Abstract
Neutrophils kill microbes with reactive oxygen species generated by the NADPH oxidase, an enzyme which moves electrons across membranes. Voltage-gated proton channels (voltage-sensing domain only protein [VSOP]/Hv1) are required for high-level superoxide production by phagocytes, but the mechanism of this effect is not established. We show that neutrophils from VSOP/Hv1−/− mice lack proton currents but have normal electron currents, indicating that these cells have a fully functional oxidase that cannot conduct protons. VSOP/Hv1−/− neutrophils had a more acidic cytosol, were more depolarized, and produced less superoxide and hydrogen peroxide than neutrophils from wild-type mice. Hydrogen peroxide production was rescued by providing an artificial conductance with gramicidin. Loss of VSOP/Hv1 also aborted calcium responses to chemoattractants, increased neutrophil spreading, and decreased neutrophil migration. The migration defect was restored by the addition of a calcium ionophore. Our findings indicate that proton channels extrude the acid and compensate the charge generated by the oxidase, thereby sustaining calcium entry signals that control the adhesion and motility of neutrophils. Loss of proton channels thus aborts superoxide production and causes a severe signaling defect in neutrophils.
Neutrophils are the first line of defense against bacterial infections. These blood cells migrate toward sites of infections, where they engulf and kill bacteria by producing reactive oxygen species (ROS) into the phagocytic vacuole (Nauseef, 2007). These bactericidal functions are essential for innate immunity, and patients who have dysfunctional neutrophils suffer from recurrent and often life-threatening microbial infections (Segal, 1987). ROS generation is mediated by the phagocyte NADPH oxidase, a multi-subunit enzyme complex that assembles at the membrane of the phagosome. The phagocyte NADPH oxidase transports electrons across the membrane of the phagosome to reduce molecular oxygen into superoxide (O2−; Babior et al., 2002).
The oxidase is electrogenic and voltage dependent (Henderson et al., 1987; DeCoursey et al., 2003; Petheo and Demaurex, 2005), and its sustained production of superoxide requires the movement of a compensating charge, i.e., efflux of positively charged ions or influx of negatively charged ions. The translocation of electrons also generates opposite effects on the cytosolic and phagosomal pH. Protons are released in the cytosol by the conversion of NADPH to NADP+ and H+ and consumed in the lumen of phagosomes to convert superoxide anions (O2−) to H2O2 and eventually HOCl. As a result, the phagocyte oxidase acidifies the cytosol (Demaurex et al., 1996) and alkalinizes phagosomes (Segal et al., 1981). Overwhelming evidence indicates that most of the compensating charge is carried by protons flowing across voltage-gated proton channels, as was initially postulated (Henderson et al., 1987). Proton channels can provide a compensating charge, prevent the acidification of the cytosol, and supply protons to phagosomes. More recently, potassium ions were proposed to provide the compensating charge and, by increasing the ionic strength within the phagosome, to activate cationic proteases required for bacterial killing (Reeves et al., 2002). However, the claim that Ca2+-activated K+ channels (BKCa) are required for bacterial killing by neutrophils (Ahluwalia et al., 2004) was disproved because neutrophils lack BKCa currents and kill microbes efficiently in the presence of BKCa inhibitors or after genetic ablation of the BKCa channel (Femling et al., 2006; Essin et al., 2007, 2009). Modeling indicates that other potassium channels and swelling-activated chloride channels (Ahluwalia, 2008) contribute no more than ∼5% of the compensating charge, which is provided essentially by proton channels (Murphy and DeCoursey, 2006). Accordingly, two recent knockout studies indicate that voltage-sensing domain only protein (VSOP)/Hv1 proton channels are required for high-level superoxide production by phagocytes (Okochi et al., 2009; Ramsey et al., 2009).
Proton currents were first recorded by Thomas and Meech (1982) in snail neurons and have since been characterized thoroughly in mammalian cells (for review see Decoursey, 2003). The molecular identity of proton channels was controversial (Maturana et al., 2002; Morgan et al., 2002; Touret and Grinstein, 2002) until the cloning by two separate laboratories of the mouse VSOP (mVSOP) and of its human homologue Hv1, (Ramsey et al., 2006; Sasaki et al., 2006), which demonstrated that this molecule can recapitulate most properties of native proton channels. Interestingly, VSOP/Hv1 closely resembles the voltage-sensing domain of voltage-gated ion channels (S1-S4) but lacks the segments that normally form the pore domain (S5-S6). A similar voltage-sensing protein exists in the sea squirt Ciona intestinalis (Ci-VSP) and contains a cytosolic phosphatase domain whose activity is regulated by the membrane voltage (Murata et al., 2005). Proton conduction in VSOP/Hv1 involves arginine residues within the S4 segment and resembles the omega pathway formed within the voltage sensors of a mutated Shaker potassium channel (Tombola et al., 2005). Further studies reported that the functional unit is a dimer and that each subunit can function independently as a proton channel, gated by its own voltage sensor (Koch et al., 2008; Lee et al., 2008; Tombola et al., 2008). The transition from dimers to monomers might explain the enhanced gating of proton channels observed in activated phagocytes (Bánfi et al., 1999; Koch et al., 2008; Musset et al., 2009; for review see Demaurex and Petheö, 2005).
The generation of viable mice bearing a disrupting mutation within the VSOP/Hv1 gene (VSOP/Hv1−/−; Okochi et al., 2009; Ramsey et al., 2009) provides a new opportunity to study the role of proton channels. In this paper, we use this genetic model to clarify the identity and function of proton channels in neutrophils. First, we show that VSOP/Hv1 is the only proton channel of neutrophils and that the oxidase does not function as a proton channel, as previously proposed by us and others. Second, we show that proton channels contribute to the maintenance of a normal cytosolic pH and membrane potential during the respiratory burst. Third, we show that VSOP/Hv1 proton channels sustain the entry of calcium ions into cells and enable neutrophils to generate calcium signals in response to chemoattractants. Finally, we show that the cytosolic pH, membrane potential, and calcium signaling defects impair actin depolymerization and translate into a failure of neutrophils to detach and to migrate efficiently. Our data reveal that VSOP/Hv1 proton channels regulate a chain of causally related functions that are critical for the microbicidal activity of neutrophils.
