Abstract
The complement system is an essential component of innate immunity, participating in the pathogenesis of inflammatory diseases and in host defense. In the lectin complement pathway, mannose-binding lectin (MBL) and ficolins act as recognition molecules, and MBL-associated serine protease (MASP) is a key enzyme; MASP-2 is responsible for the lectin pathway activation. The function of other serine proteases (MASP-1 and MASP-3) is still obscure. In this study, we generated a MASP-1– and MASP-3–deficient mouse model (Masp1/3−/−) and found that no activation of the alternative pathway was observed in Masp1/3−/− serum. Mass spectrometric analysis revealed that circulating complement factor D (Df) in Masp1/3−/− mice is a zymogen (pro-Df) with the activation peptide QPRGR at its N terminus. These results suggested that Masp1/3−/− mice failed to convert pro-Df to its active form, whereas it was generally accepted that the activation peptide of pro-Df is removed during its secretion and factor D constitutively exists in an active form in the circulation. Furthermore, recombinant MASP-1 converted pro-Df to the active form in vitro, although the activation mechanism of pro-Df by MASP-1 is still unclear. Thus, it is clear that MASP-1 is an essential protease of both the lectin and alternative complement pathways.
The complement system, consisting of >30 proteins in plasma and on the cell surface, plays roles in immunological responses, such as opsonization of pathogens, chemotaxis, and activation of leukocytes, direct killing of pathogens, bridging innate and adaptive immunity, and clearance of immune complexes and apoptotic cells (Brown, 1991; Walport, 2001a). On the other hand, inappropriate activation of complement affects the pathogenesis of inflammatory diseases (Walport, 2001b). Once the complement system is activated, a chain of reactions involving proteolysis and assembly occurs, resulting in cleavage of the third complement component (C3). The cascade up to C3 cleavage is called the activation pathway. There are three activation pathways; the classical, the alternative, and the lectin pathways. In general, the classical pathway is activated by antibody–antigen complexes and the other two, the alternative and the lectin pathways, function in innate immune defense. Recent studies reveal that C1q recognizes various ligands besides antibody–antigen complexes, resulting in the activation of complement by innate immune system (Lu et al., 2008). The alternative pathway does not involve specific recognition molecules and also functions to amplify C3 activation (amplification loop). The lectin pathway involves carbohydrate recognition by mannose-binding lectin (MBL) and ficolins, and the subsequent activation of associated enzymes, MBL-associated serine proteases (MASPs; Fujita, 2002). Three distinct MASPs, MASP-1 (Matsushita and Fujita, 1992), MASP-2 (Thiel et al., 1997), and MASP-3 (Dahl et al., 2001) have been identified in many species of vertebrates. MASP-1 and MASP-3 are produced by alternative splicing from a single MASP1/3 gene, with the result that they have a common heavy chain and individual light chains (protease domain; Dahl et al., 2001). Although MASP-1 cleaves C3, C2, factor XIII, and fibrinogen in vitro (Matsushita and Fujita, 1995; Matsushita et al., 2000; Hajela et al., 2002; Chen and Wallis, 2004; Krarup et al., 2008), the physiological significance of these events is unclear. To identify the roles of MASP-1 in vivo, we generated a MASP-1–deficient mouse in which MASP-3 is also absent (Masp1/3−/−). Using Masp1/3−/− mice, we have shown previously that MASP-1 triggers the lectin pathway by promoting activation of MASP-2 (Takahashi et al., 2008). In the present study, we found no activation of the alternative pathway in Masp1/3−/− mice and analyzed the underlying mechanisms.
RESULTS AND DISCUSSION
MASP-1– and MASP-3–deficient mice lack activation of the alternative complement pathway
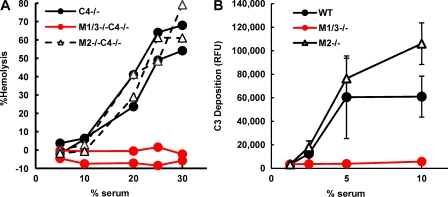
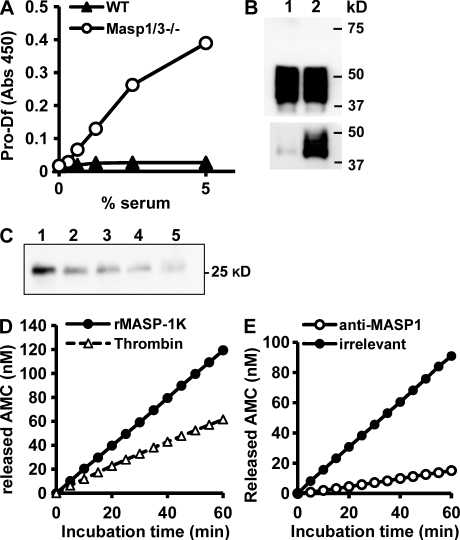
To determine whether the alternative pathway is activated in Masp1/3−/−, mouse sera were assayed for hemolytic activity against rabbit erythrocytes and for C3 deposition ability using zymosan-coated microwells. In the hemolytic assay, mice with a C4-deficient background (C4−/−) and Mg2+-EGTA–containing buffer were used to eliminate the effects of the classical and lectin pathways. Surprisingly, both assays demonstrated that sera derived from Masp1/3−/− mice had no ability to activate the alternative pathway (Fig. 1). In contrast, mice deficient in MASP-2 and small MBL-associated protein (sMAP; a truncated MASP-2:Masp2/sMap−/−; Takahashi et al., 1999; Iwaki et al., 2006) showed a considerable amount of hemolysis and C3 deposition, similar to the control mice. Therefore, these results indicate that MASP-1 and/or MASP-3 are involved in activation of the alternative pathway.
Figure 1.
