Abstract
We describe a quantitative model for assessing the cytolytic activity of antigen-specific CD8+ T cells in vitro and in vivo in which the concentration of antigen-specific CD8+ T cells determines the efficiency with which these cells kill cognate antigen–expressing melanoma cells in packed cell pellets, in three-dimensional collagen-fibrin gels in vitro, and in established melanomas in vivo. In combination with a clonogenic assay for melanoma cells, collagen-fibrin gels are 4,500–5,500-fold more sensitive than the packed cell pellet–type assays generally used to measure CD8+ T cell cytolytic activity. An equation previously used to describe neutrophil bactericidal activity in vitro and in vivo also describes antigen-specific CD8+ T cell–mediated cytolysis of cognate antigen-expressing melanoma cells in collagen-fibrin gels in vitro and in transplanted tumors in vivo. We have used this equation to calculate the critical concentration of antigen-specific CD8+ T cells, which is the concentration of these cells required to hold constant the concentration of a growing population of cognate antigen-expressing melanoma cells. It is ∼3.5 × 105/ml collagen-fibrin gel in vitro and ∼3 × 106/ml or /g melanoma for previously published studies of ex vivo–activated adoptively transferred tumor antigen–specific CD8+ T cell killing of cognate antigen–expressing melanoma cells in established tumors in vivo. The antigen-specific CD8+ T cell concentration required to kill 100% of 2 × 107/ml cognate antigen-expressing melanoma cells in collagen fibrin gels is ≥107/ml of gel.
Li et al. (2002, 2004) reported that the bactericidal activity of neutrophils depends on the absolute neutrophil concentration in fibrin gels, a condition which mimics tissue environments, and in rabbit dermis in vivo. The findings that the critical neutrophil concentration (CNC) for controlling bacterial growth in fibrin gels (1–4 × 106 neutrophils/ml of gel) is similar to the CNC in rabbit dermis in vivo (4–8 × 106 neutrophils/ml or /g dermis) showed that such gels are useful for studying neutrophil effector functions in tissue-like environments in vitro. We hypothesized that the critical concentration concept might be applicable to describing effector functions of other leukocytes, for example, the cytolytic activity of CD8+ T cells. Therefore, we examined CD8+ T cell–mediated killing of target cells expressing a cognate antigen.
We selected activated CD8+ OT-1 cells as effector cells and SIINFEKL peptide–pulsed B16 melanoma cells as target cells for these studies because both cell types and their interactions had been well characterized in vitro (Moore et al., 1988; Ochalek et al., 1988; Snyder et al., 2003; Regoes et al., 2007) and in vivo (Dobrzanski et al., 2001; Regoes et al., 2007). To quantitatively assess OT-1 cell–mediated cytolysis of SIINFEKL peptide–pulsed B16 cells, we used a newly designed three-dimensional collagen-I–fibrin gel system and a previously described clonogenic assay for B16 mouse melanoma cells (Freedman et al., 1984). We report in this paper that in every situation examined (i.e., individual SIINFEKL-B16 cells and SIINFEKL-B16 cells in spheroids [Sutherland, 1988] in collagen-fibrin gels and SIINFEKL-B16 cells in centrifugally packed cell pellets), OT-1 T cell–mediated killing of SIINFEKL-B16 cells was strictly dependent on OT-1 cell concentration. Moreover, we determined that a concentration of ≥107 OT-1 cells/ml of gel is required for them to produce sterilizing immunity versus SIINFEKL-B16 cells in vitro and that activated OT-1 cells kill SIINFEKL-B16 cells ∼10-fold more efficiently in collagen-fibrin gels in vitro than ovalbumin peptide–expressing B16 cells in tumors in vivo (Petersen et al., 2006).
RESULTS
Growth of B16 melanoma cells in collagen-fibrin gels
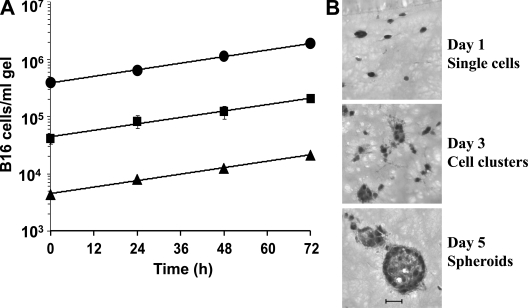
B16 melanoma cells embedded in collagen-I–fibrin gels at concentrations of 104–106/ml grow at an exponential rate and have a doubling time of ∼58 h (Fig. 1 A), which is ∼66–83% of their doubling time in vivo (Li et al., 1984). Microscopic observations of B16 cells maintained in these gels for 24 h showed mostly single B16 cells with lamellipods protruding in all directions (Fig. 1 B). By 72 h, many B16 cells aggregated into small clusters. By 96–120 h, these clusters developed into spheroids (Sutherland, 1988), varying from 50 to 100 µm in diameter. Each spheroid contained ∼100 B16 cells. Hematoxylin/eosin-stained frozen sections of gels containing single B16 cells and B16 cell spheroids revealed considerable matrix remodeling by these cells during culture (Fig. 1 B). Gels containing B16 cells at an initial concentration of >106 B16 cells/ml in the absence of OT-1 cells remained intact for slightly >120 h. After this time, the gels began to dissolve and the growth rate of B16 cells slowed. B16 cells, like many other mouse and human tumor cells, secrete proteases which are likely responsible for gel dissolution (Baramova et al., 1994; Hofmann et al., 2005; Huang et al., 2005).