RESULTS
Proton currents in resting and activated VSOP/Hv1−/− neutrophils
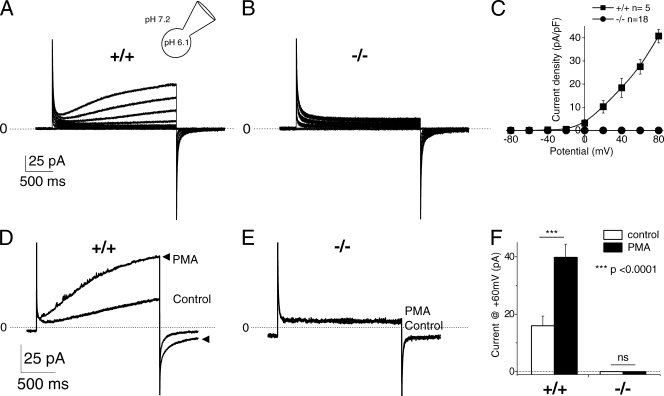
Voltage-activated proton currents (IH+) were recorded in neutrophils purified from peripheral blood using acidic pipette solutions to activate IH+ (pHi/o = 6.1/7.2). Large voltage-activated time-dependent outward currents were observed in neutrophils from WT mice (Fig. 1 A). These currents were reversibly blocked by 100 µM Zn2+, a known inhibitor of proton currents (unpublished data). In contrast, no detectable IH+ was recorded from VSOP/Hv1−/− neutrophils (Fig. 1 B). The current–voltage relationship of control neutrophils was outwardly rectifying with a threshold of activation around −30 mV, whereas the I-V curve of VSOP/Hv1−/− neutrophils was completely flat. We and others previously suggested that the phagocyte NADPH oxidase contains a built-in proton channel (Henderson et al., 1987) that opens only when the oxidase is active (Bánfi et al., 1999; Petheo et al., 2006). Whether the oxidase is a proton channel or modulates another channel has been much debated (Maturana et al., 2002; Morgan et al., 2002; Touret and Grinstein, 2002). To settle this question, we recorded proton currents in VSOP/Hv1−/− neutrophils activated by PMA to activate the oxidase and reveal its putative proton channel. As expected, PMA increased the amplitude of IH+ in control neutrophils by ∼2.5-fold, accelerated its activation kinetics by the same extent, and slowed its deactivation kinetics by ∼1.7-fold (Fig. 1, D and F). In contrast, no current was recorded from VSOP/Hv1−/− neutrophils activated with PMA (Fig. 1, E and F).
Figure 1.
Proton currents in resting and activated VSOP/Hv1−/− neutrophils. (A and B) Typical currents recorded in blood neutrophils from WT and VSOP/Hv1−/− mice, using the whole-cell configuration and acidic pipette solutions to activate proton channels (pHi/o = 6.1/7.2). Proton currents were elicited by 3.5-s depolarizing steps ranging from −80 to +80 mV, applied every 20 s from a holding potential of −60 mV. (C) Current–voltage relationship of time-dependent outward currents recorded in 5 WT and 18 VSOP/Hv1−/− blood neutrophils. (D and E) Effect of 100 nM PMA on the currents recorded at +60 mV in the perforated patch configuration (pHi/o = 7.0/7.0). The lack of proton currents in VSOP/Hv1−/− cells persisted after application of PMA to activate the phagocyte NADPH oxidase. Arrowheads in D show the PMA-activated current. The dashed line indicates zero current level. (F) Mean current amplitude at +60mV before and after PMA addition. Data are mean values ± SEM of 9 WT and 18 VSOP/Hv1−/− neutrophils that were tested in 27 independent experiments. ***, P < 0.0001, unpaired Student's t test. Five WT and nine VSOP/Hv1−/− mice were used for these experiments.
Cytosolic pH changes in VSOP/Hv1−/− neutrophils
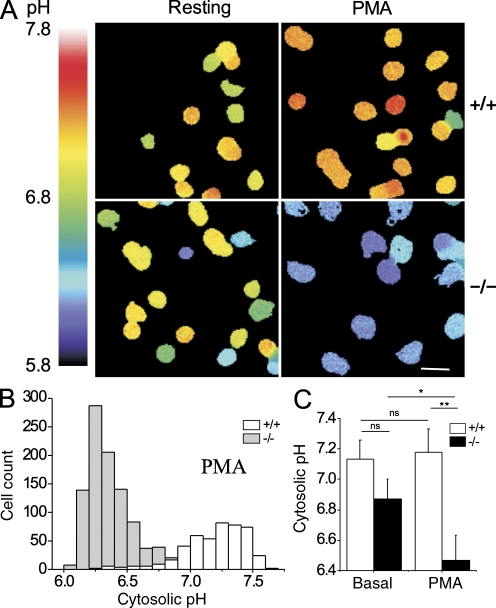
Proton channels are potent acid extruders that participate in the extrusion of the cytosolic acid generated by the phagocyte NADPH oxidase (Demaurex et al., 1996). To test whether the VSOP/Hv1 proton channel is required for acid extrusion, we measured cytosolic pH changes with BCECF during activation of neutrophils with PMA, using solutions devoid of Na+ and of bicarbonate ions to minimize the contribution of the Na+/H+ antiporter and Cl−/HCO3 exchanger. As shown in Fig. 2, VSOP/Hv1−/− neutrophils were slightly more acidic than control neutrophils before stimulation (pHcyt 6.87 ± 0.13 vs. 7.13 ± 0.13, P > 0.05) and acidified markedly upon stimulation with PMA, with their cytosolic pH decreasing by 0.4 pH U, whereas control cells alkalinized slightly (6.46 ± 0.17 vs. 7.18 ± 0.15, P < 0.01; Fig. 2, B and C). These data indicate that VSOP/Hv1 proton channels extrude the acid generated in the cytosol during the activation of neutrophils by PMA.
Figure 2.
Cytosolic pH changes in VSOP/Hv1−/− neutrophils. Bone marrow neutrophils were loaded with the pH-sensitive dye BCECF and changes in fluorescence ratio (F490/F440) measured in sodium-free solutions to minimize the contribution of Na+/H+ exchange. (A) Representative ratio images of WT and VSOP/Hv1−/− neutrophils before (left) and after (right) addition of PMA. Bar, 10 µm. (B) Cytosolic pH distribution of all the PMA-treated cells. (C) Mean cytosolic pH of resting and PMA-activated neutrophils. Data are means ± SD of three experiments with >100 cells each from two WT and two VSOP/Hv1−/− mice. **, P < 0.01; *, P < 0.05, unpaired Student's t test.
Electron current, H2O2 production, and membrane potential of VSOP/Hv1−/− neutrophils
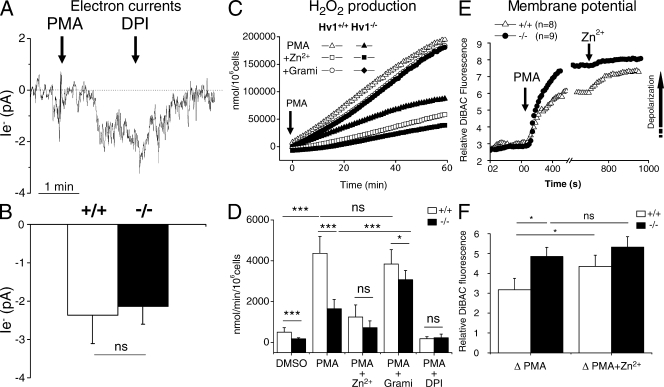
The failure to see proton currents in neutrophils activated with PMA could reflect a failure of the patch-clamped cells to mount a functional oxidase at their plasma membrane. To ensure that this was not the case, we recorded the electron currents (Ie−) generated by the electrogenic activity of the enzyme (Schrenzel et al., 1998). The perforated patch configuration was used to avoid rundown of the current that could occur in whole cell mode. As shown in Fig. 3 A, addition of 0.1 µM PMA evoked within 1 min an inward current at −60 mV that was fully inhibited by the subsequent addition of 1 µM of the oxidase inhibitor diphenyliodonium (DPI), thus fulfilling the criteria for Ie−. Importantly, the amplitude of Ie− recorded from VSOP/Hv1−/− and WT neutrophils was identical (−2.1 ± 0.4 vs. −2.3 ± 0.7 pA, n = 11 and 6, respectively; Fig. 3 B) and approached the value of −2.6 ± 0.5 pA recorded in PMA-activated mouse granulocytes by Morgan et al. (2007). The normal Ie− amplitude in VSOP/Hv1−/− neutrophils indicates that electrons are transferred from cytosolic NADPH to extracellular oxygen at normal rates, implying that VSOP/Hv1−/− neutrophils have a fully functional oxidase. The absence of proton currents in these cells thus proves that the oxidase does not contain a proton channel.