Masp1/3−/− mice show no ability to activate the alternative pathway. (A) Rabbit erythrocytes (2.5 × 106) were incubated for 1 h with mouse serum in GVB containing Mg2+-EGTA. Hemolysis was measured in a microplate reader at 405 nm. Two individual C4-deficient (C4−/−), MASP-1/-3, and C4 triple-knockout (M1/3−/−C4−/−) mice and MASP-2/sMAP and C4 triple-knockout (M2−/−C4−/−) mice were analyzed. (B) Sera from wild-type C57BL6 (WT), Masp1/3−/− (M1/3−/−), and Masp2/sMap−/− (M2−/−) mice diluted with GVB containing Mg2+-EGTA were incubated in zymosan-coated microwells for 1 h. Bound C3 was detected by fluorescein-conjugated anti–mouse C3 antibody. Values are presented as relative fluorescence units (RFUs). Data are presented as means ± SD of three individual mice for each strain. Data from one out of two (A) or three (B) independent experiments with similar results are shown.
The restoration of the alterative pathway in Masp1/3−/− mice by an active form of factor D
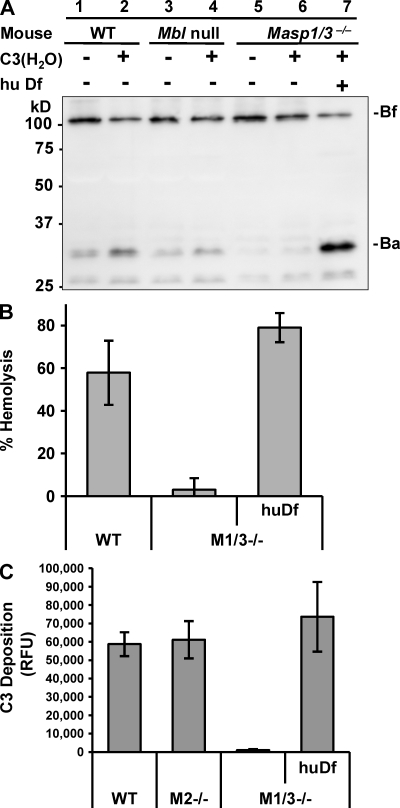
Circulating C3 is spontaneously converted to a C3b-like product called C3(H2O) upon thioester hydrolysis. In the presence of complement factor B (Bf) and complement factor D (Df), C3(H2O) is able to form the primary C3 convertase C3(H2O)Bb, which triggers the initial step of the alternative pathway (Pangburn and Müller-Eberhard, 1983). To clarify the underlying mechanisms of the aforementioned results, Bf cleavage was investigated by Western blotting. When serum from wild-type C57BL/6 mice was incubated at 37°C for 1 h, a fragment called Ba was cleaved from Bf by limited proteolysis (Fig. 2 A, lane 1). The Ba fragment was also found in serum from Mbl-null mice, in which both MBL-A and MBL-C are absent (Fig. 2 A, lane 3; Shi et al., 2004), and exogenous C3(H2O) enhanced Bf activation (Fig. 2 A, lane 2 and 4). No Ba fragment was detected in the following incubation of serum from Masp1/3−/− mice (lane 5), and a faint band of Ba fragment was detected in the presence of exogenous C3(H2O) (Fig. 2 A, lane 6). Because Bf was cleaved by Df, the addition of purified human Df (active form) to Masp1/3−/− mice sera and the following incubation resulted in the cleavage of Bf (Fig. 2 A, lane 7). A very low level of hemolytic activity against rabbit erythrocytes in the sera of Masp1/3−/− mice was compensated by adding active Df (Fig. 2 B), and the low level of C3 deposition on immobilized zymosan using serum from Masp1/3−/− mice was also restored by active Df (Fig. 2 C). These results suggest that Masp1/3−/− mice carry an abnormality in the activation of Df.
Figure 2.
Df restores the deficiency of alternative pathway activation in Masp1/3−/− mice. (A) Immunoblot analysis of Bf incubated with the sera of wild-type C57BL/6 (WT), MBL-A, and -C double-deficient (Mbl null) and Masp1/3−/− mice at 37°C for 30 min. Each serum type was incubated with (+) or without (−) 1.25 µg C3(H2O). Serum from Masp1/3−/− mice was also incubated with (+) human Df (0.2 µg). Note: an additional band (∼26 kD) was also detected with Ba, but it was not identified. (B) A hemolytic assay using rabbit erythrocytes was performed. Percent hemolysis induced by sera (25 µl) from wild-type (WT), Masp1/3−/− (M1/3−/−), and Masp1/3−/− mice supplemented with 0.2 µg human Df (huDf) is indicated. (C) A C3 deposition assay on immobilized zymosan was performed. 5 µl of sera from wild-type (WT), Masp2/sMap−/− (M2−/−), and Masp1/3−/− (M1/3−/−) mice supplemented with 0.2 µg human Df (huDf) were analyzed. Data are presented as the means ± SD of three independent experiments.
Circulating Df in Masp1/3−/− mice is a zymogen that retains activation peptide at the N terminus
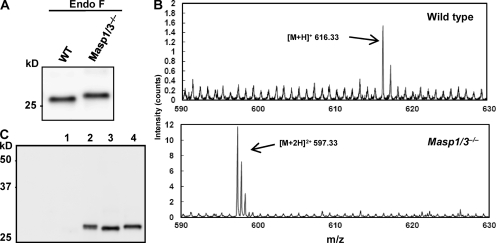
To identify the cause of this abnormality in Masp1/3−/− mice, we first determined the serum concentration of Df by sandwich ELISA. The concentration of Df in Masp1/3−/− serum (26.9 ± 7.3 mg/l; n = 5) was not significantly different from that in C57BL/6 serum (32.2 ± 16.7 mg/l; n = 5). Next, to investigate the size and modifications of Df in Masp1/3−/− serum, circulating Df was isolated by immunoprecipitation with a polyclonal anti–mouse Df antibody. Df was detected as 40–44-kD broad bands that could not be distinguished from those in wild-type mice (Fig. S1). These bands were converted to a single 26-kD band by treatment with N-glycosidase F, indicating that mouse Df is modified by N-glycosylation, as previously reported (Cook et al., 1987). The deglycosylated Df in Masp1/3−/− serum was slightly larger than that in wild-type mouse (Fig. 3, A and C).
Figure 3.
