al., 2007). In noncycling cells, the older centriole/basal body serves as the template for assembly of the primary cilium (Rieder et al., 2001).
Through its ability to serve as a multifunctional scaffold for anchoring a wide range of centrosome proteins (Table I), pericentrin is involved in essentially all of the previously described functions. In this review, we will discuss the association between pericentrin function and disease. This protein has been associated with four human disorders. We focus first on primordial dwarfism, which has been directly linked to mutations in the pericentrin gene. Although the connection between pericentrin and other diseases is not as strong, cell and molecular evidence supports a role for pericentrin in human cancer, mental disorders, and ciliopathies. Here, we first discuss each human condition, starting with a brief summary of their salient features and link to pericentrin, then explore the cellular and molecular changes that accompany them. These changes are put in context with studies from multiple experimental systems that provide insight into the mechanisms underpinning these disorders.
Table I.
Pericentrin-interacting partners and associated functions.
| Interacting partners | Description: functions linked to pericentrin | References |
| Centrosome/microtubule | ||
| γ-tubulin | γ-tubulin: microtubule nucleation/organization | Knop and Schiebel, 1997; Dictenberg et al., 1998; Takahashi et al., 2002; Zimmerman et al., 2004 |
| γ-TURC (GCP2, GCP3) | γ-tubulin ring complex (γ-tubulin complex protein): microtubule nucleation/organization | Knop and Schiebel, 1997; Takahashi et al., 2002; Zimmerman et al., 2004 |
| PCM1 | PCM protein: microtubule nucleation/organization | Li et al., 2001 |
| LIC | Cytoplasmic dynein light intermediate chain: Microtubule nucleation/organization (motor protein) | Purohit et al., 1999 |
| AKAP450/CGNAP | A-kinase anchoring protein/centrosomal and golgi N-kinase anchoring protein: microtubule nucleation/organization | Takahashi et al., 2002 |
| DISC-1 | Disrupted in Schizophrenia: microtubule nucleation/organization | Miyoshi et al., 2004; Shimizu et al., 2008 |
| Checkpoint | ||
| Chk1 | Checkpoint kinase: DNA damage induced G2/M arrest regulation | Tibelius et al., 2009 |
| Kinases | ||
| PKA | Protein kinase A: cell signaling | Diviani et al., 2000 |
| PKCβII | Protein kinase C: cell signaling | Chen et al., 2004 |
| BCR-ABL | Oncogenic tyrosine kinase: cell signaling | Patel and Gordon, 2009 |
| Nuclear | ||
| CHD3 | Chromodomain helicase DNA-binding proteins: nuclear function, microtubule nucleation | Sillibourne et al., 2007 |
| CHD4 | Chromodomain helicase DNA-binding proteins: nuclear function, microtubule nucleation | Sillibourne et al., 2007 |
| Basal body/cilia | ||
| IFT | Intraflagellar transport proteins: formation and function of cilia | Jurczyk et al., 2004 |
| PC2 | Polycystin: cilia function/signaling | Jurczyk et al., 2004 |
Pericentrin in primordial dwarfism
Pericentrin in human and mouse models of dwarfism.
Recent studies indicate that PCNT mutations are associated with two rare and complex human autosomal recessive genetic disorders with overlapping features, Majewski/microcephalic osteodysplastic primordial dwarfism type II (MOPDII) and Seckel syndrome (Griffith et al., 2008; Rauch et al., 2008). However, closer evaluation of disease features demonstrated that individuals with Seckel syndrome and PCNT mutations actually belong to the MOPDII spectrum. Moreover, a recent study demonstrates that PCNT mutations are present in all the MOPDII patients examined, confirming the genetic homogeneity of this disorder and suggesting that Seckel patients do not harbor pericentrin mutations (Willems et al., 2009). Individuals with MOPDII present with several features, including extreme proportionate short stature that begins in utero (hence the term primordial dwarfism), with some adults never reaching 20 inches in height (Majewski and Goecke, 1982; Majewski et al., 1982; Hall et al., 2004), small brain size relative to body size (microcephaly), and bony dysplasia (Majewski and Goecke, 1982; Majewski et al., 1982; Hall et al., 2004; Willems et al., 2009).
Preliminary results suggest that disruption of the PCNT gene in mice (Miyoshi et al., 2009; unpublished data; Akbarian, S., C. Lo, and G. Zheng, personal communication) may provide a good model for understanding features of human primordial dwarfism. Disruption of the gene by insertional mutagenesis using gene trap technology causes reduction in the levels of pericentrin protein and leads to embryonic lethality (unpublished data; Akbarian, S., C. Lo, and G. Zheng, personal communication). Embryos exhibit pathological features strikingly similar to the most prominent phenotypes of the human condition, including severe intrauterine growth restriction, short stature, and microcephaly (unpublished data; Akbarian, S., C. Lo, and G. Zheng, personal communication). Reproduction of many aspects of the human condition in the mouse indicates that much will be learned about the etiology of primordial dwarfism from this experimental system.
Proposed models for pericentrin function in primordial dwarfism.
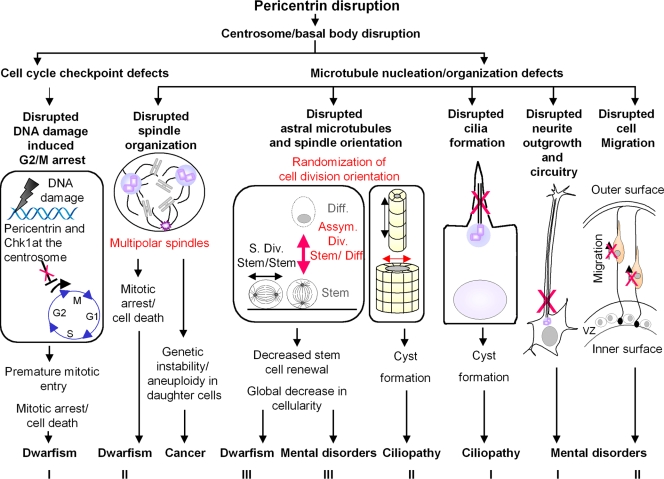
The small body and brain size caused by PCNT mutations in humans and mice suggest a dramatic reduction in the total cellularity of the growing embryo and adult organism. Several cell biological models have been proposed to explain this phenomenon and how it might contribute to the disease (Delaval and Doxsey, 2008; Griffith et al., 2008; Rauch et al., 2008). In all models, pericentrin is viewed as a multifunctional scaffold protein whose disruption results in loss of pericentrin binding proteins from the centrosome and centrosome dysfunction (Fig. 1).
Figure 1.