Figure 1.
Growth of B16 mouse melanoma cells in collagen-fibrin gels. (A) Collagen-fibrin gels containing 5 × 104 (▴), 5 × 105 (■), or 5 × 106 (•) B16 cells/ml and RPMI 1640 with 10% FBS and 5 × 10−5 M β-ME were incubated at 37°C for 3 d. Gels were harvested daily and their content of clonogenic B16 cells was assessed as described in Materials and methods. Data shown represent mean ± SEM of n = 3 experiments performed in duplicate. (B) Hematoxylin/eosin-stained frozen sections of B16 cells grown at 37°C in collagen-fibrin gels for 1, 3, and 5 d. Bar, 50 µm.
The concentration of SIINFEKL peptide required for optimal killing of B16 cells by OT-1 cells
Display of the ovalbumin peptide SIINFEKL (ova residues 257–264) in the context of B16 MHC I (H-2Kb) targets B16 cells for killing by activated OT-1 cells (Curtsinger et al., 1998; Moore et al., 1988). 96% of B16 cells incubated with 10−6 M SIINFEKL peptide for 2 h at 37°C were killed by 107 OT-1 cells/ml of gel in 24 h, whereas 85, 40, and 32% of B16 cells treated with SIINFEKL at 10−9, 10−10, and 10−12 M, respectively, were killed by these cells in the same time period (Fig. S1). In contrast, coincubation of 107 OT-1 cells/ml of gel with unpulsed B16 cells at 105/ml of gel reduced recovery of clonogenic B16 cells by ≤10%, indicating a low level of nonspecific killing of unpulsed B16 cells by activated OT-1 cells. We used B16 cells pulsed with 10−6 M SIINFEKL peptide in all subsequent experiments.
OT-1 cell concentration determines their efficiency in killing growing and nongrowing SIINFEKL-B16 cells in collagen-fibrin gels
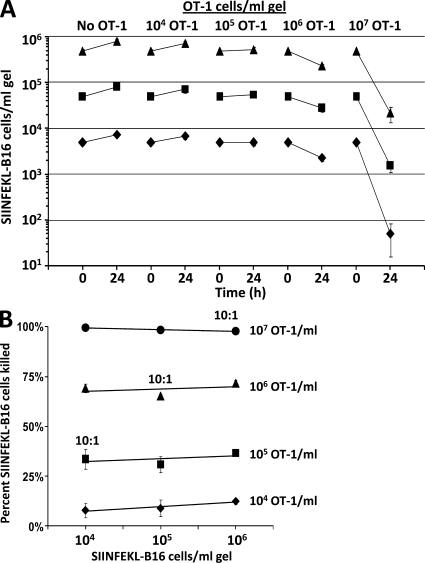
To test whether the efficiency of OT-1 cell killing of SIINFEKL-B16 cells depends on OT-1 cell concentration, we examined the killing of 5 × 103–105/ml SIINFEKL-B16 cells by 104–107/ml of activated OT-1 cells in collagen-fibrin gels. Killing of SIINFEKL-B16 cells depended on OT-1 cell concentration and was unrelated to the effector/target cell ratio (Fig. 2, A and B). For example, 107 OT-1 cells/ml of gel killed ∼98% of SIINFEKL-B16 cells in 24 h, regardless of whether the initial B16 cell concentration was 106/ml of gel, which is a 10:1 ratio of OT-1/B16 cells, or 104 B16 cells/ml of gel, which is a 1,000:1 ratio of OT-1/B16 cells (Fig. 2 B). At OT-1 cell concentrations of 105/ml or less, more clonogenic B16 cells were recovered after 24 h than were present in the inoculum (at time 0), even when the inoculum contained only 104 B16 cells/ml of gel (Fig. 2 A), indicating that at OT-1 cell concentrations ≤105/ml, the rate of B16 growth exceeded the rate of OT-1 cell killing.
