Abstract
Recent studies have challenged the view that Langerhans cells (LCs) constitute the exclusive antigen-presenting cells of the skin and suggest that the dermal dendritic cell (DDC) network is exceedingly complex. Using knockin mice to track and ablate DCs expressing langerin (CD207), we discovered that the dermis contains five distinct DC subsets and identified their migratory counterparts in draining lymph nodes. Based on this refined classification, we demonstrated that the quantitatively minor CD207+ CD103+ DDC subset is endowed with the unique capability of cross-presenting antigens expressed by keratinocytes irrespective of the presence of LCs. We further showed that Y-Ae, an antibody that is widely used to monitor the formation of complexes involving I-Ab molecules and a peptide derived from the I-E α chain, recognizes mature skin DCs that express I-Ab molecules in the absence of I-E α. Knowledge of this extra reactivity is important because it could be, and already has been, mistakenly interpreted to support the view that antigen transfer can occur between LCs and DDCs. Collectively, these data revisit the transfer of antigen that occurs between keratinocytes and the five distinguishable skin DC subsets and stress the high degree of functional specialization that exists among them.
Langerhans cells (LCs) constitute a subset of DCs. In their immature state, they reside in the stratified squamous epidermal layer of the skin and in the mucosal epithelia lining the ocular, oral, and vaginal surfaces (Iwasaki, 2007). LCs have long been regarded as the exclusive APCs of the skin, detecting pathogens that penetrate the skin barrier and, after undergoing a phase of maturation, conveying this information via lymphatic vessels to T cells present in cutaneous LNs (CLNs; Steinman and Nussenzweig, 2002; Larregina and Falo, 2005). Recent studies have shown, however, that LCs do not constitute the exclusive APCs of the skin. In addition to LCs, the skin contains a second type of DCs known as dermal DCs (DDCs). Epidermal LCs and DDCs migrate to CLNs under both steady-state and inflammatory conditions and constitute the direct precursors of the migratory LCs (mLCs) and migratory DDCs (mDDCs) found in CLNs, respectively. Some studies also suggested that migratory skin DCs play an indirect role in T cell priming, possibly by ferrying skin-derived antigens to those DCs that reside throughout their life cycle in CLNs and are denoted as lymphoid tissue–resident DCs to distinguish them from tissue-derived migratory DCs (Allan et al., 2003; Carbone et al., 2004; Allenspach et al., 2008).
Langerin (CD207) is a C-type lectin originally thought to be specifically expressed in LCs (Valladeau et al., 2000; Kissenpfennig et al., 2005a). The use of Lang-EGFP mice that express an enhanced GFP (EGFP) under the control of the langerin gene showed that CD207 alone is not a reliable marker for the identification of LCs once they have migrated outside the epidermis (Kissenpfennig et al., 2005b) and led to the identification of three subsets of CD207+ DCs in steady-state CLNs (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007; Shklovskaya et al., 2008). A minor subset corresponds to lymphoid tissue–resident CD207low CD8α+ DCs and represents ∼10% of the CD207+ DCs found in CLNs. The two other subsets account for ∼90% of the CD207+ cells present in CLNs and, consistent with their CD11cinter-to-high MHCIIhigh phenotype, originate from the skin. They result from two independent developmental pathways that coexist in steady-state conditions. The first pathway gives rise to epidermal LCs and to their migratory derivatives found in CLNs, whereas the second pathway generates the CD207+ DCs that reside in the dermis and their CD207+ mDDC progeny (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007; Shklovskaya et al., 2008). LCs are radio resistant, and their numbers are maintained through continuous in situ proliferation (Merad et al., 2002; Tripp et al., 2004; Poulin et al., 2007). In contrast, the continuous renewal of DDCs and of lymphoid tissue-resident DCs depends on blood-borne radiosensitive BM precursors (Liu et al., 2009). As a consequence, in lethally irradiated mice reconstituted with BM transplants, LCs in the epidermis and their migratory counterparts in the dermis and CLNs remain of host origin, whereas other DC subsets are primarily repopulated by donor BM–derived cells (Merad et al., 2002).
The role played by LCs and DDCs during skin immune responses remains controversial (Kaplan et al., 2008; Lee et al., 2009). Therefore, the present study intends to further analyze the phenotypic and functional complexity of the DC network present in the skin and of their migratory derivatives present in CLNs. Based on the expression of CD207, CD11b, and CD103, we identified five distinct skin DC subsets and evaluated whether some functional specialization exists among them. We examined the contribution of each of them to the presentation of keratinocyte- or LC-expressed antigens. We demonstrated that CD207+ CD103+ DDCs are endowed with the unique capability of cross-presenting a model antigen expressed by keratinocytes and showed that such a task can be accomplished irrespective of the presence of LCs. In contrast to a previous study (Ginhoux et al., 2007), we also demonstrated that DDCs do not have the capacity to capture a model antigen carried by mLCs en route to the CLNs.
RESULTS
Five distinct DC subsets coexist in steady-state dermis
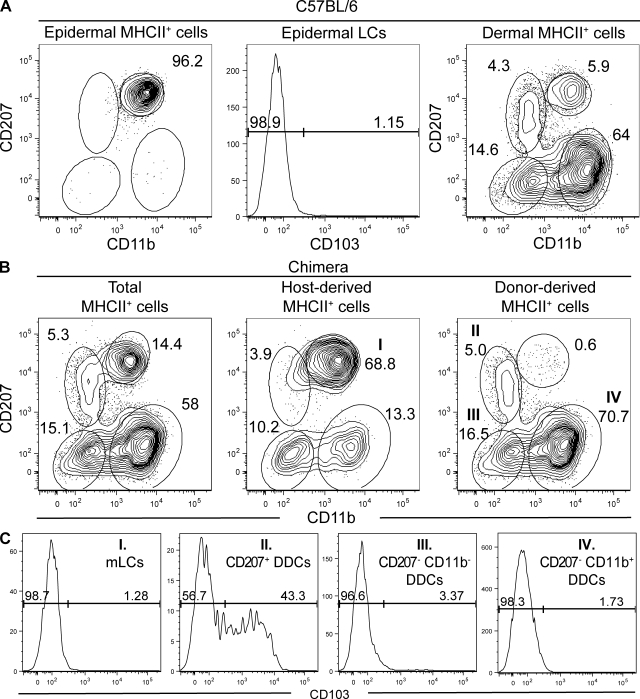
We previously characterized the DCs that migrate out of whole skin explants in response to the CCL21 chemokine (Poulin et al., 2007). However, this approach does not permit us to establish the epidermal or dermal origin of the migratory DCs and likely distorts the representation of the various skin DC subsets based on their differential ability to respond to CCL21 (Nagao et al., 2009). To circumvent these limitations, the epidermis of C57BL/6 mice was separated from the dermis using dispase treatment and the DCs associated with each compartment were extracted using collagenase digestion. CD11c expression is generally used to define DCs. However, collagenase and dispase digestion tend to affect CD11c expression, whereas MHCII expression was insensitive to such treatment. Therefore, after exclusion of rare autofluorescent cells (primarily macrophages; Ng et al., 2008), MHCIIhigh cells present in epidermal and dermal cell suspensions were analyzed for CD11b and CD207 expression. The epidermis contained a single homogeneous CD207high CD11bint DC population that did not express the integrin αE chain (CD103) and corresponds to LCs (Fig. 1 A). Among the MHCIIhigh cells present in the dermis, four DC subsets were distinguished based on their CD207high CD11bint, CD207+ CD11blow, CD207− CD11b−, and CD207− CD11b+ phenotype (Fig. 1 A).
Figure 1.
The skin contains five distinguishable DC subsets. Flow cytometry analysis of epidermal and dermal cell suspensions from C57BL/6 (B6) and B6 (CD45.1)→B6 (CD45.2) BM chimeras. After gating out autofluorescent cells using the AmCyan channel (den Haan et al., 2000; Wilson et al., 2003), MHCII+ cells were analyzed for expression of langerin (CD207) versus CD11b, and CD103 expression was determined on each of the specified DC subsets. (A) In B6 mice, the epidermis contains CD207high CD11bint LCs that are CD103− and the dermis contains four DC subsets that can be distinguished on the basis of CD11b and CD207 expression. (B) DCs found in the dermis of B6 (CD45.1)→B6 (CD45.2) BM chimeras were segregated into host-derived and donor-derived cells using CD45.1 staining (Fig. S1). Host-derived CD45.1− CD207high CD11bint cells correspond to mLCs that are in transit to CLNs. Donor-derived CD45.1+ DDCs segregate into CD207+ CD11blow, CD207− CD11b+, and CD207− CD11b− subsets. (C) Analysis of the expression of CD103 on the four DC subsets present in the dermis showed that it is only expressed on a fraction of the CD207+ CD11blow subset and thus allows us to define five skin DC subsets. The percentages of cells found in each of the specified gates are indicated. Data shown are representative of at least 12 chimeric mice corresponding to six independent experiments.
LCs are radio resistant and, thus, differ from the radio-sensitive BM-derived precursors that continuously renew the DDC pool (Merad et al., 2002). This distinct sensitivity to radiation was used to determine which subset among the two CD207+ DDC subsets corresponded to epidermal mLCs en route to the CLNs. Accordingly, lethally irradiated B6 (CD45.2) mice were reconstituted with BM cells isolated from B6 (CD45.1) mice. Analysis of those chimeras, called B6 (CD45.1)→B6 (CD45.2) chimeras, 8 wk after BM transplantation showed that the CD207high CD11bint DC subset present in the dermis was entirely of host origin and thus corresponded to mLCs (Fig. 1 B, middle; and Fig. S1), whereas the CD207+ CD11blow DC subset was of donor origin and corresponded to CD207+ DDCs (Fig. 1 B). Despite complete donor chimerism, as assessed using blood B cells, we sporadically found that, in some chimeras, up to 4% of the DDCs remained of host origin (Fig. 1 B, middle). Therefore, as recently suggested (Bogunovic et al., 2006), a small number of DDCs is likely regenerated by local radio-resistant precursors. Within cells of donor origin, two subsets of DDCs can be distinguished in addition to the CD207+ CD11blow subset, and they correspond to CD207− CD11b+ and CD207− CD11b− DDCs (Fig. 1 B). Among the four DC subsets present in the dermis, CD103 was expressed on approximately half of the CD207+ CD11blow DDCs (Fig. 1 C). Therefore, contrary to a previous study suggesting that the whole CD207+ CD11blow DDC subset expresses CD103 and that such a feature can be used to distinguish CD207+ DDCs from both mLCs and CD207− DDCs (Bursch et al., 2007), our results demonstrate that CD103 expression alone cannot be used to identify CD207+ DDCs because only half of them express such a marker.