Circulating Df in Masp1/3−/− mice is a zymogen. (A) Endogeneous Df was immunoprecipitated and immunoblotted with a polyclonal anti-Df antibody. Precipitated Df was incubated with N-glycosidase F (Endo-F) to remove N-glycosylations. Sera from wild-type (WT) and Masp1/3−/− mice were analyzed. (B) Endogenous Df was immunoprecipitated from the sera of wild-type (C57BL6; top) and Masp1/3−/− mice (bottom). V8 protease-digested fragments were analyzed by ESI-TOF-MS. The range from 590 to 630 (m/z) is presented. (C) Western blotting of Df in culture supernatants from undifferentiated 3T3-L1 cells (lane 1) and differentiated 3T3-L1 cells (lane 2) were analyzed. As a control, precipitated Df from the sera of wild-type (lane 3) and Masp1/3−/− mice (lane 4) was used in immunoblotting experiments. Data from one out of three (A and C) or two (B) independent experiments with similar results are shown.
Df is thought to circulate in blood as an activated protease (Rosen et al., 1989). A putative activation peptide QPRGR predicted from its cDNA sequence is removed during Df maturation and secretion (Cook et al., 1985). Therefore, it is probable that Df in Masp1/3−/− mice still retains the activation peptide. To verify this, we performed electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) of Df-derived fragments cleaved by V8 protease. As shown in Fig. 3 B, a molecular ion peak at 616.33 m/z ([M+H]+), corresponding to the mass of an N-terminal activated Df fragment ILGGQE, was detected after cleavage of Df from wild-type, showing that wild-type mouse has an active Df. The internal amino acid sequence of the peptide in this peak was confirmed by tandem mass spectrometry analysis (Fig. S2 and Table S1). Among V8 protease-treated fragments of Df from Masp1/3−/− mice, however, a different molecular ion peak at 597.33 m/z ([M+2H]2+) was observed. The calculated mass (1,192.64) of the peptide responsible for this peak corresponded to the sequence (p)QPRGRILGGQE, including a modified N-terminal pyroglutamate. The internal amino acid sequence in this peak was also confirmed by tandem mass spectrometry analysis (Fig. S2 and Table S1).
These results revealed that Masp1/3−/− mice failed to convert pro-Df to its active form. Thus, it seems likely that MASP-1 and/or MASP-3 is involved in the cleavage of the activation peptide from pro-Df.
3T3-L1 cells that are differentiated into adipocytes secrete pro-Df
Adipose is the main tissue that produces Df, which is secreted into the peripheral blood (Cook et al., 1987; White et al., 1992). As previously reported (Volanakis and Narayana, 1996), several studies have revealed that the activation peptide of pro-Df is removed by a putative trypsin-like protease during its secretion from adipocytes. On the other hand, pro-Df that was able to be activated by trypsin was observed in human serum (Fearon et al., 1974), suggesting that pro-Df secretes from human adipocytes. Masp1 and Masp3 mRNAs could not be detected in murine adipose tissue (Fig. S3). Therefore, it is probable that MASP-1 and/or MASP-3 cleave pro-Df in the circulation after its secretion from adipocytes. We next determined whether pro-Df was cleaved in the circulation after its secretion from adipocytes using differentiated 3T3-L1 adipocytes. As shown in Fig. 3 C, the size of the deglycosylated Df in differentiated 3T3-L1 adipocytes (lane 2) was larger than that in wild-type mouse serum (lane 3). Mass spectrometry analysis revealed that Df produced by differentiated 3T3-L1 adipocytes still retained the activation peptide, showing that pro-Df is activated after its secretion from adipocytes.
Recombinant MASP-1 cleaves and activates pro-Df
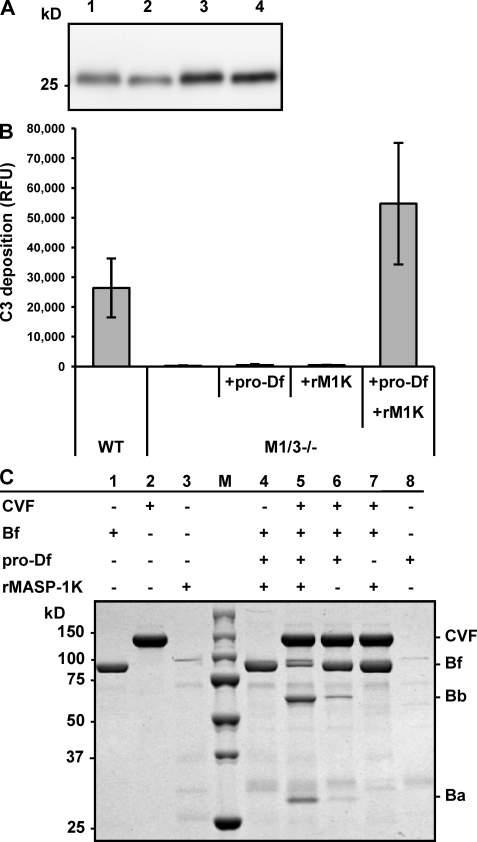
Recombinant pro-Df has been produced in insect cells using a baculovirus expression system (Rosen et al., 1989; Yamauchi et al., 1994). We also prepared recombinant mouse pro-Df containing a hexahistidine (6xHis) tag using a baculovirus system. Our mass spectrometry analysis revealed that the recombinant Df produced by this system was pro-Df with the activation peptide (unpublished data). To assess whether recombinant pro-Df is a substrate for MASP-1, recombinant pro-Df was incubated with rMASP-1K, which was modified by replacing the arginine residue at the reactive site P1 for activation of MASP-1 with lysine (Takahashi et al., 2008). rMASP-1K overcame the problem of self-activation and degradation, allowing us to produce larger amounts of the recombinant protein. As shown in Fig. 4 A (lanes 2 and 4), rMASP-1K reduced the molecular weight of deglycosylated recombinant pro-Df to that of active Df, indicating that MASP-1 is able to directly cleave the activation peptide in pro-Df.
Figure 4.