Figure 3.
Electron currents, H2O2 production, and membrane potential of VSOP/Hv1−/− neutrophils. (A and B) Electron current recorded at −60 mV in a WT blood neutrophil in the perforated patch configuration. Currents were evoked by 100 nM PMA and blocked by 1 µM DPI. (B) Mean amplitude of the PMA-activated electron currents. Data are means ± SEM of 6 WT and 11 VSOP/Hv1−/− neutrophils from five WT and nine VSOP/Hv1−/− mice that were tested in 17 independent experiments. ns, not significant at P < 0.05 by an unpaired Student's t test. (C) Time-dependent H2O2 production in WT and VSOP/Hv1−/− blood neutrophils activated with PMA in the absence or presence of 1 mM of the proton channel blocker Zn2+ or 40 µg/ml of the proton-permeable channel gramicidin. Data are from two representative experiments that were independently performed more than three times. (D) Mean H2O2 production from WT and VSOP/Hv1−/− neutrophils. Data are means ± SD of three to five separate experiments done in triplicate from six WT and five VSOP/Hv1−/− mice. ***, P < 0.0001; *, P < 0.05, unpaired Student's t test. (E and F) Membrane potential changes measured with DiBAC4(3) during sequential addition of 1 µM PMA and 100 µM Zn2+ to blood neutrophils. (E) Mean responses of eight WT and nine VSOP/Hv1−/− neutrophils from four independent experiments. PMA evoked a larger depolarization in VSOP/Hv1−/− cells. Arrow indicates the direction of depolarization. (F) Mean change in DiBAC4(3) fluorescence evoked by PMA and PMA+Zn2+. Data are means ± SEM of eight WT and nine VSOP/Hv1−/− blood neutrophils from four independent experiments using five WT and four VSOP/Hv1−/− mice. *, P < 0.05, unpaired Student's t test.
The normal Ie− amplitude of VSOP/Hv1−/− neutrophils appears at odds with the reduced superoxide production of these cells that was recently reported (Okochi et al., 2009; Ramsey et al., 2009). To confirm these studies, we measured the capacity of VSOP/Hv1−/− neutrophils to produce H2O2. As shown in Fig. 3 C, H2O2 production was reduced by 74% in our VSOP/Hv1−/− neutrophils purified from peripheral blood. Reassuringly, 1 mM of the proton channel inhibitor Zn2+ inhibited H2O2 production by control neutrophils by 80% but had only minor effects on VSOP/Hv1−/− neutrophils. To confirm that the reduced H2O2 production of VSOP/Hv1−/− neutrophils was caused by the ablation of endogenous proton channels, we exposed cells to gramicidin, a molecule that generates pores permeable to protons and other monovalent ions. As shown in Fig. 3 (C and D), gramicidin almost restored H2O2 production by VSOP/Hv1−/− neutrophils to the levels of control cells. Thus, an artificial proton conductance can restore high-level H2O2 production in cells lacking endogenous proton channels. Activation of the oxidase depolarizes the plasma membrane to voltages as high as +60 mV in physiological saline (Jankowski and Grinstein, 1999). This depolarization is mitigated by proton channels that equilibrate the membrane potential to the H+ equilibrium potential (Bánfi et al., 1999). Thus, the most likely explanation for the reduced superoxide production of VSOP/Hv1−/− neutrophils is an increased depolarization in cells lacking the charge-compensating proton channel. To test this possibility, we monitored membrane potential changes with DiBAC(4)3 during activation of neutrophils. As shown in Fig. 3 E, VSOP/Hv1−/− neutrophils depolarized significantly more than control neutrophils after PMA stimulation. Addition of Zn2+ potentiated the depolarization of control but not of VSOP/Hv1−/− neutrophils (Fig. 3 F). Subsequent addition of gramicidin collapsed the membrane potential of both control and VSOP/Hv1−/− neutrophils to the same level (unpublished data). This indicates that the reduced H2O2 production of VSOP/Hv1−/− neutrophils can be explained by the increased depolarization conferred by the lack of the proton channel.
Alteration in calcium handling in VSOP/Hv1−/− neutrophils
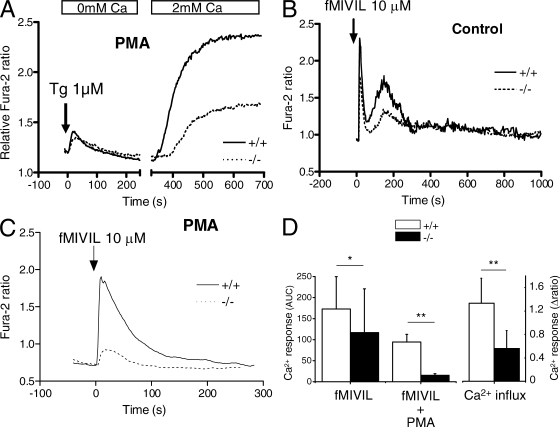
Besides decreasing superoxide production, the increased depolarization reduces the driving force for the entry of cations into cells. The lack of VSOP/Hv1 thus likely impacts on the influx of Ca2+, an ion which controls numerous cellular functions. In neutrophils, Ca2+ entry occurs predominantly across store-operated Ca2+ channels (SOCEs) activated by the depletion of intracellular Ca2+ stores (Scharff and Foder, 1993). To test whether VSOP/Hv1 ablation could have an impact on SOCE, we measured the changes in cytosolic Ca2+ concentration with fura-2. Neutrophils were exposed to PMA for 15 min to maximally activate the oxidase and depolarize the plasma membrane, thapsigargin was added in the absence of Ca2+ to deplete intracellular calcium stores, and Ca2+ was then readmitted to evoke SOCE. As shown in Fig. 4 A, thapsigargin evoked similar responses in control and VSOP/Hv1−/− neutrophils, indicating that Ca2+ release from stores is not altered by the loss of VSOP/Hv1 proton channels. However, SOCE was strongly reduced in VSOP/Hv1−/− neutrophils, as expected from the higher membrane voltage of these cells (Fig. 4, A and D). To see whether the blunted SOCE could alter Ca2+ responses to physiological agonists, we exposed cells to the bacterial peptide N-formyl-Met-Ile-Val-Ile-Leu (fMIVIL), a potent agonist of the mouse formyl peptide receptors (Southgate et al., 2008). In the absence of preactivation with PMA, fMIVIL evoked biphasic Ca2+ elevations, reflecting an initial component of Ca2+ release followed by a delayed component of Ca2+ influx, as in human neutrophils (Demaurex et al., 1992). The integrated Ca2+ responses were significantly reduced in VSOP/Hv1−/− neutrophils exposed to fMIVIL for 30 min (Fig. 4, B and D), mainly because of a reduction in the delayed influx component of the Ca2+ signal. Interestingly, the fMIVIL Ca2+ responses were monophasic in neutrophils preactivated with PMA, suggesting that Ca2+ influx activates more rapidly in neutrophils exposed to the phorbol ester, possibly because of the phosphorylation of Ca2+ handling proteins. Remarkably, the Ca2+ responses were almost abrogated in VSOP/Hv1−/− neutrophils preactivated with PMA (Fig. 4, C and D). This indicates that VSOP/Hv1−/− neutrophils cannot mount effective Ca2+ signals when their oxidase is active, most likely as a result of the decreased SOCE imparted by the enhanced depolarization.