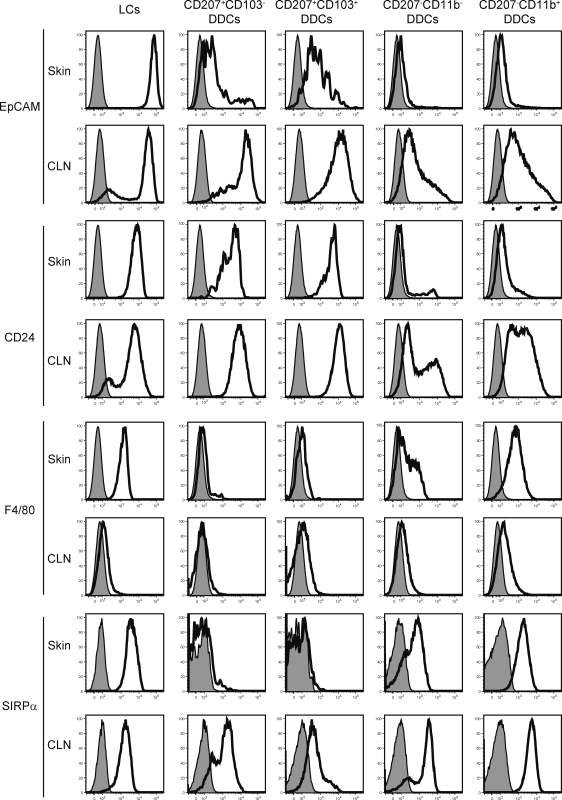
Consistent with a recent study (Nagao et al., 2009), the putative cell adhesion molecule epithelial cell adhesion molecule (EpCAM; CD326) was found to be highly expressed by LCs. Both the CD207+ CD103+ and CD207+ CD103− DDC subsets also expressed EpCAM but at lower levels than LCs (Fig. 2). As recently described (Stutte et al., 2008), CD24 expression correlated with CD207 expression and was thus highly expressed by both the LCs and CD207+ DDC subsets (Fig. 2). As summarized in Table I, five distinct subsets can thus be identified in steady-state dermis using the CD207, CD11b, and CD103 markers. CD207− CD11b+ (65.8 ± 5%) and CD207− CD11b− (16.3 ± 1.3%) DDCs constitute the majority of the DCs found in steady-state dermis, whereas CD207+ CD103− (2.8 ± 0.5%) and CD207+ CD103+ (2.6 ± 0.6%) DDCs represent quantitatively minor populations of DDCs. Finally, mLCs en route to the CLNs corresponded to 6.4 ± 2% of the DCs found in the dermis.
Figure 2.
Expression of EpCAM, CD24, F4/80, and SIRPα on the five skin DC subsets before and after their migration to CLNs. DC subsets were prepared as specified in Figs. 1 and 3 from dermis and CLNs from B6 (CD45.1)→B6 (CD45.2) BM chimeras and analyzed for the expression of EpCAM, CD24, F4/80, and SIRPα. Isotype control staining is shown by the shaded histograms. In steady-state skin, LCs and CD207− CD11b+ DDCs were homogeneously F4/80+. CD207− CD11b− DDCs showed a heterogeneous pattern of F4/80 expression, whereas both subsets of CD207+ DDCs were F4/80−. Upon migration to the CLNs, F4/80 expression was down-regulated on mLCs and CD207− mDDCs. If we except mLCs that showed a slight decrease in Sirpα levels, Sirpα expression was up-regulated or induced on all the remaining skin-derived migratory DC subsets. Data shown are representative of at least six chimeric mice corresponding to three independent experiments.
Table I.
Phenotype and frequency of the five skin DC subsets prior to and following their migration to CLNs
| Skin subset | Phenotypes | Frequency |
| % | ||
| Epidermis | ||
| Epidermal LCs | CD207++, CD11bint, CD103−, EpCAM++, CD24+, Sirpα+ | 100 ± 1 |
| Dermis | ||
| LCs in transit | CD207++, CD11bint, CD103−, EpCAM++, CD24+, Sirpα+ | 6.4 ± 1 |
| CD207+ CD103− DDCs | CD207+, CD11blow, CD103−, EpCAM−/low, CD24+, Sirpα− | 2.8 ± 0.5 |
| CD207+ CD103+ DDCs | CD207+, CD11blow, CD103+, EpCAM−/low, CD24+, Sirpα− | 2.6 ± 0.6 |
| CD207− CD11b− DDCs | CD207−, CD11b−/low, CD103−, EpCAM−, CD24−, Sirpαlow | 16.3 ± 1.3 |
| CD207− CD11b+ DDCs | CD207−, CD11bhigh, CD103−, EpCAM−, CD24−, Sirpα+ | 65.8 ± 5 |
| CLNs | ||
| mLCs | CD207+, CD11b−/low ↓, CD103−, EpCAM+, CD24+, Sirpα+ | 9.5 ± 2.9 |
| CD207+ CD103− mDDCs | CD207+, CD11b−/low, CD103−, EpCAM+ ↑, CD24+, Sirpα+ ↑ | 7.0 ± 4.2 |
| CD207+ CD103+ mDDCs | CD207+, CD11b−/low, CD103+, EpCAM+ ↑, CD24+, Sirpαlow | 13.5 ± 4.5 |
| CD207− CD11b− mDDCs | CD207−, CD11b−, CD103bim ↑, EpCAMbim ↑, CD24bim ↑, Sirpα+ ↑ | 20.8 ± 2.5 |
| CD207− CD11b+ mDDCs | CD207−, CD11b+, CD103bim ↑, EpCAMint ↑, CD24int ↑, Sirpα+ | 35.2 ± 2.6 |
The percentage ± SEM of each subset among MHCII+ (epidermis and dermis) or MHCIIhigh CD11cinter-to-high (CLN) cells were calculated from at least six independent experiments. Upward and downward pointing arrows indicate markers that were up-regulated or down-regulated, respectively, upon migration to CLNs. Bim, bimodal.
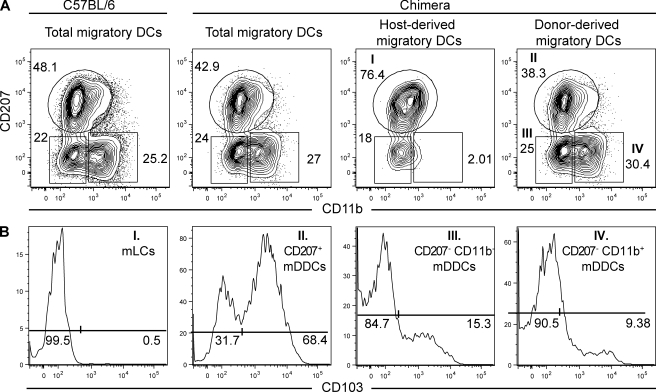
Five skin-derived DC subsets coexist in steady-state CLNs
After migration to CLNs, skin-derived DCs increase the levels of MHCII molecules that they express at their surface and can be identified as MHCIIhigh CD11cinter-to-high cells (Dakic et al., 2004; Kissenpfennig et al., 2005b). When MHCIIhigh CD11cinter-to-high skin-derived DCs were analyzed for the expression of CD207 and CD11b, CD207− CD11b+ and CD207− CD11b− mDDC clusters were readily identified (Fig. 3 A). However, in contrast to the situation observed in the dermis, CD11b did not allow discrimination of CD207+ skin-derived DCs into CD11bint and a CD11blow clusters. Instead, a single CD207+ CD11b−/low cluster was found and analysis of B6 (CD45.1)→B6 (CD45.2) BM chimeras showed that this cluster contained both radio-resistant mLCs and radio-sensitive CD207+ mDDCs (Fig. 3 A). Akin to their dermal counterparts, the radio-sensitive CD207+ mDDCs were CD11blow and could be further segregated into CD103+ and CD103− subsets (Fig. 3 B). As described in the last Results section, after in vivo diphtheria toxin (DT) treatment of Lang-DTREGFP mice, LCs and CD207+ DDCs repopulate the skin with different kinetics, and it is thus possible to obtain mice that are deprived of LCs but contain a fully reconstituted CD207+ DDC compartment in both the dermis and the CLNs. Analysis of the CD207+ DDCs present in both the dermis and the CLNs of those LC-less mice showed that they are made of CD103+ and CD103− fractions, the frequency of which is similar to that found in mice that contain a normal LC compartment (Fig. S2). Therefore, the CD207+ CD103− DDCs do not result from the contamination of the CD207+ DDC subset by CD207+ CD103− mLCs. When compared with mLCs in transit in the dermis, the mLCs found in CLNs showed down-regulated levels of CD11b (Fig. 3 A), and it is this decrease in CD11b expression that prevented the discrimination of mLCs from CD207+ mDDCs. Therefore, analysis of the CLNs of B6 (CD45.1)→B6 (CD45.2) BM chimeras demonstrates that it is still possible to identify a CLN counterpart for each of the five DC subsets identified within the dermis (Table I).
Figure 3.
Draining CLNs contain five skin-derived DC subsets. Flow cytometry analysis of the skin-derived migratory DCs (MHCIIhigh CD11cinter-to-high) present in CLNs from C57BL/6 (B6) mice and B6 (CD45.1)→B6 (CD45.2) BM chimeras. MHCIIhigh CD11cinter-to-high DCs were analyzed for the expression of CD207 versus CD11b and CD103 expression determined on each of the specified DC subsets. (A) A single CD207+ CD11b−/low DC cluster is present in the CLNs of B6 mice. Unlike DDCs, LCs are radio resistant, and this permits the use of B6 (CD45.1)→B6 (CD45.2) chimeras to split the single CD207+ CD11b−/low DC cluster into host-derived mLCs and into CD103+ and CD103− donor-derived mDDCs. Two additional mDDC subsets with a CD207+ CD11b− and CD207− CD11b+ phenotype can be identified in both C57BL/6 mice and B6 (CD45.1)→B6 (CD45.2) chimeras. (B) Expression of CD103 among the DC subsets found in the dermis of B6 (CD45.1)→B6 (CD45.2) BM chimeras. The percentages of cells found in each of the specified gates are indicated. Data shown are representative of at least 12 chimeric mice corresponding to six independent experiments.