MASP-1 activates pro-Df. (A) Recombinant pro-Df (80 ng) was incubated with 120 ng (lane 2) and 240 ng (lane 4) of recombinant MASP-1 (rMASP-1K) at 37°C for 1 h. As a control, pro-Df was incubated without rMASP-1K (lanes 1 and 3). After removing N-glycosylations by N-glycosidase F treatment, samples were separated by SDS-PAGE and immunoblotted with anti-Df antibody. Data are representative of two independent experiments. (B) A C3 deposition assay on immobilized zymosan was performed. 5 µl of the sera of wild-type (WT) and Masp1/3−/− (M1/3−/−) mice supplemented with 0.2 µg of pro-Df alone (+pro-Df), 0.03 µg of recombinant MASP-1K alone (+rM1K) or pro-Df (0.2 µg) that was preincubated with rMASP-1K (0.03 µg; +pro-Df+rM1K) are analyzed. Data are presented as the means ± SD of three independent experiments. (C) A reconstitution experiment using purified components, i.e., CVF, Bf, and recombinant pro-Df was performed. Samples were subjected to electrophoresis under nonreducing conditions and stained with Coomassie. Before reconstitution with CVF and Bf, pro-Df was preincubated with rMASP-1K (lane 4 and 5). Data are representative of two independent experiments.
Next, we elucidated whether the active Df generated by rMASP-1K can activate the alternative pathway. Incubation of recombinant pro-Df with rMASP-1K fully restored the C3 deposition activity of serum from Masp1/3−/− mice on immobilized zymosan, while neither pro-Df nor rMASP-1K alone produced an effect (Fig. 4 B). Furthermore, we reconstituted the C3 convertase of the alternative pathway by mixing purified components, including cobra-venom factor (CVF), Bf and recombinant pro-Df. CVF is a structural homologue of C3b that forms a stable complex with Bf. As shown in Fig. 4 C, the incubation of recombinant pro-Df with rMASP-1K increased the cleavage of Bf (Fig. 4 C, lane 5), compared with reconstitutions without rMASP-1K (Fig. 4 C, lane 6). These results indicate that MASP-1 is able to directly activate pro-Df to Df, which in turn activates Bf in a Bf–CVF complex. This activated Bf can cleave C3 to initiate the amplification loop of the alternative pathway.
A question was raised as to why rMASP-1K did not cleave endogenous pro-Df in serum of Masp1/3−/− mouse, as shown in Fig. 4 B. Native MASP-1 in the circulation is thought to be a zymogen. However, the major portion of rMASP-1K has been already activated during expression and purification. Therefore, the question should be explained by previous studies (Matsushita et al., 2000; Petersen et al., 2000; Rossi et al., 2001), which show that activated MASP-1 is immediately neutralized by C1 esterase inhibitor and α-2-macroglobrin.
WT serum could cleave pro-Df in Masp-1/3-deficient serum
To clarify the above question, we generated a rabbit antibody to recognize the activation peptide (QPRGR) of pro-Df. This antibody was able to detect pro-Df in serum from Masp1/3−/− mouse, but not active Df in that from WT mouse by two biochemical analyses (Fig. 5, A and B), showing that pro-Df was not detected in normal mouse serum. Using this antibody, we asked whether WT serum that contains endogenous MASP-1 has an ability to cleave endogenous pro-Df in Masp1/3−/− serum. When both sera were incubated together at 37°C, pro-Df that was captured by the specific antibody decreased in a time-dependent manner (Fig. 5 C). This result suggested that pro-Df in Masp1/3−/− serum is converted to its active-form by the endogenous MASP-1. However, it is generally accepted that most MASP-1 is circulating as a zymogen and activated through MBL and ficolins, although recombinant MASP-1 was easily auto-activated (Rossi et al., 2001; Ambrus et al., 2003; Zundel et al., 2004). Therefore, it is speculated that endogenous zymogen of MASP-1 would somehow be activated and cleave pro-Df, even in presence of inhibitors. The situation is more complicated, because free unbound MASP-1 to MBL is circulated in plasma (Terai et al., 1997; Thiel et al., 2000), and the precise roles and activation mechanisms of the free MASP-1 are also not clear. The underlying mechanism for the role of free MASP-1 is currently under investigation.
Figure 5.
Specific detection of pro-Df and kinetic analysis for cleavage of its activation peptide. (A) Pro-Df was detected by a sandwich ELISA. Endogenous Df in mouse sera was captured in anti-Df antibody-coating wells, followed by detection of pro-Df with antibody specific for the activation peptide of mouse pro-Df. (B) Sera (0.5 µl) from WT (lane 1) and Masp1/3−/− (lane 2) mice were analyzed by immunoblotting using anti-Df (top) and anti–pro-Df (bottom) under reducing conditions. (C) Masp1/3−/− serum was incubated with same volume of WT serum at 37°C for 1 h (lane 2), 2 h (lane 3), and 3 h (lane 4). As controls, Masp1/3−/− serum (lane 1) or WT serum (lane 5) was incubated for 3 h. Incubated samples corresponding to 0.5 µl of serum were immunoprecipitated with anti–pro-Df, followed by treatment of N-glycosidase-F. Captured pro-Df was detected by immunoblot with anti-Df. (D) Kinetic analysis to cleave a fluorogenic synthetic substrate (H-QPRGR-MCA) by thrombin and rMASP-1K. Each enzyme (1 nM) was used to cleave 20 µM of substrate. (E) After MASP-1 was pulled down from the purified rMASP-1K using anti–MASP-1 antibody (open circles) or the irrelevant antibody as control (filled circles), and the samples were used for cleavage of the synthetic substrate. All data represent one of similar results obtained from at least two experiments.
Kinetic activity of rMASP-1K against H-QPRGR-MCA to compare with that of thrombin
Other serum proteases that are involved in the coagulation system, such as thrombin, kallikrein, and plasmin, were able to cleave pro-Df in vitro (Yamauchi et al., 1994). To assess the specific activity of MASP-1 for cleavage of pro-Df through kinetic analysis, we prepared a fluorogenic synthetic substrate (H-QPRGR-MCA) that is identical to the activation peptide of Df. Cleavage of the synthetic substrate by rMASP-1K was almost twofold faster than cleavage by thrombin (Fig. 5 D). A substrate specificity constant, kcat/Km of rMASP-1K (29.7 ± 5.9 M−1 s−1), against the synthetic substrate was almost twofold higher than that of thrombin (16.7 ± 6.7 M−1 s−1), although Km of MASP-1K (6.2 ± 0.5 mM) was higher than that of thrombin (1.0 ± 0.4 mM). Pro-Df was stably obtained from only MASP-1/3-deficient serum; nevertheless, the clotting of MASP-1/3–deficient blood occurred normally. This observation ruled out the possibility that these serum proteases that contribute to coagulation would be physiologically involved in the cleavage of pro-Df. In addition, this activity of MASP-1K to cleave the synthetic peptide almost vanished by pulling down with anti–MASP-1 antibody (Fig. 5 E), indicating that rMASP-1K did not contain any protease to nonspecifically cleave pro-Df.