Figure 4.
Calcium handling in VSOP/Hv1−/− neutrophils. Changes in cytosolic Ca2+ were measured with fura-2. (A) SOCE in blood neutrophils exposed to PMA for 20 min to activate the oxidase. Cells were deprived of Ca2+, treated with 1 µM thapsigargin to deplete Ca2+ stores, and exposed to 2 mM Ca2+ to reveal SOCE. Traces are means of 9 WT and 14 VSOP/Hv1−/− recordings (>10 cells each) from five WT and three VSOP/Hv1−/− mice. (B and C) Calcium elevations evoked by 10 µM fMIVIL in bone marrow neutrophils pretreated or not with PMA. Traces in B are means of 19 and 64 cells measured in five and seven independent experiments from four WT and four VSOP/Hv1−/− mice. Traces in C are means of four separate recordings (>10 cells each) from two WT and two VSOP/Hv1−/− mice. The chemotactic peptide was added at t = 0 (arrows). (D) Mean changes in cytosolic Ca2+ evoked by fMIVIL (area under the curve, AUC) and by Ca2+ readmission (Δ ratio amplitude). Data are means ± SD of the experiments in A–C. **, P < 0.001; *, P < 0.05, unpaired Student's t test.
Adhesion and chemokinesis in VSOP/Hv1−/− neutrophils
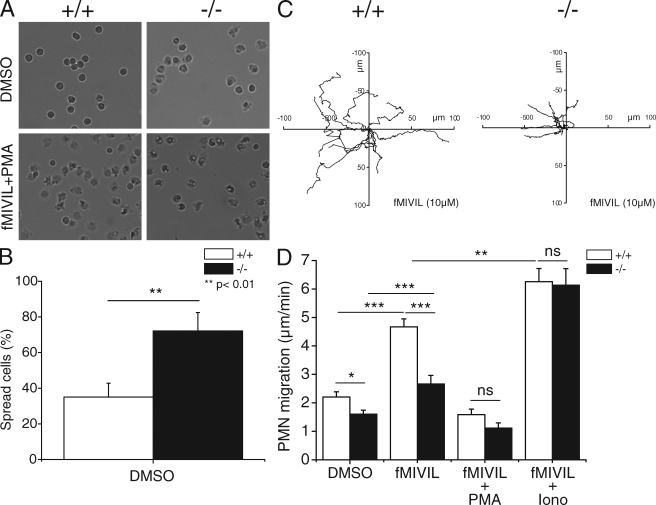
Among other functions, Ca2+ ions regulate the activity of gelsolin to depolymerize the actin cytoskeleton (Larson et al., 2005). Rapid disassembly of the actin cytoskeleton is critical in neutrophils, highly motile cells which must crawl out of blood vessels to seek bacteria inside tissues. To test whether the Ca2+ defect of VSOP/Hv1−/− neutrophils could impair actin depolymerization, we labeled cortical actin with fluorescent phalloidin during phagocytosis of zymosan particles. As shown in Fig. S1, a thicker ring of polymerized actin was observed around particles ingested by VSOP/Hv1−/− neutrophils. This indicates that cortical actin depolymerization is indeed impaired in these cells, most likely as a result of their abortive calcium signals. To test whether impaired actin depolymerization could alter the mobility of VSOP/Hv1−/− neutrophils, we measured the spreading and migration of neutrophils by transmission imaging. As shown in Fig. 5 (A and B), the majority of VSOP/Hv1−/− neutrophils (72%) spread spontaneously on BSA, a nonadhesive substrate. In contrast, only 35% of control neutrophils spread spontaneously on BSA. We next investigated the chemokinetic movement of neutrophils induced by the addition of fMIVIL. Addition of fMIVIL caused neutrophils to move in nondirectional fashion (Videos 1 and 2) and revealed a clear migration defect in neutrophils lacking VSOP/Hv1. When exposed to fMIVIL, these cells moved more slowly and migrated a smaller distance. Tracking the migration paths of cells revealed that the averaged mobility of VSOP/Hv1−/− neutrophils was reduced by 43%, from 4.67 ± 0.27 to 2.66 ± 0.30 µm/min (Fig. 5, C and D). Importantly, the spreading and mobility defects were observed in the absence of PMA and in cells exposed to fMIVIL alone. To test whether the oxidase was active in these conditions, we measured the production of ROS evoked by fMIVIL. As shown in Fig. S2, fMIVIL induced a transient production of H2O2, indicating that fMIVIL activates the oxidase, albeit less efficiently than PMA. Addition of PMA induced virtually all control and VSOP/Hv1−/− neutrophils to spread (Fig. 5 A, bottom) and prevented their migration in response to fMIVIL. Interestingly, VSOP/Hv1−/− neutrophils often exhibited a long tail that appeared stuck to the plate (Video 2), suggesting that their slower mobility was the result of an inability to detach from the substrate. To prove the causal relationship between the calcium and migration defects, we added the calcium ionophore ionomycin during fMIVIL-induced neutrophil migration, using a low dose of ionomycin (100 nM) and a low extracellular Ca2+ concentration (∼5 µM) to minimize cytotoxicity. As shown in Fig. 5 D, addition of ionomycin slightly improved the mobility of neutrophils from WT mice, confirming that the cytosolic Ca2+ clamp was effective and was not cytotoxic. Strikingly, the migration defect of VSOP/Hv1−/− neutrophils was fully restored by the addition of ionomycin (Fig. 5 D). These data indicate that lack of VSOP/Hv1 proton channels impedes the migration of neutrophils by aborting physiological Ca2+ signals.
Figure 5.
Spreading and migration of VSOP/Hv1−/− neutrophils. Bone marrow neutrophils were seeded on BSA-coated Greiner 96-well plates and fMIVIL was added to promote chemokinesis. (A) Representative phase-contrast images of neutrophils before (top) and after (bottom) stimulation with fMIVIL+PMA. The combination of fMIVIL and PMA induced 100% of the cells to spread. (B) Percentage of spread cells among naive WT and VSOP/Hv1−/− neutrophils. Data are means ± SD of 107 WT and 94 VSOP/Hv1−/− neutrophils from three independent experiments. **, P < 0.01, unpaired Student's t test. (C) Migration tracings of nine WT and nine VSOP/Hv1−/− neutrophils exposed to 10 µM fMIVIL for 45 min. (D) Mean migration speed (in micrometers per minute) of neutrophils exposed to DMSO, to fMIVIL alone, to fMIVIL together with PMA, or to fMIVIL together with 100 nM of the Ca2+ ionophore ionomycin in a buffer containing 5 µM Ca2+. Note that PMA prevented both WT and VSOP/Hv1−/− neutrophils from migrating effectively upon fMIVIL stimulation, whereas ionomycin restored normal migration in VSOP/Hv1−/− neutrophils. Data are mean ± SEM of 13–33 individual tracings for each condition from three to five independent experiments. ***, P < 0.0001; **, P < 0.001; *, P < 0.05, unpaired Student's t test. Five WT and five VSOP/Hv1−/− mice were sacrificed for this experiment.