Disruption of pericentrin function could contribute to human disease through multiple mechanisms. Models for the role of pericentrin in primordial dwarfism. Model I: PCNT disruption leads to centrosome defects including loss of centrosomal Chk1. This perturbs G2/M checkpoint activation in the presence of DNA damage (red X), allowing cells to enter mitosis with damaged DNA and leading to mitotic arrest or cell death. Model II: Centrosome defects such as loss of γ-tubulin compromise microtubule nucleation/organization, leading to spindle defects and, consequently, mitotic arrest or cell death. Model III: Defects in microtubule nucleation/organization disrupt astral microtubules, thus randomizing spindle orientation. This favors asymmetric divisions (Asym. Div, red arrow) and reduces symmetric stem cell self-renewing divisions (S. Div, symmetric division), leading to reduced stem cell number. All models would lead to a decrease in the overall cellularity of tissues. Diff, differentiated cell. Model for the role of pericentrin in cancer progression. Centrosome and associated microtubule defects give rise to disorganized spindles (e.g., multipolar). The resulting aberrant cell divisions cause chromosome losses and gains (aneuploidy), which could lead to accumulation of activated oncogenes and loss of tumor suppressors in daughter cells and thus contribute to cancer progression. Models for the role of pericentrin in mental disorders. Model I: Disruption of centrosome and associated microtubules (red X) perturbs neurite outgrowth and neuronal circuitry. Model II: Disruption of centrosome-mediated microtubule organization leads to defects in cell migration along radial glia (red X). VZ, ventricular proliferative zone. Model III: Disruption of centrosome-mediated astral microtubules depletes neuronal stem cells as described in model III for primordial dwarfism (red arrow). In all models, disruption of brain structure and neurite outgrowth, connectivity, or function would contribute to mental disorders. Models for the role of pericentrin in ciliopathies. Model I: Centrosome disruption, including mislocalization of centrosome proteins and proteins involved in cilia formation, disrupts cilia assembly (red X), leading to ciliopathy-associated phenotypes. Model II: Disruption of centrosome-associated astral microtubules causes randomization of spindle orientation (red arrow), leading to a decrease in cell divisions along the longitudinal axis of the duct (e.g., kidney). This results the expansion of the diameter of the duct, potentially contributing to cyst formation in ciliopathies.
Model 1: pericentrin, cell cycle checkpoint defects, and premature mitotic entry.
Through its protein-anchoring function, pericentrin has been implicated in the regulation of cell cycle progression, cell cycle checkpoints, and mitotic entry. Results obtained from MOPDII patient cells and from pericentrin siRNA-treated cultured human cells demonstrate a role for the protein in the ataxia telangiectasia and rad3 related (ATR)-dependent DNA damage checkpoint signaling (Griffith et al., 2008; Tibelius et al., 2009). Components of the ATR pathway localize to the centrosome (Zhang et al., 2007), including the checkpoint kinase 1 (Chk1; Krämer et al., 2004; Löffler et al., 2007). Centrosome-associated Chk1 delays the G2/M transition by inhibiting the Cdc25-dependent activation of cyclin B–Cdk1 complexes (Krämer et al., 2004). In fact, ectopic localization of Chk1 to centrosomes using Chk1 fused to a centrosome targeting polypeptide (PACT domain; Gillingham and Munro, 2000) prevents the transition from G2 to M (Krämer et al., 2004). A more recent study demonstrates that pericentrin is involved in the centrosomal anchoring of Chk1 and its associated G2/M checkpoint arrest pathway (Tibelius et al., 2009). Pericentrin depletion prevents Chk1 recruitment to centrosomes, causing premature mitotic entry, mitotic delay, and cell death. Importantly, defects in the ATR signaling checkpoint pathway are also associated with other disorders such as microcephaly, a well-documented characteristic of primordial dwarfism, thus providing support for cell cycle checkpoint disruption in primordial dwarfism (O'Driscoll et al., 2003; Alderton et al., 2004).
Collectively, these observations suggest a role for impaired checkpoint signaling in primordial dwarfism. In this model (Fig. 1), pericentrin disruption would lead to mislocalization of Chk1 from centrosomes. This would result in abnormal cell cycle checkpoint signaling during the G2-to-M transition, allowing premature entry into mitosis (Griffith et al., 2008; Tibelius et al., 2009). Unscheduled mitotic entry could induce mitotic defects, leading to mitotic arrest or cell death. This would account for the global decrease in cell number in MODPII.
Model 2: pericentrin, microtubules, and mitotic defects.
A large body of evidence supports a role for pericentrin in microtubule nucleation and spindle organization. Pericentrin homologues in a variety of organisms, including the budding yeast Saccharomyces cerevisiae (spc110p/ Nuf1p; Knop and Schiebel, 1997; Flory et al., 2000), the fission yeast Schizosaccharomyces pombe (Pcp1p; Flory et al., 2002), the filamentous fungus Aspergillus nidulans (Flory et al., 2002), and Drosophila (d-plp; Martinez-Campos et al., 2004), localize to centrosomes or the equivalent structure where they anchor proteins involved in microtubule nucleation and organization (Kilmartin and Goh, 1996; Sundberg et al., 1996; Flory et al., 2002), organize mitotic spindles, and perform mitotic functions (Doxsey et al., 1994; Sundberg et al., 1996; Sundberg and Davis, 1997; Flory et al., 2002; Martinez-Campos et al., 2004). For example, d-plp is required for efficient recruitment of PCM components to centrosomes (Martinez-Campos et al., 2004). Spc110p has affinity for the GCP2/3 orthologues in yeast (spc97p and spc98p) and anchors γ-tubulin complexes at spindle poles bodies (Knop and Schiebel, 1997), thus allowing proper mitotic spindle assembly (Sundberg et al., 1996; Sundberg and Davis, 1997). PCP1p also functions in spindle pole body assembly and microtubule nucleation (Flory et al., 2002).
In humans, the microtubule-nucleating function of pericentrin also requires its recruitment to the PCM, which is accomplished through a microtubule-based transport mechanism involving cytoplasmic dynein (Young et al., 2000). Centrosomal recruitment of pericentrin and other centrosome proteins including the pericentrin-binding protein γ-tubulin, reaches a maximum at metaphase (Dictenberg et al., 1998) in a process called “centrosome maturation” (Glover, 2005; Bettencourt-Dias and Glover, 2007). This process is critical for centrosome-based astral and spindle microtubule nucleation in mitosis. Pericentrin depletion by RNAi disrupts microtubule nucleation from mitotic centrosomes, most likely through mislocalization of the microtubule-nucleating protein γ-tubulin from spindle poles, a phenotype that seems to be mitosis specific because interphase microtubules appear largely intact (Doxsey et al., 1994; Li et al., 2001; Dammermann and Merdes, 2002; Zimmerman et al., 2004). This mitotic function of pericentrin was confirmed and extended by the recent observation that PLK1-dependent recruitment of γ-tubulin complexes to mitotic centrosomes/spindle poles requires pericentrin (Haren et al., 2009) and dynein-dependent microtubule transport. These molecular functions of pericentrin are a likely explanation for the decrease in γ-tubulin from spindle poles observed in cells from individuals with primordial dwarfism (Griffith et al., 2008). Consistent with the previously reported role for pericentrin in spindle organization in cultures of human cells (Purohit et al., 1999; Zimmerman et al., 2004), mitotic defects are observed in cells from MOPDII individuals lacking pericentrin and include disorganized mitotic spindles, chromosome misalignment, premature sister chromatid separation, and aneuploidy (Rauch et al., 2008).