Thus, we have found a novel function of MASP-1: it converts pro-Df into its active form in the circulation. We have previously demonstrated that MASP-1 contributes to the activation of the lectin pathway (Takahashi et al., 2008). To date, the three cascades leading to complement activation have been thought to be independently activated by distinct factors and to converge on the cleavage of C3. We report here that MASP-1 is the enzyme for complement activation that not only enhances the activation of MASP-2, but also activates pro-Df. Because MASP-1 and MASP-3 have a common heavy chain, it is possible that MASP-3 is involved in the activation of pro-Df. Although the preliminary experiments show that in addition to MASP-1, MASP-3 has an activity to cleave pro-Df, further investigation will be necessary to determine whether some other factor affects the activation of pro-Df through cooperation with MASP-1 and MASP-3.
Another important finding is that Df is not secreted as the active form, in contrast to previous studies (Barnum and Volanakis, 1985; White et al., 1992). As shown in Fig. 3, we demonstrated that differentiated adipocytes secreted pro-Df with the activation peptides, clearly indicating that pro-Df is cleaved after secretion. In this respect, the results of the present study clarify the role of MASPs in the MBL-dependent C2 bypass mechanism for alternative pathway-mediated C3 activation reported by Selander et al. (Selander et al., 2006). They demonstrated that human C2-deficient serum could activate C3 on solid-phase bacterial polysaccharides, and this activation was dependent on MBL, but not on MASP-1, -2 and -3. They discussed that bound MBL on solid-phase polysaccharides might provide a C3-binding site, resulting in the activation of the alternative pathway. In their study, three MASPs were depleted from serum by specific antibodies using C2-deficient sera. Our findings suggest that the Df in such serum should be already activated by MASP-1, before the activation of C3 by the MBL-dependent C2 bypass on solid-phase polysaccharides.
Interestingly, increasing evidence suggests that the alternative pathway is involved in human disease, such as inflammatory arthritis and ischemia/reperfusion injury (Thurman and Holers, 2006). It was also reported that the alternative pathway is involved in fat metabolism in adipose tissue (Paglialunga et al., 2008). Recent studies have indicated that acylation-stimulating protein (ASP), which is identical to C3adesArg, stimulates fat storage in adipocytes (Yasruel et al., 1991; Maslowska et al., 1997). ASP is a derivative of complement C3; thus, C3−/− mice are lean owing to ASP deficiency (Murray et al., 2000). Furthermore, plasma ASP levels are decreased in Bf-deficient and Df-deficient mice, indicating that the alternative pathway stimulates production of ASP (Paglialunga et al., 2008). We found that Masp1/3−/− mice are also apparently lean (Takahashi et al., 2008), strongly indicating a contribution of MASP-1 to fat metabolism via the alternative pathway activation.
In short, MASP-1, one of the enzymes in the lectin pathway, also activates zymogen of Df in the alternative pathway and plays important roles in activation of both pathways.
MATERIALS AND METHODS
Mice.
Wild-type mice, C57BL/6 (C57BL/6JJcl), were provided by CLEA Japan, Inc. Masp1/3−/− (Masp1tm1Tefu) mice (Takahashi et al., 2008) and Masp2/sMap−/− mice (Iwaki et al., 2006) have been described previously. C4−/− (C4btm1Crr) (Wessels et al., 1995) mice were provided by The Jackson Laboratory. To generate mice with a C4−/− background, Masp1/3−/− mice were backcrossed with C4−/− mice. Mice were used for experiments at 8–12 wk of age. All animal protocols were approved by the Animal Care and Use Committee in accordance with the Guidelines for the Animal Experiments of Fukushima Medical University, Japanese Government Law Concerning the Protection and Control of Animals and Japanese Government Notification on Feeding and Safekeeping of Animals.
Materials.
Purified human Df and C3 were prepared as previously described (Fujita et al., 1981; Matsushita and Okada, 1986). The fluorogenic synthetic substrate H-Gln-Pro-Arg-Gly-Arg-4-methylcoumaryl-7-amide (H-QPRGR-MCA) was synthesized by GL Biochem. 7-Amido-4-methylcoumarin (AMC) and human thrombin were purchased from Sigma-Aldrich. Recombinant mouse MASP-1K was expressed and purified as previously described (Takahashi et al., 2008). Purity of rMASP-1K was estimated as 17% in total protein by Western blotting against MASP-1 to compare the density of sequential dilution of rMASP-1K with that of quantified amount of highly purified rMASP-1i that is an inactive mutant of recombinant MASP-1 (Takahashi et al., 2008).
Assay for activation of the alternative pathway on immobilized zymosan.
Black MaxiSorp microwells for measurement of fluorescence (Nunc) were coated with 0.1 ml of 20 µg/ml zymosan (Sigma-Aldrich) in 0.1 M sodium carbonate buffer, pH 9.5, and incubated at 4°C overnight. Wells were blocked with 1% BSA in Tris-buffered saline (TBS) for 2 h at room temperature. After washing the wells four times with TBS containing 0.05% Tween 20 (TBS-T), serum samples diluted in BBS buffer (0.2 M boric acid, 0.14 M NaCl, pH 8.0) with Mg2+-EGTA were added to each well and incubated for 1 h at 37°C. Wells were washed with TBS-T and 0.1 ml of a 1/500 dilution of fluorescein-conjugated anti–mouse C3 IgG (Cappel) was added to each well and incubated for 30 min at room temperature. After washing four times with TBS-T and once with TBS, each well was filled with 0.1 ml of TBS. Fluorescence was measured using a DTX880 plate reader (Beckman Coulter; excitation at 485 nm and emission at 535 nm).
Hemolytic assay for the alternative complement pathway.