DISCUSSION
Our findings establish the mechanism by which VSOP/Hv1 proton channels sustain the activity of the phagocyte NADPH oxidase and reveal that these channels control other neutrophil functions. Cells lacking the VSOP/Hv1 molecule exhibited normal electron currents when stimulated with PMA and produced normal levels of H2O2 when an artificial proton conductance was provided by gramicidin. This indicates that these cells contain a fully functional oxidase able to transfer electrons efficiently across the plasma membrane. Yet VSOP/Hv1−/− neutrophils failed to produce adequate amounts of H2O2 when their membrane voltage was not clamped with a patch-clamp electrode or with an ionophore. We generated two pieces of evidence that can account for the reduced H2O2 production in VSOP/Hv1−/− neutrophils stimulated with PMA: (1) The lack of VSOP/Hv1 was associated with a substantial acidification of the cytosol, demonstrating that proton channels extrude the cytosolic acid generated by the consumption of NADPH during the respiratory burst; (2) VSOP/Hv1−/− neutrophils were more depolarized than control neutrophils, as predicted from the lack of the compensating charge provided by proton channels. The cytosolic acidification is detrimental to the enzymatic activity of the phagocyte NADPH oxidase (Morgan et al., 2005), whereas the membrane depolarization hinders the flow of electrons across the flavocytochrome, which is voltage dependent (DeCoursey et al., 2003; Petheo and Demaurex, 2005). These two defects can account for the 80% reduction in H2O2 production that we observed in peripheral blood neutrophils stimulated with PMA. This inhibition is more pronounced than the 30% reduction reported by Okochi et al. (2009) in thioglycollate-activated peritoneal neutrophils and slightly higher than the 65% inhibition reported by Ramsey et al. (2009) in bone marrow neutrophils. The difference might be the result of the different assays used (cytochrome c vs. Amplex red) or might reflect differences between neutrophils from marrow, blood, and peritoneum. We also observed that Ca2+ influx was reduced by ∼60% in VSOP/Hv1−/− neutrophils stimulated with PMA, most likely as a result of the membrane depolarization and cytosolic acidification. The reduced Ca2+ influx might hinder the oxidase in cells stimulated with chemoattractants, as in these conditions oxidase activity depends on the magnitude of the Ca2+ signal (Hallett et al., 1990).
The lack of proton currents in cells exhibiting normal electron currents also unambiguously establishes that the oxidase itself is not a proton channel. The notion that the oxidase contains a proton channel was postulated from thermodynamic considerations by Henderson et al. (1987), who provided several pieces of evidence for this mechanism. We did not support the hypothesis that the oxidase is a proton channel initially because cells from X-linked CGD patients lacking the gp91phox molecule had normal proton currents (Nanda et al., 1994). We revived the Henderson hypothesis when we observed that cells with an active oxidase exhibit proton currents with remarkable gating properties (Bánfi et al., 1999). These novel currents were absent in cells from X-CGD patients, indicating that they required the gp91phox molecule. The recognition that gp91phox contains a proton channel motif (Starace et al., 1997) suggested that the flavocytochrome could function as a proton channel and led to the cloning of the NOX homologues (Bánfi et al., 2000). Heterologous expression of NOX homologues in HEK-293 and CHO cells generated proton currents that recapitulated the properties of endogenous proton currents (Bánfi et al., 2000; Maturana et al., 2001), validating the channel theory. However, proton currents were not observed in COS-phox cells expressing a functional oxidase (Morgan et al., 2002), suggesting instead that the oxidase modulates a proton channel. Our findings now provide conclusive evidence that gp91phox is not a proton channel. Instead, VSOP/Hv1 is the only proton channel of phagocytes and its gating properties are enhanced when the oxidase is active (Musset et al., 2009). VSOP/Hv1 probably interacts with a component of the oxidase complex that assembles at the membrane upon phosphorylation, such as the p47phox adaptor subunit. In agreement with this scenario, electron and proton currents cosegregate in excised patches (Petheö et al., 2003), suggesting that VSOP/Hv1 is closely associated with an oxidase component.
We also demonstrate that the lack of proton channels markedly reduces the influx component of calcium signals in neutrophils. This important secondary defect could be predicted from the increased depolarization that we observed in these cells when their oxidase was activated. A role for the oxidase in limiting calcium influx was previously reported (Demaurex et al., 1994; Geiszt et al., 1997; Rada et al., 2003; for review see Rada et al., 2005) but the ability of proton channels to sustain Ca2+ influx was not testable because the proton channel inhibitor Zn2+, like most divalent cations, also inhibits SOCE channels. In this paper, we show that ablation of the VSOP/Hv1 gene is associated with a ∼60% reduction in SOCE and with a ∼85% reduction in the calcium signal evoked by the chemoattractant fMIVIL. The Ca2+ defect was more apparent when the oxidase was preactivated with PMA, as predicted from the reduced driving force for calcium ions imparted by the depolarization, but was also observed in VSOP/Hv1−/− neutrophils exposed to fMIVIL alone (Fig. 4 B). The increased cytosolic acidification might also hinder the activity of SOCE channels. This indicates that cells lacking VSOP/Hv1 proton channels cannot generate normal Ca2+ signals when their oxidase is pumping electrons across the plasma or phagosomal membrane. This signaling defect can have important consequences for the maturation of the phagosome and for the ability of neutrophils to kill microbes. Global Ca2+ elevations accelerate phagocytosis by activating calpain (Dewitt and Hallett, 2002), whereas local Ca2+ elevations around the phagosome are required for the fusion of lysosomes with phagosomes (Jaconi et al., 1990). The periphagosomal increase in Ca2+ is caused by the opening of SOCE channels on the membrane of phagosomes (Lundqvist-Gustafsson et al., 2000), small compartments which can depolarize significantly (Steinberg et al., 2007). Our observation that proton channel ablation depolarizes cells during oxidase activation suggests that phagosomes, where the oxidase normally assembles, are depolarized in cells lacking VSOP/Hv1. The loss of calcium influx at the phagosomal membrane is thus likely to impair cortical actin depolymerization. Accordingly, we observed a thicker ring of polymerized actin around zymosan particles ingested by VSOP/Hv1−/− neutrophils. The thicker cortical actin cytoskeleton might prevent the docking of oxidase subunits to the phagosome and the fusion of lysosomes with phagosomes (Jaconi et al., 1990). Whether lack of VSOP/Hv1 proton channels impairs the fusion of granules to phagosomes remains to be established, but we document here that other Ca2+-dependent functions of neutrophils are altered. VSOP/Hv1−/− neutrophils adhered more firmly to the substrate and migrated less efficiently when stimulated with the chemoattractant fMIVIL. Neutrophil migration was restored by the Ca2+ ionophore ionomycin, demonstrating that the calcium and migration defects are causally related. Thus, actin-based motility is altered in neutrophils lacking proton channels. The microbicidal activity of neutrophils in vivo requires their migration toward invading pathogens (chemotaxis), followed by the phagocytosis and killing of microbes, processes which are all orchestrated by calcium signals. In macrophages, inhibition of Ca2+ influx leads to loss of leading-edge PI3K activity, disassembly of F-actin, cessation of ruffling, and decay of chemoattractant signals (Evans and Falke, 2007). Defective bacterial killing was recently reported in VSOP/Hv1−/− neutrophils and attributed to decreased superoxide production (Ramsey et al., 2009). Our findings not only explain the decreased superoxide production, but indicate that the failure of VSOP/Hv1−/− neutrophils to kill bacteria might also be a result of the altered pH homeostasis and Ca2+ signaling of these cells, which impair their mobility and phagocytic ability. The loss of VSOP/Hv1 proton channels thus has multiple detrimental consequences on the bactericidal activity of neutrophils.