Collectively, these observations suggest a role for spindle dysfunction in primordial dwarfism. In this model (Fig. 1), pericentrin disruption would lead to mislocalization of proteins involved in microtubule nucleation/organization from spindle poles. This would result in mitotic spindle defects, chromosome missegregation and mitotic failure, which could lead to cell cycle arrest and/or cell death (Rauch et al., 2008), thus contributing to the global loss of cellularity seen in MOPDII.
Model 3: pericentrin, astral microtubules, and spindle orientation.
Evidence from the literature and ongoing experiments support a role for spindle misorientation as a contributor to MOPDII. The first clue to this idea comes from studies linking microcephaly to spindle misorientation and abnormal stem cell division. Three centrosome genes have been implicated in this disorder: ASPM, Cdk5RAP2/centrosomin, and CENPJ/SAS-4. All have well-characterized functions including astral microtubule nucleation, spindle organization, and/or orientation (do Carmo Avides and Glover, 1999; Megraw et al., 2001; Basto et al., 2006). Recent studies on these centrosome genes suggest that microcephaly results from the inability to self-renew neuronal stem cells. This appears to occur through misorientation of mitotic spindles, leading to asymmetric divisions that produce differentiating cells instead of stem cells, thus depleting the total cellularity of the stem cell compartment (Bond and Woods, 2006). This would lead to a reduction in the total number of neurons in the brain and a reduced brain size. These results from studies on microcephaly provide a testable model for the mechanism of pericentrin in primordial dwarfism. PCNT mutations could cause short stature and small brain size in primordial dwarfisms through an imbalance in symmetric versus asymmetric cell divisions resulting from spindle misorientation in all organs of the body.
Correct orientation of mitotic spindles and, subsequently, the plane of cell division are achieved through proper nucleation and organization of astral microtubules that emanate from spindle poles (Fig. 1; Toyoshima and Nishida, 2007). Loss of centrosomal γ-tubulin and astral microtubules is observed upon RNAi-mediated knockdown of pericentrin in cultured human cells (Zimmerman et al., 2004), which suggests a role for pericentrin in spindle orientation. In addition, pericentrin overexpression blocks proper mitotic spindle positioning within cells (Purohit et al., 1999).
Work in Drosophila supports a role for pericentrin in microtubule organization and mitosis. D-plp mutant embryos are defective for timely recruitment of PCM components to centrosomes (maturation) and show a reduction in centrosomal microtubules (Martinez-Campos et al., 2004). However, in this system, these defects cause relatively subtle defects in the organization of spindle poles and mitotic spindles (Martinez-Campos et al., 2004). This might be expected because, remarkably, flies completely lacking centrosomes can still complete cell division and develop normally (Basto et al., 2006). Rather than dying from mitotic dysfunction, adults in both cases die from cilia defects. However, it is important to note that flies lacking centrosomes exhibit disorganized astral microtubules and misoriented spindles, which might be expected to adversely affect the balance of symmetric and asymmetric divisions in stem cell compartments (Basto et al., 2006). However, in d-plp mutants, this detail was not examined. Thus, it is possible that d-plp disruption induces more subtle defects in spindles such as changes in spindle orientation. These would induce more moderate yet uncharacterized mitotic defects that could be manifested in specific tissues where proper spindle orientation is important.
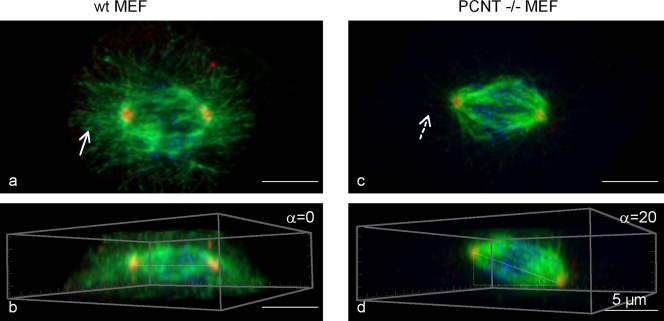
In agreement with a model in which pericentrin is required for correct spindle orientation, fibroblasts isolated from PCNT knockout mice show a significant increase in cells with disrupted astral microtubules and misoriented spindles (Fig. 2; unpublished data; Zheng, G., personal communication). Spindle misorientation is shown by an increase in the angle formed by the pole-to-pole spindle vector and the cell–substrate adhesion plane in pericentrin knockout compared with wild-type fibroblasts. Importantly, disruption of the asymmetric segregation and inheritance of centrosomes during oriented cell division was recently shown to cause premature depletion of progenitors in the ventricular zone of the neocortex (Wang et al., 2009). In vivo observations in a pericentrin knockout mouse model for primordial dwarfism show misorientation of spindles and the cell division plane in neuronal precursors (unpublished data; Akbarian, S., and G. Zheng, personal communication). However, it is not clear if centrosome inheritance is disrupted and leads to a loss of neuronal precursors in the absence of pericentrin.
Figure 2.
Fibroblasts from PCNT knockout mice show loss of astral microtubules and spindle misorientation. Top (a and c) and side (b and d) views of three-dimensional reconstructed immunofluorescence images of mitotic spindle stained for α-tubulin (green), γ-tubulin (red), and kinetochore (CREST, blue). Mitotic spindles are misoriented in PCNT−/− (b) versus wild-type cells (d). α, metaphase spindle angle relative to the bottom of the dish. Astral microtubules in the wild-type cell (a, arrow) are lost in PCNT−/− cells (b, arrow with broken line). MEF, mouse embryonic fibroblast. Images provided by S. Doxsey and G. Zheng (University of Massachusetts Medical School, Worcester MA).
Studies discussed in this section suggest a role for spindle misorientation in primordial dwarfism. In this model (Fig. 1), pericentrin disruption leads to defects in astral microtubules and misorientation of the spindle and the plane of cell division. This would reduce symmetric stem–stem cell divisions required for stem cell self-renewal (Rebollo et al., 2007; Rusan and Peifer, 2007; Yamashita et al., 2007), thus leading to depletion of the pool of progenitor cells in all organs and reducing the overall cellularity in the organism consistent with MOPDII
Summary.
What emerges from these models is a complex picture of the possible cellular and molecular events that contribute to the etiology of human primordial dwarfism (Fig. 1). All of these suggest plausible explanations for the loss of cellularity observed in these disorders and are consistent with small but near-normal organs and bodies. Additional studies will be required to determine if all functions discussed are disrupted and co-contribute to these disorders or if one predominates. It also remains a possibility that other pericentrin functions, suggested by the diversity of pericentrin interacting partners (Table I), contribute to disease etiology.
Pericentrin in human cancer
Centrosome abnormalities and chromosome instability in cancer.
Aberrant centrosome numbers were first described in human cancer cells by T. Boveri (1929). Centrosome defects were rediscovered in human carcinomas and tumor-derived cell lines in 1998 using specific markers (Lingle et al., 1998; Pihan et al., 1998) and have now become a hallmark of most solid tumors and some hematological malignancies. Aberrant centrosomes and associated abnormal mitotic spindles show a strong correlation with chromosome instability, another common feature of cancer cells (Lengauer et al., 1997; Pihan et al., 1998, 2003). Perhaps most important is the recent use of network modeling that directly links breast cancer susceptibility to centrosome defects (Pujana et al., 2007).