Rabbit erythrocytes (2.5 × 106) were incubated at 39°C for 1 h with mouse serum diluted in gelatin-veronal buffer (GVB) containing 30 mM EGTA and 35 mM MgCl2 (Mg2+-EGTA; 0.1 ml total volume). Heat-inactivated (60°C for 10 min) serum was used as a control (0% lysis). After centrifugation, supernatants (50 µl) were transferred to an ELISA plate. Hemolysis was measured in a DTX880 microplate reader at 405 nm. Percent hemolysis was calculated relative to complete lysis by water.
Expression and purification of mouse complement Df.
Full-length mouse Df cDNA was amplified by RT-PCR using total RNA isolated from C57BL/6 adipose tissue with a high-fidelity polymerase (PrimeSTAR HS; Takara Bio Inc.) and the following primers: 5′ primer (BamHI site in italics), 5′-TGCCGGATCCCAGAATGCACAGCTCCGT-3′; 3′ primer (XbaI site in italics), 5′-GGTGTCTCTAGAGGATGTCATGTTACCA-3′. To add the sequence encoding the hexahistidine tag at 3′-end of the cDNA in place of the native stop codon, a second PCR was performed using the primary amplification product as template, the same 5′ primer, and another 3′ primer (XhoI site in italics), 5′- ACTCGAGTCAATGGTGATGGTGATGATGTCTAGATGTCATGTTACCATTT-3′. The PCR amplification product was cloned into the BamHI and XhoI sites in pBacPAK9 (Takara Bio Inc.). Recombinant baculovirus encoding mouse Df was produced by transfection of Sf21 insect cells with BacPAK6 viral DNA (Bsu36I digest; Takara Bio, Inc.) together with the aforementioned transfer plasmid.
The recombinant protein was isolated from serum-free conditioned supernatant of Sf21 cells infected with the recombinant baculovirus by HisTrap (1 ml) column (GE Healthcare) and by gel filtration. Only single band was observed in rDf by SDS-PAGE and staining with CBB.
Preparation of antibodies against mouse factor Df.
Monospecific antiserum recognizing mouse factor Df was raised by immunizing a rabbit with purified recombinant Df in CFA (Invitrogen), followed by two booster injections of the same antigen in IFA (Invitrogen) at 2-wk intervals. Antiserum was collected two weeks after the last immunization. Immunoglobulin was purified using HiTrap Protein G HP columns (GE Healthcare). Affinity purification of antibodies against mouse Df was achieved using HiTrap NHS-activated HP columns (GE Healthcare) coupled to recombinant Df. Affinity-purified antibody (0.1 mg) was biotinylated using a Biotin Labeling kit-NH2 (Dojindo).
We also obtained a rabbit polyclonal antibody that recognizes the activate peptide, “QPRGR” of mouse pro-Df (anti–pro-Df). In brief, rabbits were immunized with keyhole limpet hemocyanin (KLH)–conjugated 15-mer peptide, “QPRGRILGGQEAAA.” After Igs were purified with this 15-mer peptide, the specific antibody that only recognizes the active peptide was obtained by passing twice through a column that was conjugated with a peptide, “ILGGQEAAA.”
ELISA.
Df or pro-Df concentrations in murine sera were determined with newly established sandwich ELISA using recombinant Df as standard. The affinity-purified antibodies against mouse Df were coated on MaxiSorp ELISA plates (Nunc) at 0.5 µg/well in 0.1 M carbonate buffer pH 9.6 overnight. After the plates were blocked with 1% BSA in PBS for 2h, diluted murine sera were incubated in the wells for 30 min. Captured Df was detected by biotinylated affinity-purified rabbit anti-Df or anti–pro-Df and peroxidase-conjugated Vectastain ABC kit (Vector Laboratories).
Bf activation in mouse serum.
To make C3(H2O), purified human C3 (0.25 mg/ml) was incubated at 37°C for 2 h in PBS containing 2 M potassium bromide. After incubation, it was dialyzed in TBS twice. Mouse serum (10 µl) was incubated at 37°C for 30 min with or without 1.25 µg of C3(H2O) in TBS containing 5 mM MgCl2 and 5 mM CaCl2 (final volume was 50 µl). 5 µl of each sample was separated by SDS-PAGE under reducing conditions and transferred to PVDF membranes. Factor B was detected with a rabbit anti-serum against human factor B and peroxidase-conjugated secondary antibody, and visualized by chemiluminescence (ECL Plus; GE Healthcare) using a LAS3000 imaging system (Fuji Film).
Immunoprecipitation and immunoblotting.
Mouse serum (diluted with TBS) or culture supernatant of differentiated 3T3-L1 adipocytes was incubated for 1 h at 4°C with affinity-purified rabbit anti-Df IgG (2.5 µg) and 10 µl of recombinant protein A-agarose (GE Healthcare). Beads were washed four times with TBS, and then denatured at 100°C for 5 min in 40 µl of TBS containing 0.1% SDS and 50 mM β-mercaptoethanol. Denatured samples were mixed with 2 µl of 10% Triton X-100 and 0.2 µl of N-glycosidase F (EMD) and incubated for 2 h at 37°C or overnight at room temperature. After centrifugation, 1–5 µl of supernatants was subjected to immunoblot analysis using biotinylated affinity-purified rabbit anti-Df and peroxidase-conjugated Vectastain ABC kit (Vector Laboratories). Images were visualized by chemiluminescence.
Cleavage of Bf bound to cobra venom factor in vitro.
Purified cobra venom factor (0.9 µg) was incubated overnight at 37°C with various combinations of purified human factor B (1 µg), pro-Df (0.2 µg), and rMASP-1K (0.2 µg) in 10 µl of TBS containing 10 mM MgCl2 and 10 mM CaCl2. Samples were separated by SDS-PAGE under nonreducing conditions and gels were stained with Coomassie brilliant blue R-250.
Cell culture and differentiation of 3T3-L1 cells.
The mouse 3T3-L1 cell line (JCRB9014) was obtained from the Health Science Research Resources Bank (HSRRB) in Japan. 3T3-L1 cells were cultured and differentiated into adipocytes using commercial media. In brief, at 2 d after confluence, cells were induced to differentiate with 3T3-L1 Differentiation Medium (Zen-Bio, Inc.) according to the manufacturer’s manual. After 3 d, the media were replaced with 3T3-L1 Adipocyte Maintenance Medium (Zen-Bio, Inc.). The cells were subsequently fed every 3 d with Maintenance Medium. Cultured medium from 7–9 d after differentiation was collected to detect Df.