The relative representation of the five skin-derived DC subsets present within CLNs differs from that found in the dermis (Table I). In CLNs, CD207− CD11b− and CD207− CD11b+ mDDCs corresponded to 20.8 and 35.2% of skin-derived DCs, respectively, whereas CD207+ CD103+ and CD207+ CD103− mDDCs corresponded to 13.5 and 7.0%, of skin-derived DCs, respectively. CD207+ CD103− mLCs accounted for 9.5% of the skin-derived DCs. The difference existing in the representation of those different DC subsets between the dermis and CLNs could reflect a different rate of migration from the skin or survival within the CLNs. Altogether, the MHCIIhigh CD11cinter-to-high skin-derived DCs present in CLNs are sevenfold more numerous than the MHCIIlow CD11chigh lymphoid tissue–resident DCs (Fig. S3). Furthermore, it should be noted that our MHCII-CD11c gating strategy excluded the lymphoid tissue–resident CD207+ CD8α+ DCs from the skin-derived migratory DC fraction(Fig. S3). Comparison of the phenotype of each skin DC subset before and after migration to CLNs showed that EpCAM expression was maintained at high levels on mLCs, whereas its expression increased on CD207+ mDDCs and was induced on CD207− mDDCs (Fig. 2).
Comparison of the expression of CD40, CD80, CD86, and PD-L1 (CD274) on the five skin-derived DCs after their migration to CLNs showed that they expressed higher levels of CD40, CD80, CD86, and PD-L1 at the cell surface, a phenotype matching that expected for mature DCs (Fig. S4). Akin to the situation encountered in the dermis, 31.7% of the CD207+ mDDCs were CD103−. Therefore, congruent with a recent study (Shklovskaya et al., 2008), our data show that CD103 expression does not permit the unequivocal discrimination of CD207+ DCs of epidermal and dermal origin because a substantial fraction of the CD207+ mDDCs is CD103− and will therefore contaminate the CD103− mLC fraction. Moreover, CD103 was induced on 15.3 and 9.4% of the CD207− CD11b− and CD207− CD11b+ mDDCs, respectively (Fig. 3 B), an observation which is particularly relevant for the functional studies that are described in a subsequent section. Therefore, at present, the only way to discriminate between mLCs and CD207+ CD103− mDDCs present in CLNs is through the use of BM chimeras.
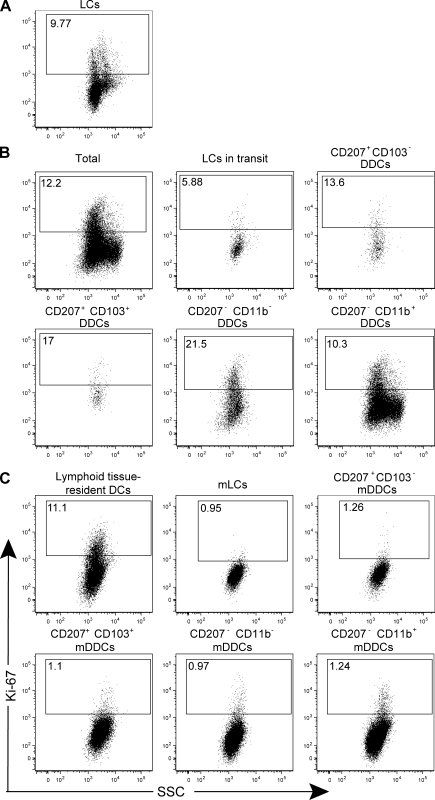
Proliferation and turnover of the five skin DC subsets before and after their migration to CLNs
The five distinguishable skin DC subsets were analyzed before and after their migration to CLNs for the expression of Ki-67, a nuclear protein specifically expressed in cells which are actively cycling (Fig. 4 and Fig. S5). Approximately 10% of epidermal LCs were Ki-67+, and this percentage dropped to 6% in the mLCs that are present in transit in the dermis and to 1% in the mLCs found in CLNs (Fig. 4, A–C). In the dermis, the highest levels of Ki-67 expression were found in the CD207+ CD103+ (17%) and CD207− CD11b− (21.5%) subsets, with the CD207+ CD103− and CD207− CD11b+ subsets showing 13.6 and 10.3% Ki-67+ cells, respectively. In CLNs, 11% of the lymphoid tissue–resident DCs were Ki-67+, whereas, at most, 1.2% of the skin-derived DC subsets expressed Ki-67. Therefore, the five identified DC subsets proliferate to a different extent when localized in the skin and largely lose this potential upon migration to the CLNs, a feature which permits us to perform, as shown in the subsequent paragraph, appropriate BrdU labeling experiments.
Figure 4.
The five skin DC subsets proliferate before their migration to LNs. (A–C) The five skin DC subsets (A and B) and their migratory counterparts found in the CLNs (C) were identified using B6 (CD45.1)→B6 (CD45.2) chimeras, as specified in Figs. 1 and 3, and analyzed for Ki-67 expression. Lymphoid tissue–resident DCs were defined on the basis of their CD11chigh MHCIIinter phenotype (Fig. S3). Positioning of the Ki-67+ gate is based on staining with isotype control antibody (Fig. S4). Data shown are representative of three independent experiments.
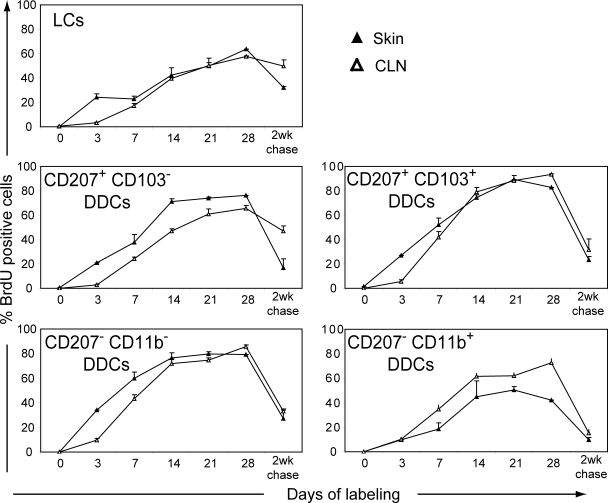
To determine the turnover of the five skin DC subsets before and after their migration to CLNs, B6 (CD45.1)→B6 (CD45.2) chimeras were continuously exposed to BrdU for up to 4 wk (Fig. 5). Consistent with previous data (Kamath et al., 2002; Vishwanath et al., 2006; Poulin et al., 2007), LCs had a slow turnover and only 50% of them were replaced after 3 wk of BrdU treatment. Removing BrdU after 4 wk of continuous administration and proceeding to a 2-wk-long chase showed that LCs are slowly replaced by unlabeled cells, with this replacement occurring first in the epidermis. Both CD207+ CD103+ and CD207− CD11b− DDCs exhibited a faster turnover than LCs, and 50% of them were replaced after 7 d of BrdU exposure and, furthermore, showed rapid loss of BrdU staining after a 2-wk-long chase. The delay that exists in the ascending part of the BrdU labeling curve corresponding to the CD207+ CD103+ mDDCs present in CLN as compared with that of the CD207+ CD103+ DDCs present in the skin suggests that they are related through a precursor–product relationship (Fig. 5). A similar delay was also found for CD207− CD11b− DDCs, the turnover of which was intermediate between the fast and the slow turnover observed for CD207+ CD103+ DDCs and LCs, respectively. Unexpectedly, the CD207− CD11b+ DDCs found in the dermis showed a slower rate of BrdU incorporation than the CD207− CD11b+ mDDCs found in CLNs. As analyzed in the Discussion section, such discrepancy is likely a result of the fact that the CD207− CD11b+ DDCs present in the dermis include CD11c− cells that are MHCIIhigh CD11b+. Altogether, these data show that most DDCs have fast labeling kinetics that are only slightly slower than those of lymphoid tissue–resident DCs (Liu et al., 2007). Moreover, the initial lag in BrdU labeling observed for most of the distinguishable subsets between their skin and CLN counterparts likely corresponds to the time taken by the BrdU-labeled cells to migrate from the skin to the CLNs.
Figure 5.
Comparison of the BrdU-labeling kinetics of the five distinct skin DC subsets before and after their migration to CLNs. BrdU was administered continuously for 28 d to a group of two B6 (CD45.1)→B6 (CD45.2) chimeric mice. After 28 d of continuous BrdU administration, a group of B6 (CD45.1)→B6 (CD45.2) chimeras was kept without BrdU and analyzed 2 wk later (2-wk chase). DC subsets were defined as described in Figs. 1 and 3, and the percentage of BrdU+ cells was determined. Data shown are representative of three independent experiments. Positioning of the positive gate is based on staining with isotype control antibody (not depicted). Error bars correspond to the SEM.