Pericentrin in solid tumors and hematological malignancies in humans.
Centrosomal abnormalities in solid tumors are accompanied by increased pericentrin levels and defects in pericentrin organization, demonstrating the utility of pericentrin as a potential marker for centrosome defects in cancer and implicating the protein in cancer progression (Pihan et al., 1998). Consistent with these observations are data from expression profiling showing that PCNT and other centrosome-associated genes are highly expressed in acute myeloid leukemia (AML). Higher levels of pericentrin also correlate with aneuploidy and centrosome aberration levels in AML (Neben et al., 2004; Krämer et al., 2005). In mantle cell lymphoma, pericentrin is part of an expression signature identifying genes associated with tetraploidization (Neben et al., 2007). In chronic myeloid leukemia (CML), blast crisis cells show disrupted/amorphous staining for pericentrin compared with normal and CML chronic phase patients, which suggests that centrosome defects are present in the advanced stage of the disease even if no altered protein expression can be detected (Giehl et al., 2005; Patel and Gordon, 2009). Additional evidence implicating pericentrin in cancer is its interaction with the CML-associated fusion protein p210 BCR-ABL (Patel and Gordon, 2009). This constitutively active tyrosine kinase activates multiple signaling cascades, leading to cell proliferation, genetic instability, and other tumor-associated features. The interaction of pericentrin with BCR-ABL suggests that this centrosomal scaffold may anchor the kinase at the centrosome, where it could modulate centrosome function, as previously suggested for other cancer-associated fusion kinases (Delaval et al., 2005). However, this remains to be tested.
Pericentrin transgenic mouse model of cancer.
Recently, a pericentrin mouse model with increased pericentrin levels was constructed to directly address the role of pericentrin up-regulation in the induction, development, and progression of cancer in vivo (Krämer, A., personal communication). The pericentrin transgenic mice develop a syndrome resembling human myelodysplasia, carcinoma, and sarcoma, thus confirming the oncogenic role of pericentrin in cancer (Krämer, A., personal communication).
Pericentrin may function as an oncogene.
Expression of pericentrin in human cultured prostate cells reproduces many features of aggressive prostate cancer, including centrosome defects, abnormal spindles, chromosome instability, and enhanced anchorage-independent growth. These observations from cell culture experiments confirm work in the mouse, and suggest that pericentrin may have oncogenic activity in human cancer, possibly through its ability to induce spindle dysfunction and aneuploidy.
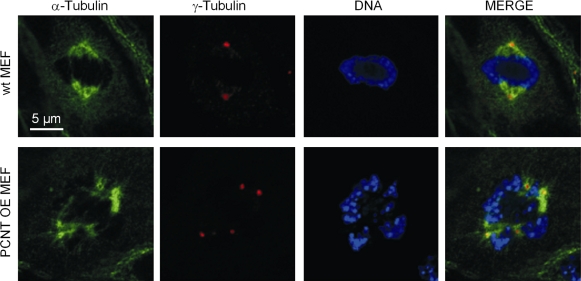
In agreement with this model, fibroblasts isolated from pericentrin-overexpressing mice are aneuploid and show supernumerary centrosomes, multipolar spindles, and bipolar spindles with clustered centrosomes (Fig. 3; Krämer, A., personal communication). A likely mechanism for the formation of supernumerary centrosomes and abnormal mitotic spindles after pericentrin disruption is cytokinesis failure, resulting in twice the number of centrosomes and chromosomes (Chen et al., 2004). They also show an increased mitotic index and decreased proliferation, potentially resulting from the observed mitotic dysfunction. Centrosome clustering may allow cancer cells with multiple centrosomes to undergo relatively normal cell divisions, but, ultimately, it is likely to lead to genetic instability, a known contributor of cancer (Quintyne et al., 2005; Rebacz et al., 2007; Kwon et al., 2008). Like fibroblasts from PCNT transgenic mice, bone marrow cells are aneuploid and also show nuclear abnormalities and increased apoptosis (Krämer, A., personal communication), which suggests that hematopoietic progenitor cells fail to undergo productive divisions and ultimately die as a result of mitotic defects and aneuploidy.
Figure 3.
Fibroblasts from PCNT transgenic mice show an increased number of centrosomes and multipolar spindles. α-tubulin (spindle, green), γ-tubulin (centrosome, red), and DAPI (DNA, blue). MEF, mouse embryonic fibroblast; OE, overexpression. Images provided by A. Krämer (Clinical Cooperation Unit Molecular Hematology/Oncology, German Cancer Research Center, Heidelberg, Germany).
Proposed model for pericentrin function in cancer.
These results support our previously proposed model to explain how disruption of pericentrin function would induce tumor formation (Fig. 1; Pihan et al., 2001; Pihan and Doxsey, 2003). Increased levels of pericentrin would cause alterations in centrosome number, structure, and function. This, in turn, would alter mitotic spindle organization and function, leading to chromosome missegregation. The resulting losses and gains of chromosomes after cell division would generate aneuploidy or chromosomal instability. Cells that accumulate chromosomes with activated oncogenes or lose chromosomes with inactivated tumor suppressors (i.e., changes in gene dosage) would survive by Darwinian selection based on their ability to outlive or outgrow their counterparts.
Pericentrin and mental disorders
Pericentrin in bipolar disorder (BPD), major depressive disorder (MDD), and schizophrenia.
Changes in pericentrin levels are associated with mental disorders. Recent studies reveal elevated pericentrin expression in the postmortem brain and peripheral blood lymphocytes in BPD and in patients with MDD (Anitha et al., 2008). More recent studies suggest a genetic linkage between the PCNT gene and MDD. Genotyping of single-nucleotide polymorphisms (SNPs) in the PCNT gene reveals significant differences in allelic frequencies between patients with MDD and controls for two SNPs (Numata et al., 2009). Furthermore, SNP and haplotype analysis reveals a significant association with schizophrenia (Anitha et al., 2009). Collectively, the data from these studies suggest that pericentrin might play a role in the pathophysiology of BPD, MDD, and schizophrenia, and could be a generalized genetic risk factor for psychiatric illnesses that also influences cognition in healthy subjects (Porteous et al., 2006).
Proposed models for pericentrin in mental disorders.
Clues to the role of pericentrin in mental disorders come from its interaction with the protein product of disrupted in schizophrenia 1(DISC1; Miyoshi et al., 2004; Shimizu et al., 2008), a gene that is a known genetic risk factor for mental disorders including schizophrenia, MDD, and BPD. DISC1 is anchored to centrosomes by pericentrin (Miyoshi et al., 2004). Expression of the DISC1 domain that binds pericentrin releases DISC1 from centrosomes and disrupts microtubules (Shimizu et al., 2008). This suggests that the DISC1–pericentrin interaction might be important for microtubule organization and that disruption of this interaction could contribute to mental disorders. In addition to its microtubule function, DISC1 associates with several proteins involved in processes essential for neuronal function, including neuronal migration, neurite outgrowth, cytoskeletal modulation, and signal transduction (Hennah et al., 2006). This suggests that pericentrin disruption could disrupt any of these processes. Three exploratory models could explain how microtubule defects in pericentrin-disrupted cells could contribute to mental disorders (Fig. 1).