In summary, we show that proton channels sustain superoxide production and calcium entry in neutrophils by preventing membrane depolarization and cellular acidification. VSOP/Hv1 is the only proton channel of neutrophils. Its deletion aborts calcium signals and inhibits neutrophil motility and, probably, also the docking of granules and oxidase subunits with phagosomes, thereby impairing the killing of microbes by neutrophils.
MATERIALS AND METHODS
Materials.
fMIVIL was synthesized at UNIGE Peptides Synthesis Platform. This pentapeptide from Listeria monocytogenes was used instead of N-formyl-Met-Leu-Phe (fMLF) because it is ∼100-fold more potent than fMLF in activating mouse neutrophils (Southgate et al., 2008). Other chemicals were purchased from Sigma-Aldrich unless indicated otherwise.
Mice.
Mice bearing a targeted disruption in the VSOP/Hv1 (VSOP/Hv1−/−, backcrossed eight times) were previously described (Okochi et al., 2009). WT mice (VSOP/Hv1+/+) were of the same genetic background (C57BL6J). Animals enrolled for the study were aged between 1 and 6 mo. All animal husbandry and experiments were approved by and performed in accordance with guidelines from the animal research committee of the University of Geneva.
Isolation of mouse neutrophils from peripheral blood.
Mouse blood (350 ± 50 µl per animal) was collected from 13 VSOP/Hv1+/+and 14 VSOP/Hv1−/− mice in EDTA-tubes by tail bleeding. Blood was collected every 2 wk from the same animals, which were bled four times on average (total 54 bleedings). The exact number of mice used for each experiment is given in the figure legends. NaCl (0.9%) was added to a final volume of 4 ml. After centrifugation at 2,000 rpm for 10 min, cells were resuspended in 1 ml HBSS (without calcium, magnesium, and phenol red) supplemented with 0.2% EDTA. The cells were overlaid afterward on a three-layer Percoll density gradient 72, 63, and 50%, respectively, diluted in HBSS (100% Percoll = nine parts Percoll and one part 10× HBSS) and centrifuged at 3,000 rpm for 25 min without breaking. Neutrophils were harvested from the 63/72% interface after carefully removing the cells from the upper phases. After one wash, remaining red cells in the neutrophils fraction were eliminated by hypotonic lysis (0.5 ml water for 35 s and 0.5 ml 1.8% NaCl). After a final wash, 100,000–500,000 cells were obtained per mouse. Neutrophils were kept in Tyrode solution containing 140 mM NaCl, 5.4 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, 10 mM Hepes, and 10 mM glucose, pH 7.2, and used within 6 h.
Isolation of mouse neutrophils from bone marrow.
Mice were sacrificed and femur and tibia from both hind legs were removed and cleared of all remaining soft tissue attachments. Then, the extreme distal tip of each extremity was cut off and the bones were flushed with HBSS medium (with calcium and magnesium) containing 0.5% BSA, 1% glucose (Sigma-Aldrich), and 20 mM Hepes, pH of 7.2, using a 25G 5/8” needle. After bone marrow was flushed on ice, marrow was centrifuged at 1,300 rpm for 10 min and a hypotonic lysis was directly performed afterward to eliminate red blood cells. The remaining cells were concentrated in 3 ml HBSS medium and treated on a three-layer Percoll density gradient exactly as described in the previous section. The total cell number obtained with this protocol was 5–8 × 106 cells. In total, 11 VSOP/Hv1−/− and 11 VSOP/Hv1+/+ mice were sacrificed. Blood and bone marrow cells preparations contained >90% neutrophils as determined by visual counting. Neutrophils viability was >95% as assessed by Trypan blue exclusion.
Electrophysiology.
Both the whole-cell and perforated patch configuration of the patch-clamp technique were used to record proton and electron currents via a patch-clamp amplifier (Axoclamp 200B; MDS Analytical Technologies) interfaced to a personal computer. Pipettes were made from GC150F-10 Harvard Apparatus borosilicate glass capillaries using a PC-10 Narishige puller. Patch electrodes of 3–8 MΩ were filled, for whole-cell recordings, with an internal solution containing: 118 mM/liter NMDG (N-methyl-d-glucamine), 63 mM/liter TMAMeSO3 (tetramethylammonium methanesulfonate), 1 mM/liter MgCl2, 119 mM/liter MES, and 1 mM/liter EGTA. This solution was adjusted to pHi 6.1 with TMAOH. Extracellular solution contained 140 mM/liter TMAMeSO3, 1.8 mM/liter CaCl2, 100 mM/liter Hepes, and 40 mM mannitol and adjusted at pHo 7.2. The osmolarities of both external and internal solutions were maintained at 300 and 290 mOsm, respectively. For perforated patch recordings, the pipette solution contained 130 mM/liter TMAMeSO3, 25 mM/liter (NH4)2SO4, 2 mM/liter MgCl2, 10 mM/liter Hepes, 1 mM/liter EGTA, and 0.5 mg/ml nystatin. Extracellular solution contained 130 mM/liter TMAMeSO3, 25 mM/liter (NH4)2SO4, 2 mM/liter MgCl2, 1.5 mM/liter CaCl2, 10 mM/liter Hepes, 1 mM/liter EGTA and 10 mM/liter glucose. The pH of both solutions was adjusted at 7.0. No liquid junction potential correction was applied and all experiments were performed at room temperature (20–25°C).
pH measurements.
Neutrophils were loaded with 2 µM BCECF/AM for 20 min, seeded on 25-mm glass coverslips that were inserted in a thermostatic chamber (Harvard Apparatus), and imaged on an Axiovert S100 TV through a 40× 1.3 NA oil-immersion objective (Carl Zeiss, Inc.) using a cooled 16-bit charge-coupled device back-illuminated frame transfer camera (MicroMax; Roper Industries). Cells were alternately excited at 490 and 440 nm with a monochromator (DeltaRam; Photon Technology International) and images acquired using a 535 ± 35-nm emission filter (Omega Optical). Analysis was performed with the MetaFluor 6.2 software (MDS ANalytical Technologies). To exclude any contribution of the Na+/H+ antiporter, experiments were performed in Na+-free medium containing: 140 mM/liter NMDG-Cl, 5 mM/liter KCl, 1 mM/liter MgCl2, 1.8 mM/liter CaCl2, 10 mM/liter Hepes, and 10 mM/liter glucose, pH 7.4. For calibration, 5 µg/ml nigericin and 5 µM monensin (Sigma-Aldrich) were added to solutions containing 140 mM/liter KCl, 1 mM/liter MgCl2, 0.2 mM/liter EGTA, and 20 mM/liter Hepes, pH 7.0–7.5, or MES, pH 6.0–6.5.
Measurement of H2O2 production.