Figure 2.
OT-1 cell concentration determines the efficiency of killing of SIINFEKL-B16 cells growing in collagen-fibrin gels. (A) Collagen-fibrin gels containing 5 × 104 (♦), 5 × 105 (■), or 5 × 106 (▴) SIINFEKL-B16 cells/ml of gel, with or without 104, 105, 106, or 107 OT-1 cells/ml of gel, were overlaid with 0.5 ml RPMI 1640 with 10% FBS and 5 × 10−5 M β-ME and incubated at 37°C. Shown is the number of clonogenic B16 cells recovered from gels at time 0 and after a 24-h incubation at 37°C with the indicated concentration of OT-1 cells. Data shown represent mean ± SEM of n = 3 experiments performed in duplicate. (B) Relationship between OT-1 cell concentration, initial SIINFEKL-B16 cell concentration, and percentage of B16 cells killed.
Addition of β-ME to the culture medium improved survival and effector activity of OT-1 cells (Cerottini et al., 1974). It also improved B16 cell growth in the first 24 h after their placement into collagen-fibrin gels. In the absence of β-ME, B16 cells showed a small (∼20%) decline in number during their first 24 h in these gels (not depicted and Table S1). We used this observation to compare the efficiency of OT-1 cell killing of nongrowing (without β-ME) versus growing (with β-ME) B16 cells in collagen-fibrin gels. OT-1 cells killed approximately the same percentage of nongrowing SIINFEKL-B16 cells as growing SIINFEKL-B16 cells (Fig. 2 B and Table S1). However, with nonproliferating B16 cells (without β-ME in the medium), we observed a net reduction in B16 cells at every OT-1 cell concentration. OT-1 cell concentration also determined the efficiency with which these cells killed SIINFEKL-B16 cells in two-dimensional cultures (unpublished data). Thus, in both two- and three-dimensional cultures, OT-1 cell concentration was the critical determinant of killing of SIINFEKL-B16 cells.
The OT-1 critical T-cell concentration (CTC)
As noted in the Introduction, the CNC is the concentration of neutrophils required to hold constant the concentration of growing bacteria. We hypothesized that the critical concentration concept also applies to OT-1 cell killing of SIINFEKL-B16 cells. Indeed, inspection of Fig. 2 A shows that the CTC for killing SIINFEKL-B16 cells lies between 105 and 106 OT-1 cells/ml collagen-fibrin gel. 105 OT-1 cells/ml of gel killed SIINFEKL-B16 cells at a slightly slower rate than the rate of B16 cell growth, whereas 106 OT-1 cells/ml killed SIINFEKL-B16 cells at a substantially higher rate than B16 cell growth (Fig. 2 A). Additional experiments (unpublished data) showed that 3–4 × 105 OT-1 cells/ml of gel were required to hold SIINFEKL-B16 melanoma cell concentration constant. Therefore, the experimentally determined CTC for OT-1 cell killing of SIINFEKL-B16 cells is ∼3.5 × 105 OT-1 cells/ml of gel.
Naive T cells from wild-type C57BL/6 mouse spleen had no effect on the efficiency with which OT-1 cells killed SIINFEKL peptide-pulsed B16 cells
It was possible that nutrient deprivation by high concentrations (i.e., ≥107 cells/ml) of OT-1 cells was responsible for B16 cell killing. To test this possibility, we incubated 106 SIINFEKL-B16 cells/ml of gel with 104–106 OT-1 cells/ml of gel without and with sufficient naive or mitogen-activated C57BL/6 lymphocytes to bring the total number of lymphocytes to 107/ml of gel. The presence of 107 naive or mitogen-activated lymphocytes had no effect on viability or growth of 106 SIINFEKL-B16 cells/ml of gel, and a 9–900-fold excess of naive or mitogen-activated lymphocytes in combination with activated OT-1 cells had no effect on the efficiency of OT-1 cell killing of 106 SIINFEKL-pulsed B16 cells/ml of gel (Table S2). Thus, killing of SIINFEKL-B16 cells cocultivated with high concentrations of OT-1 cells was not the result of nutrient depletion by the OT-1 cells.