Mature DCs from Eα-deficient mice express Y-Ae–recognizable pMHCII complexes
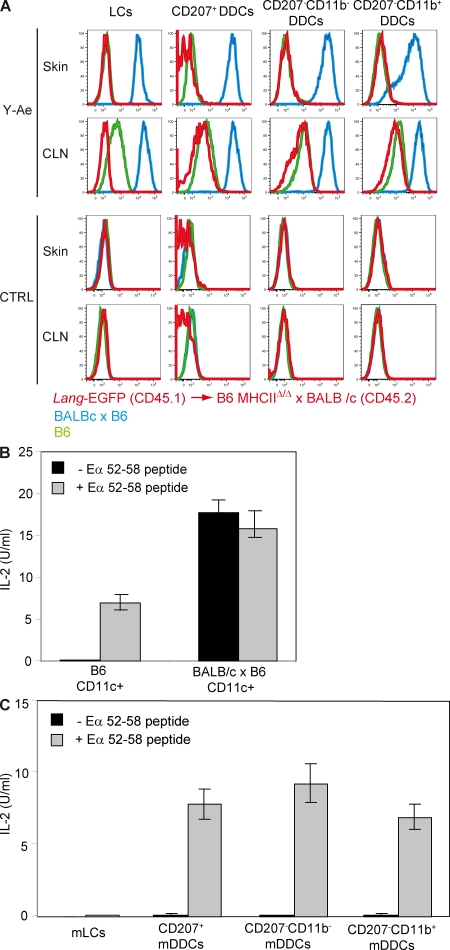
It has been suggested that LCs in transit to CLNs can transfer the antigens they have captured in the epidermis to the DDCs that continuously crawl through the interstitial space of the dermis (Ng et al., 2008). Such exchanges may amplify immune responses by recruiting more skin-derived DCs to present antigens in CLNs (Villablanca and Mora, 2008). To demonstrate that CD207+ and CD207− DDCs can capture LC-derived antigens and subsequently convey them to CLNs under steady-state conditions, Ginhoux et al. (2007) took advantage of the specificity of the Y-Ae monoclonal antibody. Y-Ae recognizes peptide–MHCII (pMHCII) complexes made of I-Ab molecules and of a peptide corresponding to residues 52–68 of the I-E α chain (Eα52-68; Murphy et al., 1992). F1 mice resulting from the cross of BALB/c (I-Ed+) mice expressing the Eα52-68 peptide and of B6 mice that express a null allele of the Ab gene and are thus deprived of I-Ab molecules were lethally irradiated and reconstituted with BM cells isolated from B6 Lang-EGFP mice. In these B6 Lang-EGFP→C57BL/6 I-Ab– x BALB/c chimeras, radio-resistant LCs are deprived of I-Ab molecules but have the potential to transfer Eα52-68 peptides to donor-derived CD207+ and CD207− DDCs that are the only skin DCs that express I-Ab molecules. Both CD207+ and CD207− DDCs were found to be positive for Y-Ae, a feature thought to establish their capacity to capture and present the LC-derived Eα52-58 peptide. Based on those results it was concluded that, under steady-state conditions, DDCs interact with mLCs in the dermis and migrate to the CLNs laden with LC-derived antigenic peptides (Ginhoux et al., 2007).
Although B6 mice express I-Ab molecules, they do not have a functional I-Eα gene (Mathis et al., 1983) and cannot generate Eα52-68–I-Ab complexes. As a result, their B cells show no detectable Y-Ae staining. However, upon LPS treatment, activated B cells from B6 mice stain with Y-Ae (Viret and Janeway, 2000). This unexpected reactivity depends on the expression of the MHCII exchange factor H-2M and thus corresponds to the recognition of pMHCII complexes involving I-Ab and endogenous peptides distinct from Eα52-58 (Viret and Janeway, 2000). Furthermore, Y-Ae was also found to be capable of staining mature DCs present in late cultures of B6 BM progenitors (Viret and Janeway, 2000). This prompted us to determine whether Y-Ae–recognizable epitopes can be found on skin DCs that originate from B6 mice and that have matured under physiological in vivo conditions, a key control which was not included in the experiments of Ginhoux et al. (2007). Therefore, we compared Y-Ae expression on the various skin-derived DCs of B6 mice before and after their migration to CLNs (Fig. 6 A). Consistent with their capacity to coexpress Eα52-68 peptides and I-Ab molecules, mLCs, CD207+, CD207− CD11b−, and CD207− CD11b+ mDDCs isolated from CLNs of B6 x BALB/c F1 mice displayed high levels of Y-Ae staining and their immature precursors present in the skin also exhibited strong Y-Ae staining (Fig. 6 A). The level of Y-Ae staining was, however, higher on the skin-derived DC subsets isolated from the CLNs of B6 x BALB/c F1 mice as compared with their dermal counterparts, a finding which is consistent with the fact that upon migration to CLNs, skin-derived DCs express up-regulated levels of MHCII and enhanced antigen-processing activity (Kissenpfennig et al., 2005b; Spörri and Reis e Sousa, 2005; Jiang et al., 2007). As expected, DCs isolated from the skin and CLNs of I-Ab–negative BALB/c mice showed no Y-Ae staining (unpublished data). Although LCs, CD207+, CD207− CD11b−, and CD207− CD11b+ DDCs isolated from the skin of Eα52-68-negative B6 mice showed no detectable staining with Y-Ae, their mature derivatives isolated from steady-state B6 CLNs showed readily detectable Y-Ae reactivity (Fig. 6 A). Importantly, the homogeneous density distribution of Y-Ae staining displayed by those mature DC subsets was markedly lower than that observed on the corresponding DC subsets isolated from CLNs of mice expressing Eα52-68–I-Ab complexes. Therefore, the observations of Viret and Janeway (2000) also apply to in vivo conditions in that Y-Ae–recognizable epitopes are readily detectable on mLCs, CD207+, CD207− CD11b−, and CD207− CD11b+ mDDCs that are found in steady-state CLNs of mice that express I-Ab molecules in the absence of Eα chains and, thus, of Eα52-68 peptides. The absence of detectable Y-Ae staining on immature skin DCs from B6 mice is likely a result of their lower expression of MHCII molecules or of those endogenous peptides that confer Y-Ae reactivity once bound to I-Ab.
Figure 6.
DDCs do not present Eα52-68 LC-derived peptides. (A) Flow cytometry analysis of LCs, CD207+, CD207− CD11b−, and CD207− CD11b+ DDCs isolated from Langerin-EGFP→B6 MHCIIΔ/Δ x BALB/c chimeras (red) and from B6 (green) and B6 x BALB/c (blue) mice before (skin) and after (CLN) their migration to CLN. Cells were separately stained with Y-Ae and isotype control staining (CTRL). (B) CD11c+ DCs isolated from B6 and B6 x BALB/c mice were cultured together with H30 T cells in the presence or absence of 3 µg/ml Eα52-68 peptide. The content of IL-2 present in supernatant was determined after 24 h of culture. (C) LCs, CD207+, CD207− CD11b−, and CD207− CD11b+ DDCs isolated from the CLNs of Langerin-EGFP→B6 MHCIIΔ/Δ x BALB/c chimeras were cultured together with H30 T cells that are specific for Eα52-68–I-Ab complexes and in the presence or absence of 3 µg/ml Eα52-68 peptide. The content of IL-2 present in supernatant was determined after 24 h of culture. Data shown are representative of three independent experiments. Error bars correspond to SEM.
DDCs do not capture LC-derived Eα52-68 peptides
The results mentioned in the previous section suggest that the low levels of Y-Ae reactivity observed by Ginhoux et al. (2007) on CD207+ mDDCs present in the CLNs of B6 Lang-EGFP→C57BL/6 I-Ab– x BALB/c chimeras might not result from the capture of LC-derived Eα52-68 peptides but might correspond to the expression of I-Ab molecules bound to endogenous peptides. To validate this alternative hypothesis, F1 mice resulting from the cross of BALB/c mice and of B6 mice deprived of MHCII molecules (B6 MHCIIΔ/Δ; Madsen et al., 1999) were lethally irradiated and reconstituted with BM cells isolated from Lang-EGFP (CD45.1) mice. In these chimeras, LCs are deprived of I-Ab molecules but will have the potential to transfer Eα52-68 peptides to DDCs. LCs, CD207+, CD207− CD11b−, and CD207− CD11b+ DDCs isolated from the skin of Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) chimeras 8 wk after reconstitution showed no detectable staining with Y-Ae (Fig. 6 A). In contrast, CD207+, CD207− CD11b−, and CD207− CD11b+ mDDCs isolated from the CLNs of Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) showed levels of Y-Ae staining that, although lower than those observed on the corresponding DC subsets isolated from B6 x BALB/c F1 mice, were identical to those isolated from Eα52-68-negative B6 mice (Fig. 6 A).
To formally demonstrate that the low levels of Y-Ae staining observed on CD207+, CD207− CD11b−, and CD207− CD11b+ mDDCs isolated from the CLNs of Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) chimeras do not involve Eα52-68 peptides, we analyzed whether they were capable of stimulating T cells specific for Eα52-68–I-Ab complexes. In contrast to the Y-Ae antibody, 1H3.1, a T cell hybridoma derived from a B6 mouse immunized with Eα52-68 peptides (Rudensky et al., 1991a,b), and H30 T cells, which result from transfection of the TCR-α and TCR-β chains of 1H3.1 into a TCR-negative T cell hybridoma variant, display an exquisite specificity for Eα52-68–I-Ab complexes present at the surface of DCs derived from B6 x BALB/c F1 CLNs (Fig. 6 B; Viret and Janeway, 2000). When CD207+, CD207− CD11b−, and CD207− CD11b+ mDDCs isolated from Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) CLNs were cultured in the presence of 1H3.1 or H30 T cells, none of them were capable of eliciting the production of IL-2, supporting the view that the low levels of Y-Ae reactivity they displayed do not involve the Eα52-68 peptide (Fig. 6 C). Pulsing CD207+, CD207− CD11b−, and CD207− CD11b+ mDDCs with Eα52-68 peptides conferred upon them the ability to activate 1H3.1 and H30 T cells (Fig. 6 C). Therefore, the DC isolation procedure fully preserved their ability to stimulate T cells. Importantly, when B6 splenocytes were pulsed with decreasing amounts of Eα52-68 peptide, 1H3.1 and H30 T cells were capable of producing IL-2, even at peptide doses that do not generate a detectable signal after staining with Y-Ae (Viret and Janeway, 2000). Therefore, these results cannot be accounted for by the poor sensitivity of 1H3.1 T cells, and they demonstrate that the presence of Eα52-68-independent Y-Ae–recognizable epitopes at the surface of the mature skin-derived DCs found in the CLNs of Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) prevent reaching any conclusion on the capacity of DDCs to capture LC-derived peptides and to convey them to CLNs.