Mass spectrometry.
Partial purification of mouse Df was performed by binding to a HiTrap NHS-activated HP column (GE Healthcare) coupled to anti-Df antibody. Eluted samples were separated by SDS-PAGE and stained. Bands were excised and digested with V8 protease (Sigma-Aldrich). Extracted peptides were analyzed by QSTAR electrospray ionization time-of-flight tandem MS (Applied Biosystems).
Enzyme assays.
Thrombin or rMASP-1K was incubated in phosphate-buffered saline containing 0.05% Tween 20 and 0.1 mM bestatin in a total volume of 100 µl at 25°C, using as a substrate H-QPRGR-MCA (20–2,000 µM). Fluorescence was monitored with time at excitation and emission wavelengths of 360 and 465 nm using a DTX880 (Beckman Coulter). To obtain a standard curve, dilutions of AMC were prepared in the same assay buffer and corresponding fluorescence was measured. All enzyme kinetic parameters were computed by fitting data to the Michaelis-Menten equation and determined from Lineweaver-Burk blot.
Statistical analysis.
The statistical significance of differences was assessed by the Student’s t test. P values <0.05 were considered significant.
Online supplemental material.
Fig. S1 shows immunoprecipitation and immunoblot of mouse Df from mouse sera. Fig. S2 shows tandem mass profiles from molecular ion peaks from V8-protease digested Df fragments of WT and Masp1/3−/− mice. Fig. S3 shows expression of Df and Masp1 in mouse adipose and liver tissue by RT-PCR. Table S1 shows summary of tandem mass profiles presented in Fig. S2. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090633/DC1.
Acknowledgments
We thank K. Takahashi of Massachusetts General Hospital, Boston for kindly providing Mbl null sera.
This work was supported by parts of Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by the Core Research for Evolutional, Science, and Technology, Japan Science and Technology Agency.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ASP
- acylation-stimulating protein
- Bf
- complement factor B
- CVF
- cobra-venom factor
- Df
- complement factor D
- GVB
- gelatin-veronal buffer
- MASP
- MBL-associated serine protease
- MBL
- mannose-binding lectin
- sMAP
- small MBL-associated protein
- TBS
- Tris-buffered saline
References
- Ambrus G., Gál P., Kojima M., Szilágyi K., Balczer J., Antal J., Gráf L., Laich A., Moffatt B.E., Schwaeble W., et al. 2003. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: a study on recombinant catalytic fragments. J. Immunol. 170:1374–1382 [DOI] [PubMed] [Google Scholar]
- Barnum S.R., Volanakis J.E. 1985. In vitro biosynthesis of complement protein D by U937 cells. J. Immunol. 134:1799–1803 [PubMed] [Google Scholar]
- Brown E.J. 1991. Complement receptors and phagocytosis. Curr. Opin. Immunol. 3:76–82 10.1016/0952-7915(91)90081-B [DOI] [PubMed] [Google Scholar]
- Chen C.B., Wallis R. 2004. Two mechanisms for mannose-binding protein modulation of the activity of its associated serine proteases. J. Biol. Chem. 279:26058–26065 10.1074/jbc.M401318200 [DOI] [PubMed] [Google Scholar]
- Cook K.S., Groves D.L., Min H.Y., Spiegelman B.M. 1985. A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc. Natl. Acad. Sci. USA. 82:6480–6484 10.1073/pnas.82.19.6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K.S., Min H.Y., Johnson D., Chaplinsky R.J., Flier J.S., Hunt C.R., Spiegelman B.M. 1987. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 237:402–405 10.1126/science.3299705 [DOI] [PubMed] [Google Scholar]
- Dahl M.R., Thiel S., Matsushita M., Fujita T., Willis A.C., Christensen T., Vorup-Jensen T., Jensenius J.C. 2001. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 15:127–135 10.1016/S1074-7613(01)00161-3 [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Austen K.F., Ruddy S. 1974. Properdin factor D: characterization of its active site and isolation of the precursor form. J. Exp. Med. 139:355–366 10.1084/jem.139.2.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. 2002. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2:346–353 10.1038/nri800 [DOI] [PubMed] [Google Scholar]
- Fujita T., Takata Y., Tamura N. 1981. Solubilization of immune precipitates by six isolated alternative pathway proteins. J. Exp. Med. 154:1743–1751 10.1084/jem.154.6.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajela K., Kojima M., Ambrus G., Wong K.H., Moffatt B.E., Ferluga J., Hajela S., Gál P., Sim R.B. 2002. The biological functions of MBL-associated serine proteases (MASPs). Immunobiology. 205:467–475 10.1078/0171-2985-00147 [DOI] [PubMed] [Google Scholar]
- Iwaki D., Kanno K., Takahashi M., Endo Y., Lynch N.J., Schwaeble W.J., Matsushita M., Okabe M., Fujita T. 2006. Small mannose-binding lectin-associated protein plays a regulatory role in the lectin complement pathway. J. Immunol. 177:8626–8632 [DOI] [PubMed] [Google Scholar]
- Krarup A., Gulla K.C., Gál P., Hajela K., Sim R.B. 2008. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim. Biophys. Acta. 1784:1294–1300 [DOI] [PubMed] [Google Scholar]
- Lu J.H., Teh B.K., Wang L., Wang Y.N., Tan Y.S., Lai M.C., Reid K.B. 2008. The classical and regulatory functions of C1q in immunity and autoimmunity. Cell. Mol. Immunol. 5:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowska M., Sniderman A.D., Germinario R., Cianflone K. 1997. ASP stimulates glucose transport in cultured human adipocytes. Int. J. Obes. Relat. Metab. Disord. 21:261–266 10.1038/sj.ijo.0800396 [DOI] [PubMed] [Google Scholar]