Neutrophils were assayed in HBSS medium with a cell density of 20,000 cells/well in a 96-well flat-bottom plate in a 200-µl final volume. For H2O2 measurements, cells were incubated in the presence of 25 µM Amplex red and 0.005 U/ml horseradish peroxidase. The inhibitors Zinc (1 mM) and DPI (10 µM) were added to the wells 10 min before the recordings. 0.1 µM PMA was added at time t = 0 and fluorescence measured at 590 nm on a FLUOstar (BMG Labtech) microplate reader every minute for 1 h at 37°C. Each condition was performed in triplicate. Six different concentrations of H2O2 were used to obtain a calibration curve. The fMIVIL effect on ROS production was measured with the chemiluminescent probe 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4(2H,3H)dione (L-012) (Imada et al., 1999). Chemiluminescence was measured every 8 s during 15 min on the FLUOstar microplate reader.
Measurements of membrane potential.
The voltage-sensitive bis-oxonol fluorescent dye DiBAC(4)3 was used to study membrane potential changes of neutrophils. Cells were seeded on 25-mm glass coverslips and the acquisition started directly after loading the cells with 250 nM of the dye in Tyrode medium. Cells were excited at 500 nm through a 430/500/575-nm triple band beamsplitter (Chroma Technology Corp.) and images acquired at 535 ± 30 nm.
Calcium measurements.
Neutrophils were loaded with 2 µM Fura-2/AM for 20 min. When indicated, cells were activated by adding 0.1 µM PMA 10 min before starting the acquisition. Fura-2 fluorescence was imaged using alternate excitation at 340 and 380 nm and 510 ± 40 nm emission.
Spreading and motility assays.
Bone marrow neutrophils were seeded in a Greiner 96-well flat-bottom plate at 500,000 cells/well in HBSS medium supplemented with 0.5% BSA, 1% glucose, and 20 mM Hepes. Neutrophils were prewarmed at 37°C. The wells were coated with 0.5% BSA and the plate was preheated for 15 min at 37°C. Agonists were added to each well (200 µl/well) and the adhesion and chemokinesis of neutrophils imaged with an ImageXpress Micro (MDS Analytical Technologies) automated fluorescence imaging system. Data were analyzed with the MetaXpress software (MDS Analytical Technologies). Images were taken every 15 s during 45 min.
Online supplemental material.
Fig. S1 shows actin polymerization in VSOP/Hv1−/− neutrophils. Fig. S2 shows the effect of fMIVIL on ROS production. Video 1 shows VSOP/Hv1+/+ neutrophil migration upon fMIVIL stimulation. Video 2 shows the slower migration of neutrophils lacking VSOP/Hv1. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091837/DC1.
Acknowledgments
The authors would like to thank C. Castelbou for his excellent technical assistance.
This research was supported by grant 3100A0-118393 from the Swiss National Science Foundation (to N. Demaurex) and by grants from the Japan Ministry of Education, Culture, Sports, Science and Technology (to Y. Okamura).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- DPI
- diphenyliodonium
- fMIVIL
- N-formyl-Met-Ile-Val-Ile-Leu
- ROS
- reactive oxygen species
- SOCE
- store-operated Ca2+ channel
- VSOP
- voltage-sensing domain only protein
References
- Ahluwalia J. 2008. Chloride channels activated by swell can regulate the NADPH oxidase generated membrane depolarisation in activated human neutrophils. Biochem. Biophys. Res. Commun. 365:328–333 10.1016/j.bbrc.2007.10.176 [DOI] [PubMed] [Google Scholar]
- Ahluwalia J., Tinker A., Clapp L.H., Duchen M.R., Abramov A.Y., Pope S., Nobles M., Segal A.W. 2004. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 427:853–858 10.1038/nature02356 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Babior B.M., Lambeth J.D., Nauseef W. 2002. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397:342–344 10.1006/abbi.2001.2642 [DOI] [PubMed] [Google Scholar]
- Bánfi B., Schrenzel J., Nüsse O., Lew D.P., Ligeti E., Krause K.H., Demaurex N. 1999. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J. Exp. Med. 190:183–194 10.1084/jem.190.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánfi B., Maturana A., Jaconi S., Arnaudeau S., Laforge T., Sinha B., Ligeti E., Demaurex N., Krause K.H. 2000. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 287:138–142 10.1126/science.287.5450.138 [DOI] [PubMed] [Google Scholar]
- Decoursey T.E. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83:475–579 [DOI] [PubMed] [Google Scholar]
- DeCoursey T.E., Morgan D., Cherny V.V. 2003. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 422:531–534 10.1038/nature01523 [DOI] [PubMed] [Google Scholar]
- Demaurex N., Petheö G.L. 2005. Electron and proton transport by NADPH oxidases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:2315–2325 10.1098/rstb.2005.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Schlegel W., Varnai P., Mayr G., Lew D.P., Krause K.H. 1992. Regulation of Ca2+ influx in myeloid cells. Role of plasma membrane potential, inositol phosphates, cytosolic free [Ca2+], and filling state of intracellular Ca2+ stores. J. Clin. Invest. 90:830–839 10.1172/JCI115958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Monod A., Lew D.P., Krause K.H. 1994. Characterization of receptor-mediated and store-regulated Ca2+ influx in human neutrophils. Biochem. J. 297:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Downey G.P., Waddell T.K., Grinstein S. 1996. Intracellular pH regulation during spreading of human neutrophils. J. Cell Biol. 133:1391–1402 10.1083/jcb.133.6.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S., Hallett M.B. 2002. Cytosolic free Ca2+ changes and calpain activation are required for β integrin–accelerated phagocytosis by human neutrophils. J. Cell Biol. 159:181–189 10.1083/jcb.200206089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essin K., Salanova B., Kettritz R., Sausbier M., Luft F.C., Kraus D., Bohn E., Autenrieth I.B., Peschel A., Ruth P., Gollasch M. 2007. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am. J. Physiol. Cell Physiol. 293:C45–C54 10.1152/ajpcell.00450.2006 [DOI] [PubMed] [Google Scholar]
- Essin K., Gollasch M., Rolle S., Weissgerber P., Sausbier M., Bohn E., Autenrieth I.B., Ruth P., Luft F.C., Nauseef W.M., Kettritz R. 2009. BK channels in innate immune functions of neutrophils and macrophages. Blood. 113:1326–1331 10.1182/blood-2008-07-166660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.H., Falke J.J. 2007. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc. Natl. Acad. Sci. USA. 104:16176–16181 10.1073/pnas.0707719104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femling J.K., Cherny V.V., Morgan D., Rada B., Davis A.P., Czirják G., Enyedi P., England S.K., Moreland J.G., Ligeti E., et al. 2006. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J. Gen. Physiol. 127:659–672 10.1085/jgp.200609504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszt M., Kapus A., Német K., Farkas L., Ligeti E. 1997. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease. J. Biol. Chem. 272:26471–26478 10.1074/jbc.272.42.26471 [DOI] [PubMed] [Google Scholar]
- Hallett M.B., Davies E.V., Campbell A.K. 1990. Oxidase activation in individual neutrophils is dependent on the onset and magnitude of the Ca2+ signal. Cell Calcium. 11:655–663 10.1016/0143-4160(90)90020-U [DOI] [PubMed] [Google Scholar]
- Henderson L.M., Chappell J.B., Jones O.T. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada I., Sato E.F., Miyamoto M., Ichimori Y., Minamiyama Y., Konaka R., Inoue M. 1999. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal. Biochem. 271:53–58 10.1006/abio.1999.4107 [DOI] [PubMed] [Google Scholar]