CD207+ CD103+ DDCs cross-present keratinocyte-derived antigens
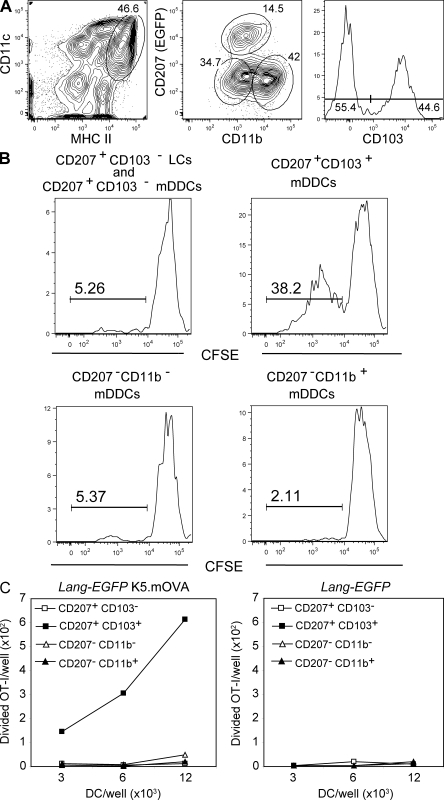
To determine the relative ability of the five skin DC subsets to cross-present self antigens expressed in keratinocytes, we used the K5.mOVA transgenic mouse model, in which a portion of the ovalbumin protein (OVA) is fused to the transmembrane domain of the transferrin receptor to provide membrane localization and expressed under the control of the K5 keratin promoter (Azukizawa et al., 2003). Earlier studies have established that both mLCs and mDDCs were capable of cross-presenting OVA and of triggering the proliferation of OT-I CD8+ T cells that were specific for H-2Kb–OVA complexes (Azukizawa et al., 2003; Waithman et al., 2007). The involvement of LCs in cross-presentation was likely a result of the fact that earlier studies were unaware of the existence of CD103+ DCs that also expressed CD207, and a recent study showed that CD103+ mDDCs isolated from the CLNs of K5.mOVA mice constitute the only skin-derived DCs capable of cross-presenting OVA (Bedoui et al., 2009). Importantly, the elegant sorting scheme used by Bedoui et al. (2009) to reach such conclusions did not take into account the presence of CD103+ cells among CD207− CD11b+ and CD207− CD11b− mDDCs (Fig. 3 C). Therefore, to prevent the contamination of CD207+ CD103+ mDDCs by those CD207− CD11b+ and CD207− CD11b− mDDCs that express CD103, we developed a sorting scheme allowing the isolation of homogeneous populations of CD207 (EGFP)+ CD103+, CD207−CD11b−, and CD207−CD11b+ mDDCs from the CLNs of Lang-EGFP K5.mOVA mice (Fig. 7 A). Our sorting scheme also yielded a fourth CD207+CD103− fraction that comprises mLCs as well as CD207 (EGFP)+CD103− mDDCs. To analyze their respective capacity to cross-present OVA expressed by keratinocytes, each of the sorted subsets was cultured at various ratios with CFSE-labeled OT-I CD8+ T cells. After 60 h of culture, the magnitude of OT-I proliferation was assessed. CD207+ CD103+ mDDCs constituted the sole subset capable of cross-presenting OVA (Fig. 7, B and C). As expected, DC subsets sorted from Lang-EGFP mice did not induce any OT-I proliferation (Fig. 7 C), but upon incubation with the OVA257-264 peptide all the sorted DC subsets were able to induce OT-I proliferation (Fig. S6). The induction of granzyme was also analyzed, and CD207+ CD103+ mDDCs isolated from the CLNs of Lang-EGFP K5.mOVA mice were again the only DC population capable of inducing granzyme A (unpublished data). Consistent with previous studies (Waithman et al., 2007), OVA expressed in keratinocytes was not cross-presented by CD11chigh MHCIIinter lymphoid tissue-resident DCs (unpublished data). Therefore, even when depleted of contaminating CD207− CD11b+ CD103+ and CD207− CD11b− CD103+ cells, CD207+ CD103+ mDDCs remain the only skin migratory DCs capable of cross-presenting a keratinocyte-derived antigen.
Figure 7.
CD207+ CD103+ mDDCs cross-present a keratinocyte-derived self antigen. (A) Gating strategy for isolation of CD207+ CD103+, CD207− CD11b−, and CD207− CD11b+ mDDCs from Lang-EGFP K5.mOVA and Lang-EGFP CLNs. Skin-derived DCs were identified on the basis of their MHCIIhigh CD11cinter-to-high phenotype. Expression of CD207 (EGFP) and CD11b was assessed among MHCIIhigh CD11cinter-to-high DCs, leading to the isolation of CD207− CD11b−/low and CD207− CD11b+ mDDCs. CD207+ cells were categorized on the basis of CD103 expression. CD207+ CD103+ cells corresponded to CD207+ CD103+ mDDCs, whereas CD207+ CD103− cells corresponded to a mix of mLCs and CD207+ CD103− mDDCs. (B) DC subsets, sorted according to the scheme described in A, were cultured with CFSE-labeled OT-I transgenic T cells. Histograms corresponding to CFSE dilution are shown after gating on cells that stain positive with H-2Kb-OVA257-264 tetramers. (C) The absolute numbers of divided OT-I transgenic T cells, in response to DC subsets sorted from Lang-EGFP K5.mOVA or Lang-EGFP CLNs, are presented. Data are representative of three independent experiments.
LCs are dispensable for cross-presentation of keratinocyte-specific antigen by CD207+ DDCs
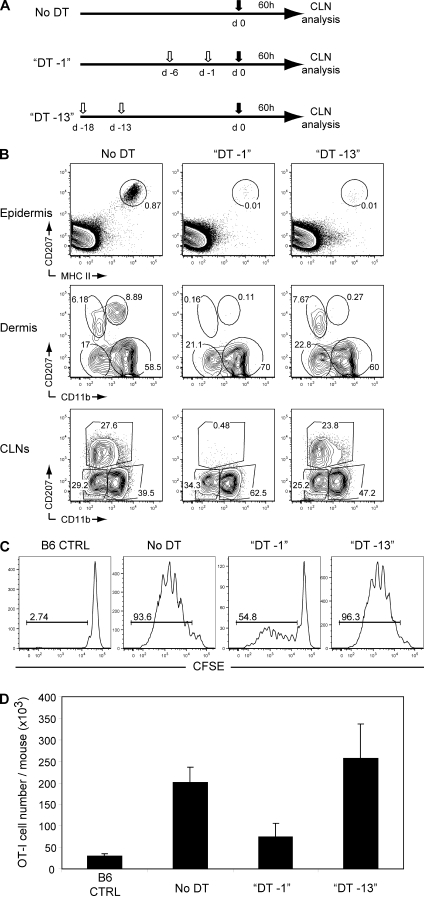
Considering that LCs are closely intermingled with keratinocytes (Kissenpfennig et al., 2005b), it is possible that mLCs transferred OVA to the CD207+ CD103+ DDCs, thereby allowing them to play a dominant role in the cross-presentation of keratinocyte-expressed OVA. To determine whether CD207+ CD103+ DDCs do require the help of LCs to cross-present keratinocyte-expressed OVA, we used Lang-DTREGFP x B6 K5.mOVA mice in which LCs and CD207+ DDCs are equally sensitive to DT treatment and can thus be chronically depleted by systemic administration of DT (Kissenpfennig et al., 2005b). After in vivo DT treatment, LCs and CD207+ DDCs repopulate the skin with different kinetics. For instance, CD207+ DDCs reappear in the dermis as soon as 3–4 d after DT administration, whereas epidermal LCs are undetectable for up to 20 d after DT administration (Bursch et al., 2007; Poulin et al., 2007; Wang et al., 2008). Therefore, we took advantage of such distinct kinetics of reappearance to assess whether CD207+ DDCs constitute the main subtype able to cross-present keratinocyte-derived OVA in vivo and perform this exclusive task irrespective of the presence of LCs. A group of Lang-DTREGFP x B6 K5.mOVA mice received a last DT injection 13 d before adoptive transfer of CFSE-labeled OT-I T cells (DT -13 mice; Fig. 8 A). At the time of OT-I T cell transfer, such timing of DT administration resulted in the complete elimination of LCs for >2 wk but allowed the reconstitution of the CD207+ DDC compartment in both the dermis and the CLNs (Fig. 8 B). We also prepared Lang-DTREGFP x B6 K5.mOVA mice that received a last DT injection 1 d before transfer of CFSE-labeled OT-I CD8+ T cells, a protocol which depletes both LCs and CD207+ DDCs at the time of OT-I T cell transfer (DT -1 mice; Fig. 8, A and B). Upon transfer into B6 control mice, OT-I T cells did not show any sign of proliferation (Fig. 8 C, left). In contrast, OT-I T cells robustly proliferated upon transfer into untreated Lang-DTREGFP x B6 K5.mOVA mice and a proliferation of the same magnitude occurred upon transfer in Lang-DTREGFP x B6 K5.mOVA mice that received a last DT injection on day 13 before transfer of CFSE-labeled OT-I T cells (Fig. 8 C). In contrast, in Lang-DTREGFP x B6 K5.mOVA mice that received a last DT injection on day −1 before transfer of CFSE-labeled OT-I T cells, both the percentage of dividing OT-I T cells and the yield of OT-I T cells were dramatically decreased (Fig. 8, C and D). Altogether, these results demonstrate that CD207+ DDCs are the dominant skin-migratory subtype capable of cross-presenting an antigen whose expression is limited to keratinocytes. Moreover, mLCs do not need to ferry OVA into the dermis to make it available to CD207+ DDCs.
Figure 8.
CD207+ CD103+ mDDCs cross-present a keratinocyte-derived self antigen regardless of the presence of mLCs. (A) Experimental scheme for the depletion of LCs alone or of both LCs and CD207+ mDDCs in Lang-DTREGFP x B6 K5.mOVA mice. 1 µg DT (open arrows) was administered i.p. 18 and 13 (DT–13) or 6 and 1 (DT–1) d before adoptive transfer of 5 × 106 CFSE-labeled OT-I CD8+ T cells (filled arrows). (B) Kinetics of reappearance of the various skin (epidermis and dermis) or skin-derived (CLN) DC subsets after DT ablation. DCs from DT–13 and DT–1 Lang-DTREGFP x B6 K5.mOVA mice were gated as MHCII+ (epidermis and dermis) and MHCIIhigh CD11cinter-to-high (CLNs) cells and then analyzed using CD207-MHCII (epidermis) and CD207)-CD11b (dermis and CLN) dot plots. Untreated Lang-DTREGFP x B6 K5.mOVA (no DT) mice are also shown. Windows correspond to LCs (epidermis) and to mLCs, CD207+ DDCs, CD207− CD11b−, and CD207− CD11b+ (dermis). In the CLNs, windows correspond to CD207− CD11b−, CD207− CD11b+, and mLCs + CD207+ DDCs. Numbers indicate the percentage of cells in the specified windows. (C and D) 60 h after adoptive transfer in the specified mice, the OT-I CD8+ T cells present in pooled skin-draining LNs were analyzed by flow cytometry for the percentage (C) and yield (D) of proliferating OT-I CD8+ T cells. Numbers in C indicate percent proliferated cells ± SEM. Data shown are representative of three independent experiments.