- Matsushita M., Fujita T. 1992. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176:1497–1502 10.1084/jem.176.6.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Fujita T. 1995. Cleavage of the third component of complement (C3) by mannose-binding protein-associated serine protease (MASP) with subsequent complement activation. Immunobiology. 194:443–448 [DOI] [PubMed] [Google Scholar]
- Matsushita M., Okada H. 1986. Alternative complement pathway activation by C4b deposited during classical pathway activation. J. Immunol. 136:2994–2998 [PubMed] [Google Scholar]
- Matsushita M., Thiel S., Jensenius J.C., Terai I., Fujita T. 2000. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J. Immunol. 165:2637–2642 [DOI] [PubMed] [Google Scholar]
- Murray I., Havel P.J., Sniderman A.D., Cianflone K. 2000. Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology. 141:1041–1049 10.1210/en.141.3.1041 [DOI] [PubMed] [Google Scholar]
- Paglialunga S., Fisette A., Yan Y., Deshaies Y., Brouillette J.F., Pekna M., Cianflone K. 2008. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am. J. Physiol. Endocrinol. Metab. 294:E521–E529 10.1152/ajpendo.00590.2007 [DOI] [PubMed] [Google Scholar]
- Pangburn M.K., Müller-Eberhard H.J. 1983. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann. N. Y. Acad. Sci. 421:291–298 10.1111/j.1749-6632.1983.tb18116.x [DOI] [PubMed] [Google Scholar]
- Petersen S.V., Thiel S., Jensen L., Vorup-Jensen T., Koch C., Jensenius J.C. 2000. Control of the classical and the MBL pathway of complement activation. Mol. Immunol. 37:803–811 10.1016/S0161-5890(01)00004-9 [DOI] [PubMed] [Google Scholar]
- Rosen B.S., Cook K.S., Yaglom J., Groves D.L., Volanakis J.E., Damm D., White T., Spiegelman B.M. 1989. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 244:1483–1487 10.1126/science.2734615 [DOI] [PubMed] [Google Scholar]
- Rossi V., Cseh S., Bally I., Thielens N.M., Jensenius J.C., Arlaud G.J. 2001. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J. Biol. Chem. 276:40880–40887 10.1074/jbc.M105934200 [DOI] [PubMed] [Google Scholar]
- Selander B., Mårtensson U., Weintraub A., Holmström E., Matsushita M., Thiel S., Jensenius J.C., Truedsson L., Sjöholm A.G. 2006. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J. Clin. Invest. 116:1425–1434 10.1172/JCI25982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Takahashi K., Dundee J., Shahroor-Karni S., Thiel S., Jensenius J.C., Gad F., Hamblin M.R., Sastry K.N., Ezekowitz R.A. 2004. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199:1379–1390 10.1084/jem.20032207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Endo Y., Fujita T., Matsushita M. 1999. A truncated form of mannose-binding lectin-associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Int. Immunol. 11:859–863 10.1093/intimm/11.5.859 [DOI] [PubMed] [Google Scholar]
- Takahashi M., Iwaki D., Kanno K., Ishida Y., Xiong J., Matsushita M., Endo Y., Miura S., Ishii N., Sugamura K., Fujita T. 2008. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J. Immunol. 180:6132–6138 [DOI] [PubMed] [Google Scholar]
- Terai I., Kobayashi K., Matsushita M., Fujita T. 1997. Human serum mannose-binding lectin (MBL)-associated serine protease-1 (MASP-1): determination of levels in body fluids and identification of two forms in serum. Clin. Exp. Immunol. 110:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel S., Vorup-Jensen T., Stover C.M., Schwaeble W., Laursen S.B., Poulsen K., Willis A.C., Eggleton P., Hansen S., Holmskov U., et al. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 386:506–510 10.1038/386506a0 [DOI] [PubMed] [Google Scholar]
- Thiel S., Petersen S.V., Vorup-Jensen T., Matsushita M., Fujita T., Stover C.M., Schwaeble W.J., Jensenius J.C. 2000. Interaction of C1q and mannan-binding lectin (MBL) with C1r, C1s, MBL-associated serine proteases 1 and 2, and the MBL-associated protein MAp19. J. Immunol. 165:878–887 [DOI] [PubMed] [Google Scholar]
- Thurman J.M., Holers V.M. 2006. The central role of the alternative complement pathway in human disease. J. Immunol. 176:1305–1310 [DOI] [PubMed] [Google Scholar]
- Volanakis J.E., Narayana S.V. 1996. Complement factor D, a novel serine protease. Protein Sci. 5:553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walport M.J. 2001a. Complement. First of two parts. N. Engl. J. Med. 344:1058–1066 10.1056/NEJM200104053441406 [DOI] [PubMed] [Google Scholar]
- Walport M.J. 2001b. Complement. Second of two parts. N. Engl. J. Med. 344:1140–1144 10.1056/NEJM200104123441506 [DOI] [PubMed] [Google Scholar]
- Wessels M.R., Butko P., Ma M., Warren H.B., Lage A.L., Carroll M.C. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA. 92:11490–11494 10.1073/pnas.92.25.11490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.T., Damm D., Hancock N., Rosen B.S., Lowell B.B., Usher P., Flier J.S., Spiegelman B.M. 1992. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J. Biol. Chem. 267:9210–9213 [PubMed] [Google Scholar]
- Yamauchi Y., Stevens J.W., Macon K.J., Volanakis J.E. 1994. Recombinant and native zymogen forms of human complement factor D. J. Immunol. 152:3645–3653 [PubMed] [Google Scholar]
- Yasruel Z., Cianflone K., Sniderman A.D., Rosenbloom M., Walsh M., Rodriguez M.A. 1991. Effect of acylation stimulating protein on the triacylglycerol synthetic pathway of human adipose tissue. Lipids. 26:495–499 10.1007/BF02536592 [DOI] [PubMed] [Google Scholar]
- Zundel S., Cseh S., Lacroix M., Dahl M.R., Matsushita M., Andrieu J.P., Schwaeble W.J., Jensenius J.C., Fujita T., Arlaud G.J., Thielens N.M. 2004. Characterization of recombinant mannan-binding lectin-associated serine protease (MASP)-3 suggests an activation mechanism different from that of MASP-1 and MASP-2. J. Immunol. 172:4342–4350 [DOI] [PubMed] [Google Scholar]