Model 1: PCNT and neurite outgrowth and interconnectivity.
First (Fig. 1), disruption of microtubule integrity in neurons could affect neurite outgrowth and interconnectivity during development (Matsuzaki and Tohyama, 2007). Microtubules play an essential role both in formation and maintenance of neuronal processes including axonal outgrowth and fasciculation, and dendritic arborization. It is worth noting that the pericentrin-binding region of DISC1 overlaps with the region interacting with FEZ1 (Miyoshi et al., 2004), a schizophrenia susceptibility gene that plays a vital role in axonal outgrowth and fasciculation (Yamada et al., 2004). These three proteins may cooperate in establishing and maintaining axons and dendrites.
Model 2: PCNT and cell migration.
Second (Fig. 1), disruption of the centrosome and associated microtubules could affect cell migration (Higginbotham and Gleeson, 2007). An increasing number of psychiatric susceptibility genes regulate neuronal migration, which is important for development and organization of the brain. This includes PCM1 (Gurling et al., 2006), a pericentrin interacting protein (Li et al., 2001) that is also a member of the DISC1 interactome (Kamiya et al., 2008). Suppression of PCM1 in the developing cerebral cortex leads to neuronal migration defects (Kamiya et al., 2008). Therefore, through its interaction with PCM1 and DISC1, pericentrin could affect neuronal migration.
Model 3: PCNT and spindle orientation.
Third (Fig. 1), disruption of mitotic astral microtubules in pericentrin-depleted cells (Zimmerman et al., 2004) is likely to contribute to aberrant spindle orientation (see “Pericentrin in primordial dwarfism”) and consequently could disrupt the asymmetric segregation and inheritance of centrosomes in neuronal stem/progenitor cells. This could decrease neuronal precursor self-renewal and disrupt cellular balances affecting neuron number and tissue organization. In fact, disruption of asymmetric inheritance of centrosomes has recently been shown to lead to premature depletion of progenitors in the developing mammalian neocortex (Wang et al., 2009).
Additional studies are required to directly demonstrate a role for pericentrin in these disorders and test whether pericentrin, DISC1, PCM1, and FEZ1 function together in the same pathway or individually to contribute to cortical development and the pathogenesis of neurodevelopmental disorders.
Pericentrin in ciliopathies
Disruption of cilia formation or function has long been associated with ciliopathies (Hildebrandt and Otto, 2005). PCNT has not been directly linked to ciliopathies in humans, but recent evidence demonstrates that disruption of the gene contributes to ciliopathy-associated phenotypes in model organisms, including Drosophila and mice (Martinez-Campos et al., 2004; Miyoshi et al., 2009).
Pericentrin in mouse and fly models of ciliopathy.
Pericentrin localizes to the base of primary cilia in several mouse embryonic tissues, which suggests a role for the protein in cilia formation (Miyoshi et al., 2006b). In fact, hypomorphic mutations in the mouse PCNT gene lead to compromised assembly of the olfactory cilia of chemosensory neurons in the nasal olfactory epithelium and reduced olfactory performance (Miyoshi et al., 2009). In vivo studies in Drosophila also demonstrate a role for the pericentrin-like protein (d-plp) in cilia formation. d-plp mutant flies are severely uncoordinated, a phenotype associated with malformed sensory cilia that results in disturbed neuronal function and that is consistent with the mouse model. In contrast to the mouse model, d-plp mutant flies are also defective for the formation of motile cilia and flagella (Martinez-Campos et al., 2004). One possible explanation for the difference in results in the two model organisms is the hypomorphic mutation in the mouse and presumably higher protein levels that may allow normal function of motile cilia and flagella.
Pericentrin and cilia formation in cultured cells.
Studies in cultured cells provide additional insight into the role of pericentrin in cilia assembly and function. In noncycling cells, depletion of pericentrin by RNAi shows a dramatic reduction in the assembly of primary cilia (Jurczyk et al., 2004; Graser et al., 2007; Mikule et al., 2007). Mechanistically, pericentrin could function in cilia assembly through its association with known cilia proteins such intraflagellar transport (IFT) proteins and polycystin-2 (Jurczyk et al., 2004). Along these lines, pericentrin showed codependency with IFT proteins in localization to centrosomes (Jurczyk et al., 2004).
Proposed models for pericentrin in ciliopathy and associated cyst formation.
Even without direct evidence for a role for pericentrin in ciliopathies in humans, observations from a pericentrin knockout mice model (unpublished data; Lo, C., and G. Zheng, personal communication) associate the loss of pericentrin with the formation of cysts in the kidney, a phenotype typically associated with ciliopathy.
Model 1: PCNT and cilia formation.
Cilia disruption has long been associated with cystic kidneys (Hildebrandt and Otto, 2005). This suggests a model (Fig. 1) in which disruption of pericentrin would reduce recruitment of protein complexes involved in primary cilia formation to the basal bodies at the base of the primary cilium (centrosome) and consequently lead to loss of cilia and cyst formation.
Model 2: PCNT and spindle orientation.
An alternative model (Fig. 1) for cyst formation in ciliopathies would be misorientation of cell division. Recent work in several mouse models of ciliopathy suggests that misoriented cell division contributes to ciliopathies and cyst formation (Fischer et al., 2006; Jonassen et al., 2008; Patel et al., 2008; Saburi et al., 2008). In these studies, cell division in the epithelial layer is oriented perpendicular to the longitudinal dimension, thus increasing the diameter of ducts. Preliminary observations suggest that pericentrin knockout mice exhibit misoriented cell divisions (unpublished data; Zheng, G., personal communication). This finding, together with the role of pericentrin in spindle positioning previously described (see Pericentrin in primordial dwarfism), suggests that the protein might also contribute to cyst formation in mice through spindle and cell division misorientation.
Concluding remarks
In this review, we discuss diverse human disorders linked to pericentrin by genetics (MOPDII), protein level changes, or association with results from experimental systems. Where possible, we detail and integrate the cell and molecular changes that characterize both human conditions and experimental models of these disorders. What emerges from this review of published and ongoing work (at an early stage in our understanding of the etiology of pericentrin-associated disorders) is a complex picture potentially involving disruption of multiple pathways either alone or together to generate phenotypes likely to contribute to disease etiology (Fig. 1).
A unifying theme underlying the diverse deficiencies associated with pericentrin-associated disorders is loss of microtubule integrity resulting from compromised centrosome function. As a multifunctional scaffold protein, pericentrin binds proteins of diverse cellular pathways. In this model, pericentrin disruption would mislocalize proteins essential for microtubule integrity (γ-tubulin and DISC1) or cilia proteins (IFT) from centrosomes/spindle poles/basal bodies. The resulting loss of microtubule integrity could explain the defects observed both in interphase/G0 (e.g., cilia, neuronal outgrowth, and motility) and mitosis (spindle disorganization and misorientation). Mitotic dysfunction is the feature that appears in all four disorders either through spindle disorganization leading to cell death or aneuploidy, or through spindle misorientation leading to depletion of stem cells. A second potential pathway cocontributing to disease phenotypes is disruption of centrosome proteins involved in cell cycle regulation (Chk1).