DISCUSSION
We identified up to five different DC subsets in mouse steady-state dermis by combining CD207, CD11b, and CD103 expression, with one of those subsets corresponding to epidermal LCs in transit to CLNs. A similar analysis performed in steady-state CLNs revealed the presence of a smaller number of skin-derived DC subsets. This is a result of the fact that CD11b expression loses its discriminatory power in the CLNs and does not permit mLCs to be distinguished from CD207+ mDDCs. Regardless of this technical limitation, analysis of B6 (CD45.1)→B6 (CD45.2) BM chimeras demonstrated that CLNs do contain a counterpart of each of the five different DC subsets identified in the dermis. Note that the bimodal CD103 expression found on both CD207− CD11b+ and CD207− CD11b− mDDC subsets can be further used to distinguish up to seven skin-derived DC subsets within steady-state CLNs. In a few studies, CD45+ dermal cells have been divided into CD11c+ CD11b−-to-+ and CD11c− CD11b+ subsets (Bogunovic et al., 2006; Jakubzick et al., 2008). The CD11c+ CD11b−-to-+ subset expresses high levels of MHCII molecules and corresponds to DDCs (Jakubzick et al., 2008). The CD11c− CD11b+ subset likely corresponds to dermal macrophages (Dupasquier et al., 2004; Jakubzick et al., 2008). However, a fair fraction of it also expresses high levels of MHCII molecules and has been categorized as DDCs in some studies (Jakubzick et al., 2008) but not others (Bogunovic et al., 2006). The enzymatic treatment required to prepare epidermal and dermal cell suspensions complicates the usage of the CD11c marker (Stutte et al., 2008) and led us to limit its usage to the characterization of the DCs found in CLNs. Accordingly, the CD207− CD11b+ subset present within our CD45+ MHCIIhigh dermal cells likely includes both CD11c+ CD11b+ and CD11c− CD11b+ cells. In the case that MHCIIhigh CD207− CD11c− CD11b+ cells correspond to dermal macrophages (Bogunovic et al., 2006), they are expected to have a low rate of BrdU labeling and to reside permanently in the dermis. Therefore, the presence of those last cells in the dermis and only in the dermis likely accounts for the inconsistency in the BrdU incorporation rate noted for the CD207− CD11b+ subset found in the skin and in the CLNs. Settling this important issue will require the identification of specific markers allowing us to discriminate DDCs from dermal macrophages.
Because of the lack of appropriate cell surface markers, it is impossible to discriminate mLCs from CD207+ mDDCs in nonchimeric mouse CLNs. However, the distinct repopulation kinetics of LCs and of CD207+ DDCs that follow DT treatment provide an opportunity to analyze the response to OVA expressed in keratinocytes when only LCs were absent or when both LCs and CD207+ DDCs were absent. DT administration 1 d before adoptive transfer of OT-I T cells into Lang-DTREGFP x B6 K5.mOVA mice resulted in the depletion of both LCs and CD207+ DDCs and in dramatically reduced response against OVA. Considering that the CD207+ CD103+ mDDCs isolated from the CLNs of K5.mOVA mice are the only DC subset that can cross-present OVA in vitro, it is paradoxical to see that low levels of proliferation still occurred among the OT-I T cells that were transfered into Lang-DTREGFP x B6 K5.mOVA mice 1 d after DT treatment. Such residual T cell proliferation might be proper for the Lang-DTREGFP x B6 K5.mOVA model and result from the fact that by undergoing apoptosis, the DT-sensitive CD207+ CD103+ mDDCs present in CLNs release their OVA content and thereby confer some adventitious cross-presentation ability to the quantitatively major CD207− migratory DCs that are DT resistant and present in the CLNs. When DT was applied 13 d before adoptive transfer of OT-I T cells into Lang-DTREGFP x B6 K5.mOVA mice, OT-I T cell proliferation was unaffected, suggesting that the CD207+ DDCs that have fully reappeared at that time play a predominant role in cross-presenting a model antigen whose expression is limited to keratinocytes. Among the skin DC subsets sorted from K5.mOVA CLNs, CD207+ CD103+ mDDCs were the only cells capable of cross-presenting OVA ex vivo and are likely responsible for OT-I T cell activation in Lang-DTR-EGFP K5.mOVA mice that were DT-treated 13 d before adoptive transfer of OT-I T cells. Therefore, as previously shown in the case of contact hypersensitivity (Bennett et al., 2007; Bursch et al., 2007), CD207+ CD103+ mDDCs and mLCs appear to be endowed with distinct immune functions. Importantly, the unique ability of CD207+ CD103+ mDDCs to cross-present a keratinocyte-derived antigen is not associated with the expression of increased levels of CD40, CD80, CD86, PD-L1, and MHCII molecules. Akin to K5.mOVA mice, K14 mice also express OVA in keratinocytes and, upon extraction from the epidermis, their LCs were found to be capable of cross-presenting OVA as assessed using OT-I T cell proliferation (Stoitzner et al., 2006; Bursch et al., 2009). Therefore, provided that the use of the K14 and K5 promoters does not result in gross differences in the levels of OVA expression, our data and those of Bedoui et al. (2009) suggest that epidermal LCs lose such property upon migration to CLNs.
The possibility for CD207+ DDCs to capture an antigen whose expression is limited to keratinocytes in the absence of LCs constitutes an unexpected finding. Considering that LCs maintain tight contacts with keratinocytes and that the basal membrane found at the dermoepidermal junction insulates keratinocytes from DDCs, LCs might have captured OVA and, upon migration to the dermis, transferred it to DDCs. LCs are, however, dispensable for the cross-presentation observed in the K5-mOVA model, and it thus remains to be determined how CD207+ CD103+ DDCs can acquire OVA from keratinocytes. Furthermore, it has been noted that CD207+ DDCs are often found adjacent to hair follicles (Bursch et al., 2007; Poulin et al., 2007) and that in rare instances, CD207+ DDCs were even found interdigitating with follicular epidermis (Bursch et al., 2007). Whether such anatomical localization favors a preferential access to OVA and accounts for the fact that CD207+ DDCs constitute the dominant skin-migratory subtype capable of cross-presenting antigen expressed by keratinocytes remains, however, to be determined. It should be also noted that DCs found in the lamina propria of the small intestine can constitutively transport apoptotic epithelial cells to the mesenteric LNs (Huang et al., 2000), a property which might correlate with their ability to send dendrites through the basal membrane and the mucosal epithelium to sample luminal antigens (Chieppa et al., 2006). Provided that mouse keratinocytes express MHCII molecules, it may have been expected that the CD207+ CD103+ DDCs would be able to acquire Eα peptides from keratinocytes of Lang-EGFP B6 (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) chimeras and to cross-present them. The genotype of the recipient and of the transplanted BM cells used in the Lang-EGFP B6 (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) chimeras was chosen to prevent the occurrence of cutaneous graft-versus-host reaction and, consistent with this expectation, analysis of the skin of those chimeras 8 wk after BM transplantation revealed no signs of inflammation. Because resting keratinocytes are normally MHCII-negative and only express MHCII molecules during cutaneous graft-versus-host reaction (Breathnach and Katz, 1983), it is thus likely that, at the time of analysis, the keratinocytes of Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) chimeras were Eα negative, an attribute accounting for the fact that the CD207+ CD103+ mDDCs were unable to trigger T cells specific for Ea52-68-Ab pMHCII complexes.
Lymphoid tissue–resident CD8α+ DCs have become a focus of intensive investigation because they are prone to cross-present antigens, a property important for inducing peripheral tolerance and for fighting infections and tumors (Hildner et al., 2008). Interestingly, a recent study showed that among CD8α+ splenic DCs it is the CD207+ CD103+ subset that is responsible for phagocytosis of apoptotic bodies and for the induction of tolerance via cross-presentation of antigens associated with apoptotic bodies (Qiu et al., 2009). Resting CD207+ CD103+ CD8α+ DCs are located in the marginal zone of the spleen, a position which might facilitate the clearance of circulating apoptotic cells, and they migrate to the T cell zone upon stimulation with microbial products (Idoyaga et al., 2009; Qiu et al., 2009). The lung-derived CD207+ CD103+ DCs that are found in the bronchial LNs are also capable of cross-presenting antigens to CD8+ T cells (del Rio et al., 2007). There thus exists an interesting parallel in the biology of the CD207+ CD103+ CD8α+ DCs found in the spleen and of the CD207+ CD103+ present in CLNs and bronchial LNs. It has been suggested that the special ability of CD207+ CD8α+ DCs to cross-present antigens is a result of the fact that they are the only DCs to possess specialized intracellular machinery dedicated to cross-presentation (Schnorrer et al., 2006; Savina et al., 2009). Such machinery might require the expression of langerin (CD207), a C-type lectin which participates in the formation of Birbeck granules. However, we previously showed that cross-presentation is not affected in mice deprived of CD207 (Kissenpfennig et al., 2005a). Therefore, the common denominator that renders these DC subsets prone to cross-present antigens remains to be found.
In conclusion, our data show that the quantitatively minor CD207+ CD103+ DDC subset has the capability of cross-presenting antigens expressed by keratinocytes and that this task can be accomplished irrespective of the presence of LCs. Importantly, because of the presence in CLNs of both CD207+ CD103− and CD207− CD103+ mDDC subsets, we showed that the appropriate definition of the CD207+ CD103+ DDC subset requires the simultaneous analysis of CD11b, CD207, and CD103 expression. Our data also raise a note of caution on the use of the Y-Ae antibody to unravel the antigen transfers that may occur between distinct subsets of mature DCs. The possibility to identify five distinct subsets of skin DCs and their migratory derivatives may contribute to an understanding of those that pick up vaccine antigens delivered via the skin and initiate productive immunity rather than tolerance.
MATERIALS AND METHODS
Mice.
Mice were housed under specific pathogen-free conditions and handled in accordance with French and European directives. Protocols were approved by Direction Départementale des Services Vétérinaires des Bouches du Rhône (agreement 13–22). C57BL/6 (B6) female mice that express the CD45.1 (B6 [CD45.1]) or CD45.2 (B6 [CD45.2]) allele were purchased from Charles River. Lang-EGFP and Lang-DTREGFP mice have been previously described (Kissenpfennig et al., 2005b) and were crossed onto the B6 (CD45.2) background for at least 10 generations. The resulting Lang-EGFP (CD45.2) and Lang-DTREGFP (CD45.2) mice were further crossed onto the B6 (CD45.1) background to obtain Lang-EGFP (CD45.1) and Lang-DTREGFP (CD45.1) mice. MHCIIΔ/Δ mice backcrossed on a B6 background have been previously described (Madsen et al., 1999). B6 K5.mOVA mice have been previously described (Azukizawa et al., 2003). OT-I mice have been previously described (Hogquist et al., 1994).