- Jaconi M.E., Lew D.P., Carpentier J.L., Magnusson K.E., Sjögren M., Stendahl O. 1990. Cytosolic free calcium elevation mediates the phagosome–lysosome fusion during phagocytosis in human neutrophils. J. Cell Biol. 110:1555–1564 10.1083/jcb.110.5.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski A., Grinstein S. 1999. A noninvasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 274:26098–26104 10.1074/jbc.274.37.26098 [DOI] [PubMed] [Google Scholar]
- Koch H.P., Kurokawa T., Okochi Y., Sasaki M., Okamura Y., Larsson H.P. 2008. Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 105:9111–9116 10.1073/pnas.0801553105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L., Arnaudeau S., Gibson B., Li W., Krause R., Hao B., Bamburg J.R., Lew D.P., Demaurex N., Southwick F. 2005. Gelsolin mediates calcium-dependent disassembly of Listeria actin tails. Proc. Natl. Acad. Sci. USA. 102:1921–1926 10.1073/pnas.0409062102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Letts J.A., Mackinnon R. 2008. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. USA. 105:7692–7695 10.1073/pnas.0803277105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist-Gustafsson H., Gustafsson M., Dahlgren C. 2000. Dynamic ca(2+)changes in neutrophil phagosomes A source for intracellular ca(2+)during phagolysosome formation? Cell Calcium. 27:353–362 10.1054/ceca.2000.0130 [DOI] [PubMed] [Google Scholar]
- Maturana A., Arnaudeau S., Ryser S., Banfi B., Hossle J.P., Schlegel W., Krause K.H., Demaurex N. 2001. Heme histidine ligands within gp91(phox) modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 276:30277–30284 10.1074/jbc.M010438200 [DOI] [PubMed] [Google Scholar]
- Maturana A., Krause K.H., Demaurex N. 2002. NOX family NADPH oxidases: do they have built-in proton channels? J. Gen. Physiol. 120:781–786 10.1085/jgp.20028713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Cherny V.V., Price M.O., Dinauer M.C., DeCoursey T.E. 2002. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 119:571–580 10.1085/jgp.20018544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Cherny V.V., Murphy R., Katz B.Z., DeCoursey T.E. 2005. The pH dependence of NADPH oxidase in human eosinophils. J. Physiol. 569:419–431 10.1113/jphysiol.2005.094748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Cherny V.V., Finnegan A., Bollinger J., Gelb M.H., DeCoursey T.E. 2007. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2 alpha activity. J. Physiol. 579:327–344 10.1113/jphysiol.2006.124248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Iwasaki H., Sasaki M., Inaba K., Okamura Y. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 435:1239–1243 10.1038/nature03650 [DOI] [PubMed] [Google Scholar]
- Murphy R., DeCoursey T.E. 2006. Charge compensation during the phagocyte respiratory burst. Biochim. Biophys. Acta. 1757:996–1011 10.1016/j.bbabio.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Musset B., Cherny V.V., Morgan D., DeCoursey T.E. 2009. The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett. 583:7–12 10.1016/j.febslet.2008.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Romanek R., Curnutte J.T., Grinstein S. 1994. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J. Biol. Chem. 269:27280–27285 [PubMed] [Google Scholar]
- Nauseef W.M. 2007. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 219:88–102 10.1111/j.1600-065X.2007.00550.x [DOI] [PubMed] [Google Scholar]
- Okochi Y., Sasaki M., Iwasaki H., Okamura Y. 2009. Voltage-gated proton channel is expressed on phagosomes. Biochem. Biophys. Res. Commun. 382:274–279 10.1016/j.bbrc.2009.03.036 [DOI] [PubMed] [Google Scholar]
- Petheo G.L., Demaurex N. 2005. Voltage- and NADPH-dependence of electron currents generated by the phagocytic NADPH oxidase. Biochem. J. 388:485–491 10.1042/BJ20041889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petheö G.L., Maturana A., Spät A., Demaurex N. 2003. Interactions between electron and proton currents in excised patches from human eosinophils. J. Gen. Physiol. 122:713–726 10.1085/jgp.200308891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petheo G.L., Girardin N.C., Goossens N., Molnár G.Z., Demaurex N. 2006. Role of nucleotides and phosphoinositides in the stability of electron and proton currents associated with the phagocytic NADPH oxidase. Biochem. J. 400:431–438 10.1042/BJ20060578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B.K., Geiszt M., Van Bruggen R., Nemet K., Roos D., Ligeti E. 2003. Calcium signalling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clin. Exp. Immunol. 132:53–60 10.1046/j.1365-2249.2003.02138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B.K., Geiszt M., Hably C., Ligeti E. 2005. Consequences of the electrogenic function of the phagocytic NADPH oxidase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:2293–2300 10.1098/rstb.2005.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey I.S., Moran M.M., Chong J.A., Clapham D.E. 2006. A voltage-gated proton-selective channel lacking the pore domain. Nature. 440:1213–1216 10.1038/nature04700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey I.S., Ruchti E., Kaczmarek J.S., Clapham D.E. 2009. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. USA. 106:7642–7647 10.1073/pnas.0902761106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves E.P., Lu H., Jacobs H.L., Messina C.G., Bolsover S., Gabella G., Potma E.O., Warley A., Roes J., Segal A.W. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297 10.1038/416291a [DOI] [PubMed] [Google Scholar]
- Sasaki M., Takagi M., Okamura Y. 2006. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 312:589–592 10.1126/science.1122352 [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. 1993. Regulation of cytosolic calcium in blood cells. Physiol. Rev. 73:547–582 [DOI] [PubMed] [Google Scholar]
- Schrenzel J., Serrander L., Bánfi B., Nüsse O., Fouyouzi R., Lew D.P., Demaurex N., Krause K.H. 1998. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 392:734–737 10.1038/33725 [DOI] [PubMed] [Google Scholar]
- Segal A.W. 1987. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 326:88–91 10.1038/326088a0 [DOI] [PubMed] [Google Scholar]
- Segal A.W., Geisow M., Garcia R., Harper A., Miller R. 1981. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 290:406–409 10.1038/290406a0 [DOI] [PubMed] [Google Scholar]
- Southgate E.L., He R.L., Gao J.L., Murphy P.M., Nanamori M., Ye R.D. 2008. Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 181:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace D.M., Stefani E., Bezanilla F. 1997. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron. 19:1319–1327 10.1016/S0896-6273(00)80422-5 [DOI] [PubMed] [Google Scholar]
- Steinberg B.E., Touret N., Vargas-Caballero M., Grinstein S. 2007. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc. Natl. Acad. Sci. USA. 104:9523–9528 10.1073/pnas.0700783104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.C., Meech R.W. 1982. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 299:826–828 10.1038/299826a0 [DOI] [PubMed] [Google Scholar]
- Tombola F., Pathak M.M., Isacoff E.Y. 2005. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 45:379–388 10.1016/j.neuron.2004.12.047 [DOI] [PubMed] [Google Scholar]
- Tombola F., Ulbrich M.H., Isacoff E.Y. 2008. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 58:546–556 10.1016/j.neuron.2008.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret N., Grinstein S. 2002. Voltage-gated proton “channels”: a spectator's viewpoint. J. Gen. Physiol. 120:767–771 10.1085/jgp.20028706 [DOI] [PMC free article] [PubMed] [Google Scholar]