The proposed theme that links all disorders does not explain the lack of overlap of some phenotypes. For example, why is cancer not a feature of MOPDII if multipolar spindle formation is a common phenotype to explain both dwarfism and cancer? Future studies are required to further test this model and others to gain a full understanding of the cellular and molecular underpinnings of the disorders associated with mutated, deleted, or elevated levels of pericentrin in human disease.
Acknowledgments
We are particularly grateful to A. Kramer, C. Lo, S. Akbarian, J. Raff, T. Davis, and W. Zimmerman for thoughtful discussions on this manuscript. We also would like to thank A. Kramer, C. Lo, and, from S. Doxsey's laboratory, G. Zheng and S. Redick for sharing unpublished results.
This work was supported by funding from the National Institutes of Health (GM051994-14) to S.J. Doxsey and the Polycystic Kidney Disease Foundation to B. Delaval.
Footnotes
Abbreviations used in this paper:
- ATR
- ataxia telangiectasia and rad3 related
- BPD
- bipolar disorder
- CML
- chronic myeloid leukemia
- IFT
- intraflagellar transport
- MDD
- major depressive disorder
- MOPDII
- Majewski/microcephalic osteodysplastic primordial dwarfism type II
- PCM
- pericentriolar material
- SNP
- single-nucleotide polymorphism
References
- Alderton G.K., Joenje H., Varon R., Børglum A.D., Jeggo P.A., O'Driscoll M. 2004. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum. Mol. Genet. 13:3127–3138 10.1093/hmg/ddh335 [DOI] [PubMed] [Google Scholar]
- Anitha A., Nakamura K., Yamada K., Iwayama Y., Toyota T., Takei N., Iwata Y., Suzuki K., Sekine Y., Matsuzaki H., et al. 2008. Gene and expression analyses reveal enhanced expression of pericentrin 2 (PCNT2) in bipolar disorder. Biol. Psychiatry. 63:678–685 10.1016/j.biopsych.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Anitha A., Nakamura K., Yamada K., Iwayama Y., Toyota T., Takei N., Iwata Y., Suzuki K., Sekine Y., Matsuzaki H., et al. 2009. Association studies and gene expression analyses of the DISC1-interacting molecules, pericentrin 2 (PCNT2) and DISC1-binding zinc finger protein (DBZ), with schizophrenia and with bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B:967–976 10.1002/ajmg.b.30926 [DOI] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. 2006. Flies without centrioles. Cell. 125:1375–1386 10.1016/j.cell.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M., Glover D.M. 2007. Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 8:451–463 10.1038/nrm2180 [DOI] [PubMed] [Google Scholar]
- Bond J., Woods C.G. 2006. Cytoskeletal genes regulating brain size. Curr. Opin. Cell Biol. 18:95–101 10.1016/j.ceb.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Boveri T. 1929. The Origin of Malignant Tumors. Baltimore: Williams & Wilkins Company, 82 pp [Google Scholar]
- Chen D., Purohit A., Halilovic E., Doxsey S.J., Newton A.C. 2004. Centrosomal anchoring of protein kinase C betaII by pericentrin controls microtubule organization, spindle function, and cytokinesis. J. Biol. Chem. 279:4829–4839 10.1074/jbc.M311196200 [DOI] [PubMed] [Google Scholar]
- Dammermann A., Merdes A. 2002. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159:255–266 10.1083/jcb.200204023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaval B., Doxsey S. 2008. Genetics. Dwarfism, where pericentrin gains stature. Science. 319:732–733 10.1126/science.1154513 [DOI] [PubMed] [Google Scholar]
- Delaval B., Létard S., Lelièvre H., Chevrier V., Daviet L., Dubreuil P., Birnbaum D. 2005. Oncogenic tyrosine kinase of malignant hemopathy targets the centrosome. Cancer Res. 65:7231–7240 10.1158/0008-5472.CAN-04-4167 [DOI] [PubMed] [Google Scholar]
- Dictenberg J.B., Zimmerman W., Sparks C.A., Young A., Vidair C., Zheng Y., Carrington W., Fay F.S., Doxsey S.J. 1998. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141:163–174 10.1083/jcb.141.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani D., Langeberg L.K., Doxsey S.J., Scott J.D. 2000. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr. Biol. 10:417–420 10.1016/S0960-9822(00)00422-X [DOI] [PubMed] [Google Scholar]
- do Carmo Avides M., Glover D.M. 1999. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 283:1733–1735 10.1126/science.283.5408.1733 [DOI] [PubMed] [Google Scholar]
- Doxsey S.J., Stein P., Evans L., Calarco P.D., Kirschner M. 1994. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 76:639–650 10.1016/0092-8674(94)90504-5 [DOI] [PubMed] [Google Scholar]
- Doxsey S., Zimmerman W., Mikule K. 2005. Centrosome control of the cell cycle. Trends Cell Biol. 15:303–311 10.1016/j.tcb.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J.F., Torres V., Yaniv M., Pontoglio M. 2006. Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38:21–23 10.1038/ng1701 [DOI] [PubMed] [Google Scholar]
- Flory M.R., Davis T.N. 2003. The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics. 82:401–405 10.1016/S0888-7543(03)00119-8 [DOI] [PubMed] [Google Scholar]
- Flory M.R., Moser M.J., Monnat R.J., Jr., Davis T.N. 2000. Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. USA. 97:5919–5923 10.1073/pnas.97.11.5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory M.R., Morphew M., Joseph J.D., Means A.R., Davis T.N. 2002. Pcp1p, an Spc110p-related calmodulin target at the centrosome of the fission yeast Schizosaccharomyces pombe. Cell Growth Differ. 13:47–58 [PubMed] [Google Scholar]
- Giehl M., Fabarius A., Frank O., Hochhaus A., Hafner M., Hehlmann R., Seifarth W. 2005. Centrosome aberrations in chronic myeloid leukemia correlate with stage of disease and chromosomal instability. Leukemia. 19:1192–1197 10.1038/sj.leu.2403779 [DOI] [PubMed] [Google Scholar]
- Gillingham A.K., Munro S. 2000. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D.M. 2005. Polo kinase and progression through M phase in Drosophila: a perspective from the spindle poles. Oncogene. 24:230–237 10.1038/sj.onc.1208279 [DOI] [PubMed] [Google Scholar]
- Graser S., Stierhof Y.D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M., Nigg E.A. 2007. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179:321–330 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith E., Walker S., Martin C.A., Vagnarelli P., Stiff T., Vernay B., Al Sanna N., Saggar A., Hamel B., Earnshaw W.C., et al. 2008. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 40:232–236 10.1038/ng.2007.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurling H.M., Critchley H., Datta S.R., McQuillin A., Blaveri E., Thirumalai S., Pimm J., Krasucki R., Kalsi G., Quested D., et al. 2006. Genetic association and brain morphology studies and the chromosome 8p22 pericentriolar material 1 (PCM1) gene in susceptibility to schizophrenia. Arch. Gen. Psychiatry. 63:844–854 10.1001/archpsyc.63.8.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.G., Flora C., Scott C.I., Jr., Pauli R.M., Tanaka K.I. 2004. Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am. J. Med. Genet. A. 130A:55–72 10.1002/ajmg.a.30203 [DOI] [PubMed] [Google Scholar]