Transplantation of BM cells.
7–8-wk-old B6 (CD45.2) mice were lethally irradiated with two doses of 550 rads each, 5 h apart, and then injected i.v. with 2 × 106 BM cells obtained from adult B6 (CD45.1) or Lang-DTR-EGFPDTR (CD45.1) mice. 7–8 wk after reconstitution, the level of blood chimerism was determined and, at that time, >99% of blood B cells were of donor origin. Chimeras were kept on antibiotic-containing water (0.2% Bactrim, Roche, Germany) during the whole experiment.
DC isolation.
DCs were isolated from lymphoid organs as previously described (Vremec et al., 2000). In brief, the organs were first cut in small pieces and incubated with a mixture of type II collagenase (Worthington Biochemical Corporation) and of DNase (Sigma-Aldrich). Light density cells were purified by centrifugation on a Nycoprep solution (d = 1.068; Abcys). Skin DCs were extracted from mouse ears. In brief, ears were split in two parts (dorsal and ventral) and incubated overnight in PBS containing 2.5 mg/ml dispase II (Roche) to allow separation of dermal and epidermal sheets. The separated epidermal and dermal sheets were then cut into small pieces and incubated for 1 h at 37°C with a solution of RPMI containing 1 mg/ml DNase and 1 mg/ml collagenase type IV (Worthington Biochemical Corporation) to obtain homogeneous cell suspension. In the experiments shown in Fig. 6, CD11c+ DCs were purified from the specified organs using MACS.
Antibodies.
Anti-CD11c (HL3), anti-CD8α (53–6.7), anti-MHCII (M5/114 and AF6-120.1), anti-CD45.2 (104), anti-CD45.1 (A20), anti-CD24 (M1/69), anti-SIRPα (P84), anti-EpCAM, anti-CD103 (M290), anti-CD5 (53–7.3), anti-Ki67, anti-CD40 (1C10), anti-CD80 (16-10A1), anti-CD86 (GL1), anti–PD-L1(MIH5), and anti-Vα2 (B20.1) antibodies were all purchased from BD, except for anti-CD11b (M1/70) and anti–Ea52-68 (Y-Ae) antibodies, which were purchased from eBioscience, anti-F4/80 antibody, which was purchased from AbD Serotec, and anti-CD207 (929F3) antibody, which was purchased from Dendritics. H-2Kb-OVA257-264-PE tetramers were purchased from Beckman Coulter.
Flow cytometry.
Before staining, cells were preincubated on ice for at least 10 min with the 2.4 G2 antibody to block Fc receptors. Multiparameter FACs analysis was performed using a FACSCanto system (BD). Analysis was performed using FlowJo software (Tree Star, Inc.). For each experiment, control mice, B6 (CD45.2), B6 (CD45.1), and Lang-DTR-EGFP mice were included to define the appropriate gates. Autofluorescent cells were gated out by using the AmCyan channel.
Sorting of DC.
DCs isolated from the CLNs of Lang-EGFP x B6 K5.mOVA mice were stained with MHCII, CD11c, CD11b, and CD103 antibodies. Cells were subsequently sorted on a FACSAria system (BD). Skin-derived DCs (MHCIIhigh CD11clow to high) were displayed using a CD207 (EGFP) versus CD11b plot. CD207− DDCs were then separated into CD11b− and CD11b+ fractions and CD207+ DCs separated into CD103− and CD103+ fractions. DCs isolated from the CLNs of Lang-EGFP (CD45.1)→B6 MHCIIΔ/Δ x BALB/c (CD45.2) chimeras were stained with MHCII, CD11c, CD11b, CD45.1, and CD103. Skin-derived DCs (MHCIIhigh CD11clow-to-high) were first separated into CD45.1+ and CD45.1− fractions. CD45.1− cells were then displayed using a CD207 (EGFP) versus CD11b plot. CD207− DDCs were then separated into CD11b− and CD11b+ fractions and CD207+ DCs further separated into CD103− and CD103+ fractions.
Preparation and adoptive transfer of CFSE-labeled OT-I T cells.
OT-I T cells were purified from pooled LNs and spleen by density gradient using Lympholyte M followed by negative selection using a T cell isolation kit (Invitrogen). Purity was determined by staining with Vα2 and CD8α antibodies. For CFSE labeling, purified OT-I T cells were resuspended in PBS and labeled with 2.5 µM CFSE (5- and 6-carboxy-fluorescein diacetate succinimidyl ester; Invitrogen) for 10 min at room temperature. 5 × 106 CFSE-labeled cells were injected i.v. into the specified unirradiated recipient mice. 60 h after injection, single-cell suspension from pooled CLNs were stained with PE-labeled H-2Kb-OVA257-264 tetramers and Vα2, CD8α, and CD5 antibodies to detect the OT-I transferred cells. The percentage and absolute numbers of OT-I T cells that have proliferated were determined.
Culture of DC subpopulations with CFSE-labeled OT-I T cells.
3 × 103 to 12 × 103 cells of each DC subpopulation were cocultured with 50 × 103 CFSE-labeled OT-I cells in 100 µl in the presence or absence of 1 nM OVA257-264 peptide. After 60 h of culture, proliferation was measured by flow cytometry as a loss of CFSE staining.
Functional assays.
The 1H3.1 T cell hybridoma derives from a B6 mouse immunized with the Eα52-68 peptide and is specific for Eα52-68–I-Ab complexes (Rudensky et al., 1991a,b). H30 T cells result from the transfection of the TCR-α and TCR-β chains of 1H3.1 into a TCR-negative T cell hybridoma variant (Viret and Janeway, 2000). H30 or 1H3.1 cells (105 cells/well) were stimulated for 24 h with the specified DCs (104 cells/well) in the presence or absence of synthetic Eα52-68 peptide. Supernatants were harvested and the level of IL-2 was determined using the CTL-L cell line and the Cell Titer Glow Assay (Promega). Values were converted into IL-2 units using a recombinant mouse IL-2 standard (BD).
In vivo depletion of LCs or of CD207+ DDCs.
For systemic in vivo depletion of CD207+ DCs, Lang-DTREGFP mice were injected i.p. with 1 µg DT (ServiBio) using the specified timing of administration. Mice were analyzed at different time points after the last DT injection.
BrdU labeling in vivo and cell cycle analyses.
Mice were injected i.p. with 1.5 mg BrdU (Sigma-Aldrich) to ensure its immediate availability, and their drinking water was supplemented for up to 28 d with 0.8 mg/ml of BrdU and 2% glucose and changed daily. After 28 d of continuous BrdU labeling, some mice received BrdU-free drinking water for an additional 2 wk and the loss of BrdU-positive DCs was analyzed. DCs from epidermis, dermis, and CLNs were first stained for surface markers and then permeabilized for BrdU staining (BrdU labeling Flow kit; BD) and langerin staining. Ki-67 staining (BD) was performed using the staining protocol corresponding to the BrdU labeling Flow kit.
Online supplemental material.
Fig. S1 shows the extent of chimerism 8 wk after BM transplantation. Fig. S2 shows that both CD207+ CD103+ and CD207+ CD103− DDCs can be found in the dermis of mice deprived of LCs. Fig. S3 shows the MHCII-CD11c contour plots of DCs found in the CLNs of Lang-EGFP mice. Fig. S4 shows expression of CD40, CD80, CD86, and PD-L1 on the five skin DC subsets before and after their migration to CLNs. Fig. S5 shows isotype control staining profile of the five skin DC subsets before and after their migration to CLNs. Fig. S6 shows OT-I proliferation in response to the OVA257-264 peptide. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091964/DC1.
Acknowledgments
We thank Marie Malissen, Florent Ginhoux, Lee Leserman, and Jean Davoust for discussion. We thank Marc Barad, Pierre Grenot, and Atika Zouine for assistance with flow cytometry and cell sorting.