- Haren L., Stearns T., Lüders J. 2009. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One. 4:e5976 10.1371/journal.pone.0005976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennah W., Thomson P., Peltonen L., Porteous D. 2006. Genes and schizophrenia: beyond schizophrenia: the role of DISC1 in major mental illness. Schizophr. Bull. 32:409–416 10.1093/schbul/sbj079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H.R., Gleeson J.G. 2007. The centrosome in neuronal development. Trends Neurosci. 30:276–283 10.1016/j.tins.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F., Otto E. 2005. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat. Rev. Genet. 6:928–940 10.1038/nrg1727 [DOI] [PubMed] [Google Scholar]
- Jonassen J.A., San Agustin J., Follit J.A., Pazour G.J. 2008. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 183:377–384 10.1083/jcb.200808137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczyk A., Gromley A., Redick S., San Agustin J., Witman G., Pazour G.J., Peters D.J., Doxsey S. 2004. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J. Cell Biol. 166:637–643 10.1083/jcb.200405023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A., Tan P.L., Kubo K., Engelhard C., Ishizuka K., Kubo A., Tsukita S., Pulver A.E., Nakajima K., Cascella N.G., et al. 2008. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch. Gen. Psychiatry. 65:996–1006 10.1001/archpsyc.65.9.996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J.V., Goh P.Y. 1996. Spc110p: assembly properties and role in the connection of nuclear microtubules to the yeast spindle pole body. EMBO J. 15:4592–4602 [PMC free article] [PubMed] [Google Scholar]
- Knop M., Schiebel E. 1997. Spc98p and Spc97p of the yeast gamma-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 16:6985–6995 10.1093/emboj/16.23.6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A., Mailand N., Lukas C., Syljuåsen R.G., Wilkinson C.J., Nigg E.A., Bartek J., Lukas J. 2004. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat. Cell Biol. 6:884–891 10.1038/ncb1165 [DOI] [PubMed] [Google Scholar]
- Krämer A., Neben K., Ho A.D. 2005. Centrosome aberrations in hematological malignancies. Cell Biol. Int. 29:375–383 10.1016/j.cellbi.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Kwon M., Godinho S.A., Chandhok N.S., Ganem N.J., Azioune A., Thery M., Pellman D. 2008. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 22:2189–2203 10.1101/gad.1700908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer C., Kinzler K.W., Vogelstein B. 1997. Genetic instability in colorectal cancers. Nature. 386:623–627 10.1038/386623a0 [DOI] [PubMed] [Google Scholar]
- Li Q., Hansen D., Killilea A., Joshi H.C., Palazzo R.E., Balczon R. 2001. Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1. J. Cell Sci. 114:797–809 [DOI] [PubMed] [Google Scholar]
- Lingle W.L., Lutz W.H., Ingle J.N., Maihle N.J., Salisbury J.L. 1998. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA. 95:2950–2955 10.1073/pnas.95.6.2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler H., Bochtler T., Fritz B., Tews B., Ho A.D., Lukas J., Bartek J., Krämer A. 2007. DNA damage-induced accumulation of centrosomal Chk1 contributes to its checkpoint function. Cell Cycle. 6:2541–2548 [DOI] [PubMed] [Google Scholar]
- Lüders J., Stearns T. 2007. Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8:161–167 10.1038/nrm2100 [DOI] [PubMed] [Google Scholar]
- Majewski F., Goecke T. 1982. Studies of microcephalic primordial dwarfism I: approach to a delineation of the Seckel syndrome. Am. J. Med. Genet. 12:7–21 10.1002/ajmg.1320120103 [DOI] [PubMed] [Google Scholar]
- Majewski F., Ranke M., Schinzel A. 1982. Studies of microcephalic primordial dwarfism II: the osteodysplastic type II of primordial dwarfism. Am. J. Med. Genet. 12:23–35 10.1002/ajmg.1320120104 [DOI] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., Raff J.W. 2004. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 165:673–683 10.1083/jcb.200402130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Maller J.L. 2004. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 306:885–888 10.1126/science.1103544 [DOI] [PubMed] [Google Scholar]
- Matsuzaki S., Tohyama M. 2007. Molecular mechanism of schizophrenia with reference to disrupted-in-schizophrenia 1 (DISC1). Neurochem. Int. 51:165–172 10.1016/j.neuint.2007.06.018 [DOI] [PubMed] [Google Scholar]
- Megraw T.L., Kao L.R., Kaufman T.C. 2001. Zygotic development without functional mitotic centrosomes. Curr. Biol. 11:116–120 10.1016/S0960-9822(01)00017-3 [DOI] [PubMed] [Google Scholar]
- Mikule K., Delaval B., Kaldis P., Jurcyzk A., Hergert P., Doxsey S. 2007. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat. Cell Biol. 9:160–170 10.1038/ncb1529 [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Asanuma M., Miyazaki I., Diaz-Corrales F.J., Katayama T., Tohyama M., Ogawa N. 2004. DISC1 localizes to the centrosome by binding to kendrin. Biochem. Biophys. Res. Commun. 317:1195–1199 10.1016/j.bbrc.2004.03.163 [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Asanuma M., Miyazaki I., Matsuzaki S., Tohyama M., Ogawa N. 2006a. Characterization of pericentrin isoforms in vivo. Biochem. Biophys. Res. Commun. 351:745–749 10.1016/j.bbrc.2006.10.101 [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Onishi K., Asanuma M., Miyazaki I., Diaz-Corrales F.J., Ogawa N. 2006b. Embryonic expression of pericentrin suggests universal roles in ciliogenesis. Dev. Genes Evol. 216:537–542 10.1007/s00427-006-0065-8 [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Kasahara K., Miyazaki I., Shimizu S., Taniguchi M., Matsuzaki S., Tohyama M., Asanuma M. 2009. Pericentrin, a centrosomal protein related to microcephalic primordial dwarfism, is required for olfactory cilia assembly in mice. FASEB J. 23:3289–3297 10.1096/fj.08-124420 [DOI] [PubMed] [Google Scholar]
- Neben K., Tews B., Wrobel G., Hahn M., Kokocinski F., Giesecke C., Krause U., Ho A.D., Krämer A., Lichter P. 2004. Gene expression patterns in acute myeloid leukemia correlate with centrosome aberrations and numerical chromosome changes. Oncogene. 23:2379–2384 10.1038/sj.onc.1207401 [DOI] [PubMed] [Google Scholar]
- Neben K., Ott G., Schweizer S., Kalla J., Tews B., Katzenberger T., Hahn M., Rosenwald A., Ho A.D., Müller-Hermelink H.K., et al. 2007. Expression of centrosome-associated gene products is linked to tetraploidization in mantle cell lymphoma. Int. J. Cancer. 120:1669–1677 10.1002/ijc.22404 [DOI] [PubMed] [Google Scholar]
- Numata S., Iga J., Nakataki M., Tayoshi S., Tanahashi T., Itakura M., Ueno S., Ohmori T. 2009. Positive association of the pericentrin (PCNT) gene with major depressive disorder in the Japanese population. J. Psychiatry Neurosci. 34:195–198 [PMC free article] [PubMed] [Google Scholar]
- O'Connell C.B., Khodjakov A.L. 2007. Cooperative mechanisms of mitotic spindle formation. J. Cell Sci. 120:1717–1722 10.1242/jcs.03442 [DOI] [PubMed] [Google Scholar]
- O'Driscoll M., Ruiz-Perez V.L., Woods C.G., Jeggo P.A., Goodship J.A. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 33:497–501 10.1038/ng1129 [DOI] [PubMed] [Google Scholar]
- Patel H., Gordon M.Y. 2009. Abnormal centrosome-centriole cycle in chronic myeloid leukaemia? Br. J. Haematol. 146:408–417 10.1111/j.1365-2141.2009.07772.x [DOI] [PubMed] [Google Scholar]
- Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. 2008. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17:1578–1590 10.1093/hmg/ddn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan G., Doxsey S.J. 2003. Mutations and aneuploidy: co-conspirators in cancer? Cancer Cell. 4:89–94 10.1016/S1535-6108(03)00195-8 [DOI] [PubMed] [Google Scholar]
- Pihan G.A., Purohit A., Wallace J., Knecht H., Woda B., Quesenberry P., Doxsey S.J. 1998. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 58:3974–3985 [PubMed] [Google Scholar]
- Pihan G.A., Purohit A., Wallace J., Malhotra R., Liotta L., Doxsey S.J. 2001. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 61:2212–2219 [PubMed] [Google Scholar]
- Pihan G.A., Wallace J., Zhou Y., Doxsey S.J. 2003. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 63:1398–1404 [PubMed] [Google Scholar]
- Porteous D.J., Thomson P., Brandon N.J., Millar J.K. 2006. The genetics and biology of DISC1—an emerging role in psychosis and cognition. Biol. Psychiatry. 60:123–131 10.1016/j.biopsych.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Pujana M.A., Han J.D., Starita L.M., Stevens K.N., Tewari M., Ahn J.S., Rennert G., Moreno V., Kirchhoff T., Gold B., et al. 2007. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat. Genet. 39:1338–1349 10.1038/ng.2007.2 [DOI] [PubMed] [Google Scholar]
- Purohit A., Tynan S.H., Vallee R., Doxsey S.J. 1999. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147:481–492 10.1083/jcb.147.3.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne N.J., Reing J.E., Hoffelder D.R., Gollin S.M., Saunders W.S. 2005. Spindle multipolarity is prevented by centrosomal clustering. Science. 307:127–129 10.1126/science.1104905 [DOI] [PubMed] [Google Scholar]
- Rauch A., Thiel C.T., Schindler D., Wick U., Crow Y.J., Ekici A.B., van Essen A.J., Goecke T.O., Al-Gazali L., Chrzanowska K.H., et al. 2008. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 319:816–819 10.1126/science.1151174 [DOI] [PubMed] [Google Scholar]
- Rebacz B., Larsen T.O., Clausen M.H., Rønnest M.H., Löffler H., Ho A.D., Krämer A. 2007. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 67:6342–6350 10.1158/0008-5472.CAN-07-0663 [DOI] [PubMed] [Google Scholar]
- Rebollo E., Sampaio P., Januschke J., Llamazares S., Varmark H., González C. 2007. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev. Cell. 12:467–474 10.1016/j.devcel.2007.01.021 [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Faruki S., Khodjakov A. 2001. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11:413–419 10.1016/S0962-8924(01)02085-2 [DOI] [PubMed] [Google Scholar]
- Rusan N.M., Peifer M. 2007. A role for a novel centrosome cycle in asymmetric cell division. J. Cell Biol. 177:13–20 10.1083/jcb.200612140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S.E., Harrison R., Mount R., McNeill H. 2008. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 40:1010–1015 10.1038/ng.179 [DOI] [PubMed] [Google Scholar]
- Shimizu S., Matsuzaki S., Hattori T., Kumamoto N., Miyoshi K., Katayama T., Tohyama M. 2008. DISC1-kendrin interaction is involved in centrosomal microtubule network formation. Biochem. Biophys. Res. Commun. 377:1051–1056 10.1016/j.bbrc.2008.10.100 [DOI] [PubMed] [Google Scholar]
- Sillibourne J.E., Delaval B., Redick S., Sinha M., Doxsey S.J. 2007. Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity. Mol. Biol. Cell. 18:3667–3680 10.1091/mbc.E06-07-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg H.A., Davis T.N. 1997. A mutational analysis identifies three functional regions of the spindle pole component Spc110p in Saccharomyces cerevisiae. Mol. Biol. Cell. 8:2575–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg H.A., Goetsch L., Byers B., Davis T.N. 1996. Role of calmodulin and Spc110p interaction in the proper assembly of spindle pole body compenents. J. Cell Biol. 133:111–124 10.1083/jcb.133.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Yamagiwa A., Nishimura T., Mukai H., Ono Y. 2002. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell. 13:3235–3245 10.1091/mbc.E02-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibelius A., Marhold J., Zentgraf H., Heilig C.E., Neitzel H., Ducommun B., Rauch A., Ho A.D., Bartek J., Krämer A. 2009. Microcephalin and pericentrin regulate mitotic entry via centrosome-associated Chk1. J. Cell Biol. 185:1149–1157 10.1083/jcb.200810159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F., Nishida E. 2007. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 26:1487–1498 10.1038/sj.emboj.7601599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tsai J.W., Imai J.H., Lian W.N., Vallee R.B., Shi S.H. 2009. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 461:947–955 10.1038/nature08435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M., Geneviève D., Borck G., Baumann C., Baujat G., Bieth E., Edery P., Farra E., Gérard M., Héron D., et al. 2009. Molecular analysis of Pericentrin gene (PCNT) in a series of 24 Seckel/ MOPD II families. J. Med. Genet. In press [DOI] [PubMed] [Google Scholar]
- Yamada K., Nakamura K., Minabe Y., Iwayama-Shigeno Y., Takao H., Toyota T., Hattori E., Takei N., Sekine Y., Suzuki K., et al. 2004. Association analysis of FEZ1 variants with schizophrenia in Japanese cohorts. Biol. Psychiatry. 56:683–690 10.1016/j.biopsych.2004.08.015 [DOI] [PubMed] [Google Scholar]
- Yamashita Y.M., Mahowald A.P., Perlin J.R., Fuller M.T. 2007. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 315:518–521 10.1126/science.1134910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A., Dictenberg J.B., Purohit A., Tuft R., Doxsey S.J. 2000. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell. 11:2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Hemmerich P., Grosse F. 2007. Centrosomal localization of DNA damage checkpoint proteins. J. Cell. Biochem. 101:451–465 10.1002/jcb.21195 [DOI] [PubMed] [Google Scholar]
- Zimmerman W.C., Sillibourne J., Rosa J., Doxsey S.J. 2004. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell. 15:3642–3657 10.1091/mbc.E03-11-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]