This work was supported by Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, European Communities Framework Program 7 (MUGEN Network of Excellence and MASTERSWITCH Integrating Project [HEALTH-F2-2008-223404]), Institut National du Cancer (Melan-Imm project), Agence Nationale pour le Recherche (ANR; DCINVIVO project), and by postdoctoral fellowships from Fondation pour la Recherche Médicale (L.F. Poulin), MUGEN (L.F. Poulin), Association pour la R Recherche sur le Cancer (M. Guilliams), Ministère de la Recherche (S. Tamoutounour), and ANR (E. Devilard).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CLN
- cutaneous LN
- DDC
- dermal DC
- DT
- diphtheria toxin
- EGFP
- enhanced GFP
- EpCAM
- epithelial cell adhesion molecule
- LC
- Langerhans cell
- mDDC
- migratory DDC
- mLC
- migratory LC
References
- Allan R.S., Smith C.M., Belz G.T., van Lint A.L., Wakim L.M., Heath W.R., Carbone F.R. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 301:1925–1928 doi: 10.1126/science.1087576 [DOI] [PubMed] [Google Scholar]
- Allenspach E.J., Lemos M.P., Porrett P.M., Turka L.A., Laufer T.M. 2008. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 29:795–806 doi: 10.1016/j.immuni.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azukizawa H., Kosaka H., Sano S., Heath W.R., Takahashi I., Gao X.H., Sumikawa Y., Okabe M., Yoshikawa K., Itami S. 2003. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur. J. Immunol. 33:1879–1888 doi: 10.1002/eji.200323630 [DOI] [PubMed] [Google Scholar]
- Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R.S., Wojtasiak M., Shortman K., Carbone F.R., et al. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488–495 doi: 10.1038/ni.1724 [DOI] [PubMed] [Google Scholar]
- Bennett C.L., Noordegraaf M., Martina C.A., Clausen B.E. 2007. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J. Immunol. 179:6830–6835 [DOI] [PubMed] [Google Scholar]
- Bogunovic M., Ginhoux F., Wagers A., Loubeau M., Isola L.M., Lubrano L., Najfeld V., Phelps R.G., Grosskreutz C., Scigliano E., et al. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J. Exp. Med. 203:2627–2638 doi: 10.1084/jem.20060667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach S.M., Katz S.I. 1983. Keratinocytes synthesize Ia antigen in acute cutaneous graft-vs-host disease. J. Immunol. 131:2741–2745 [PubMed] [Google Scholar]
- Bursch L.S., Wang L., Igyarto B., Kissenpfennig A., Malissen B., Kaplan D.H., Hogquist K.A. 2007. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204:3147–3156 doi: 10.1084/jem.20071966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch L.S., Rich B.E., Hogquist K.A. 2009. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J. Immunol. 182:4657–4664 doi: 10.4049/jimmunol.0803656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F.R., Belz G.T., Heath W.R. 2004. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 25:655–658 doi: 10.1016/j.it.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Chieppa M., Rescigno M., Huang A.Y., Germain R.N. 2006. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203:2841–2852 doi: 10.1084/jem.20061884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic A., Shao Q.X., D’Amico A., O’Keeffe M., Chen W.F., Shortman K., Wu L. 2004. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 172:1018–1027 [DOI] [PubMed] [Google Scholar]
- del Rio M.L., Rodriguez-Barbosa J.I., Kremmer E., Förster R. 2007. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178:6861–6866 [DOI] [PubMed] [Google Scholar]
- den Haan J.M., Lehar S.M., Bevan M.J. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696 doi: 10.1084/jem.192.12.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupasquier M., Stoitzner P., van Oudenaren A., Romani N., Leenen P.J. 2004. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J. Invest. Dermatol. 123:876–879 doi: 10.1111/j.0022-202X.2004.23427.x [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Collin M.P., Bogunovic M., Abel M., Leboeuf M., Helft J., Ochando J., Kissenpfennig A., Malissen B., Grisotto M., et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204:3133–3146 doi: 10.1084/jem.20071733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K., Edelson B.T., Purtha W.E., Diamond M., Matsushita H., Kohyama M., Calderon B., Schraml B.U., Unanue E.R., Diamond M.S., et al. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 322:1097–1100 doi: 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27 doi: 10.1016/0092-8674(94)90169-4 [DOI] [PubMed] [Google Scholar]
- Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191:435–444 doi: 10.1084/jem.191.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idoyaga J., Suda N., Suda K., Park C.G., Steinman R.M. 2009. Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA. 106:1524–1529 doi: 10.1073/pnas.0812247106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. 2007. Mucosal dendritic cells. Annu. Rev. Immunol. 25:381–418 doi: 10.1146/annurev.immunol.25.022106.141634 [DOI] [PubMed] [Google Scholar]
- Jakubzick C., Bogunovic M., Bonito A.J., Kuan E.L., Merad M., Randolph G.J. 2008. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 205:2839–2850 doi: 10.1084/jem.20081430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A., Bloom O., Ono S., Cui W., Unternaehrer J., Jiang S., Whitney J.A., Connolly J., Banchereau J., Mellman I. 2007. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 27:610–624 doi: 10.1016/j.immuni.2007.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath A.T., Henri S., Battye F., Tough D.F., Shortman K. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 100:1734–1741 [PubMed] [Google Scholar]
- Kaplan D.H., Kissenpfennig A., Clausen B.E. 2008. Insights into Langerhans cell function from Langerhans cell ablation models. Eur. J. Immunol. 38:2369–2376 doi: 10.1002/eji.200838397 [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A., Aït-Yahia S., Clair-Moninot V., Stössel H., Badell E., Bordat Y., Pooley J.L., Lang T., Prina E., Coste I., et al. 2005a. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol. Cell. Biol. 25:88–99 doi: 10.1128/MCB.25.1.88-99.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhé C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D., et al. 2005b. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654 doi: 10.1016/j.immuni.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Larregina A.T., Falo L.D., Jr 2005. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J. Invest. Dermatol. 124:1–12 doi: 10.1111/j.1523-1747.2004.23554.x [DOI] [PubMed] [Google Scholar]
- Lee H.K., Zamora M., Linehan M.M., Iijima N., Gonzalez D., Haberman A., Iwasaki A. 2009. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 206:359–370 doi: 10.1084/jem.20080601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Waskow C., Liu X., Yao K., Hoh J., Nussenzweig M. 2007. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8:578–583 doi: 10.1038/ni1462 [DOI] [PubMed] [Google Scholar]
- Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. 2009. In vivo analysis of dendritic cell development and homeostasis. Science. 324:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen L., Labrecque N., Engberg J., Dierich A., Svejgaard A., Benoist C., Mathis D., Fugger L. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA. 96:10338–10343 doi: 10.1073/pnas.96.18.10338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D.J., Benoist C., Williams V.E., II, Kanter M., McDevitt H.O. 1983. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc. Natl. Acad. Sci. USA. 80:273–277 doi: 10.1073/pnas.80.1.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Manz M.G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I.L., Cyster J.G., Engleman E.G. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141 doi: 10.1038/ni852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D.B., Rath S., Pizzo E., Rudensky A.Y., George A., Larson J.K., Janeway C.A., Jr 1992. Monoclonal antibody detection of a major self peptide. MHC class II complex. J. Immunol. 148:3483–3491 [PubMed] [Google Scholar]
- Nagao K., Ginhoux F., Leitner W.W., Motegi S., Bennett C.L., Clausen B.E., Merad M., Udey M.C. 2009. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl. Acad. Sci. USA. 106:3312–3317 doi: 10.1073/pnas.0807126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.G., Hsu A., Mandell M.A., Roediger B., Hoeller C., Mrass P., Iparraguirre A., Cavanagh L.L., Triccas J.A., Beverley S.M., et al. 2008. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 4:e1000222 doi: 10.1371/journal.ppat.1000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin L.F., Henri S., de Bovis B., Devilard E., Kissenpfennig A., Malissen B. 2007. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204:3119–3131 doi: 10.1084/jem.20071724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C.H., Miyake Y., Kaise H., Kitamura H., Ohara O., Tanaka M. 2009. Novel subset of CD8alpha+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J. Immunol. 182:4127–4136 doi: 10.4049/jimmunol.0803364 [DOI] [PubMed] [Google Scholar]
- Rudensky A.Y., Preston-Hurlburt P., Hong S.C., Barlow A., Janeway C.A., Jr 1991a. Sequence analysis of peptides bound to MHC class II molecules. Nature 353:622–627 doi: 10.1038/353622a0 [DOI] [PubMed] [Google Scholar]
- Rudensky A.Y., Rath S., Preston-Hurlburt P., Murphy D.B., Janeway C.A., Jr 1991b. On the complexity of self. Nature 353:660–662 doi: 10.1038/353660a0 [DOI] [PubMed] [Google Scholar]
- Savina A., Peres A., Cebrian I., Carmo N., Moita C., Hacohen N., Moita L.F., Amigorena S. 2009. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 30:544–555 doi: 10.1016/j.immuni.2009.01.013 [DOI] [PubMed] [Google Scholar]
- Schnorrer P., Behrens G.M., Wilson N.S., Pooley J.L., Smith C.M., El-Sukkari D., Davey G., Kupresanin F., Li M., Maraskovsky E., et al. 2006. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. USA. 103:10729–10734 doi: 10.1073/pnas.0601956103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklovskaya E., Roediger B., Fazekas de St Groth B. 2008. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J. Immunol. 181:418–430 [DOI] [PubMed] [Google Scholar]
- Spörri R., Reis e Sousa C. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163–170 doi: 10.1038/ni1162 [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Nussenzweig M.C. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 99:351–358 doi: 10.1073/pnas.231606698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner P., Tripp C.H., Eberhart A., Price K.M., Jung J.Y., Bursch L., Ronchese F., Romani N. 2006. Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. USA. 103:7783–7788 doi: 10.1073/pnas.0509307103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutte S., Jux B., Esser C., Förster I. 2008. CD24a expression levels discriminate Langerhans cells from dermal dendritic cells in murine skin and lymph nodes. J. Invest. Dermatol. 128:1470–1475 doi: 10.1038/sj.jid.5701228 [DOI] [PubMed] [Google Scholar]
- Tripp C.H., Chang-Rodriguez S., Stoitzner P., Holzmann S., Stössel H., Douillard P., Saeland S., Koch F., Elbe-Bürger A., Romani N. 2004. Ontogeny of Langerin/CD207 expression in the epidermis of mice. J. Invest. Dermatol. 122:670–672 doi: 10.1111/j.0022-202X.2004.22337.x [DOI] [PubMed] [Google Scholar]
- Valladeau J., Ravel O., Dezutter-Dambuyant C., Moore K., Kleijmeer M., Liu Y., Duvert-Frances V., Vincent C., Schmitt D., Davoust J., et al. 2000. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 12:71–81 doi: 10.1016/S1074-7613(00)80160-0 [DOI] [PubMed] [Google Scholar]
- Villablanca E.J., Mora J.R. 2008. A two-step model for Langerhans cell migration to skin-draining LN. Eur. J. Immunol. 38:2975–2980 doi: 10.1002/eji.200838919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret C., Janeway C.A., Jr 2000. Functional and phenotypic evidence for presentation of E alpha 52-68 structurally related self-peptide(s) in I-E alpha-deficient mice. J. Immunol. 164:4627–4634 [DOI] [PubMed] [Google Scholar]
- Vishwanath M., Nishibu A., Saeland S., Ward B.R., Mizumoto N., Ploegh H.L., Boes M., Takashima A. 2006. Development of intravital intermittent confocal imaging system for studying Langerhans cell turnover. J. Invest. Dermatol. 126:2452–2457 doi: 10.1038/sj.jid.5700448 [DOI] [PubMed] [Google Scholar]
- Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978–2986 [DOI] [PubMed] [Google Scholar]
- Waithman J., Allan R.S., Kosaka H., Azukizawa H., Shortman K., Lutz M.B., Heath W.R., Carbone F.R., Belz G.T. 2007. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J. Immunol. 179:4535–4541 [DOI] [PubMed] [Google Scholar]
- Wang L., Bursch L.S., Kissenpfennig A., Malissen B., Jameson S.C., Hogquist K.A. 2008. Langerin expressing cells promote skin immune responses under defined conditions. J. Immunol. 180:4722–4727 [DOI] [PubMed] [Google Scholar]
- Wilson N.S., El-Sukkari D., Belz G.T., Smith C.M., Steptoe R.J., Heath W.R., Shortman K., Villadangos J.A. 2003. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 102:2187–2194 doi: 10.1182/blood-2003-02-0513 [DOI] [PubMed] [Google Scholar]