The concentration of activated OT-1 cells remains constant for at least 72 h of co-culture with SIINFEKL peptide–pulsed B16 cells in collagen-fibrin gels
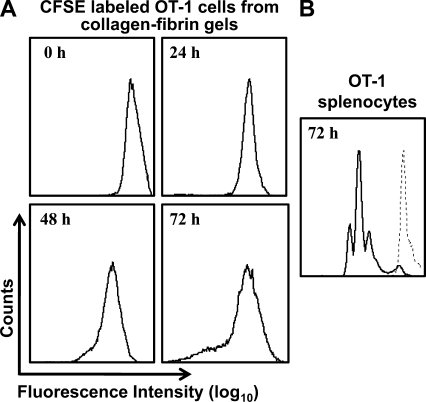
To determine whether the concentration of activated OT-1 cells changed during their incubation with SIINFEKL-B16 cells, we coincubated CFSE-labeled or unlabeled OT-1 cells with SIINFEKL-B16 cells in collagen-fibrin gels for 24–72 h, lysed the gels, and assayed the number of B16 cells and CFSE-labeled OT-1 cells by clonogenic assay and FACS, respectively. CFSE-labeled OT-1 cells killed SIINFEKL-B16 cells with the same efficiency as unlabeled OT-1 cells (unpublished data). FACS analysis of the CFSE-labeled cells showed that >90% of the OT-1 cells remained viable at 24–72 h and that their exposure to SIINFEKL-B16 cells in the presence or absence of IL-2 did not stimulate a significant number of them to divide (Fig. 3 A and not depicted). Control experiments in which CFSE-labeled naive OT-1 splenocytes were incubated with SIINFEKL peptide showed dilution of the CFSE label (Fig. 3 B), confirming that proliferation of the CFSE-labeled cells could be detected by this assay, had it occurred.
Figure 3.
CFSE-labeled OT-1 cells remain viable but do not divide when coincubated with SIINFEKL-B16 cells in collagen-fibrin gels. (A) 106 SIINFEKL-B16 cells/ml were coincubated with 107/ml CFSE-labeled OT-1 cells in collagen-fibrin gels in the presence or absence of 100 U/ml IL-2. Gels were lysed as described at 24, 48, and 72 h. The released cells were incubated in propidium iodide and analyzed by FACS. (B) CFSE-labeled naive OT-1 splenocytes were incubated with 0.75 µg/ml SIINFEKL peptide at 37°C for 72 h, after which the cells were isolated and analyzed by FACS. Shown is a representative experiment of n = 3 experiments performed in duplicate.
Mechanism of OT-1 cell killing of peptide-pulsed B16 cells
Snyder et al. (2003) reported that activated OT-1 cell killing of SIINFEKL-sensitized targets is perforin dependent. Indeed, coincubation of 106 OT-1 cells and 105 SIINFEKL-B16 cells/ml collagen-fibrin gel in medium containing 10 or 100 nM concanamycin A (CMA), an inhibitor of vacuolar-type H+-ATPases (Kataoka et al., 1996) and of perforin-mediated killing, inhibited killing of the B16 cells by 19 and 87%, respectively (Fig. S2). CMA at >100 nM was toxic to B16 cells after 24 h (as measured by the clonogenic assay; unpublished data).
OT-1 cell concentration determines the efficiency of killing of SIINFEKL peptide–pulsed B16 cells in packed cell pellet–type assays
Release of 51Cr from 51Cr-labeled target cells coincubated with activated CD8+ T lymphocytes in packed pellets is a standard technique for assessing CD8+ T lymphocyte cytolytic activity (Brunner et al., 1968; Martz, 1975). Target cell killing in these packed cell pellet–type assays is generally reported as a function of effector/target cell ratio. Maximal target cell killing often plateaus at <100% in these assays, even when the effector/target cell ratio is very high (e.g., 100:1). This plateau effect is inconsistent with the concept that the extent of target cell killing is dependent on effector/target cell ratio. It is consistent, however, with a determinative role for effector cell concentration in target cell killing. This occurs because as effector cell concentration rises, it reaches close to the maximum effector cell concentration that can be attained in a packed pellet of effector cells alone. For example, the maximum concentration of activated OT-1 cells in a centrifugally packed pellet is 5.9 × 109 OT-1 cells/ml, whereas the maximum concentration of OT-1 cells in a packed pellet containing OT-1 cells and 104 B16 cells is ∼5 × 109 OT-1 cells/ml.
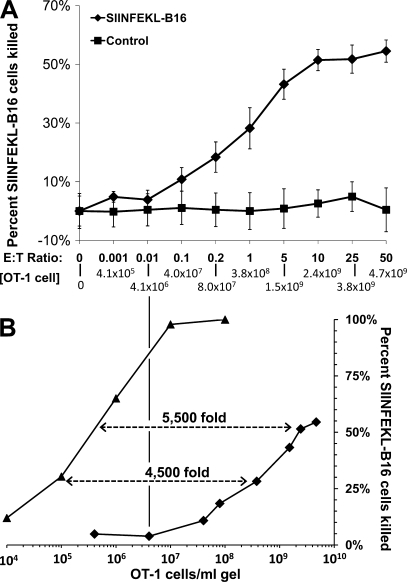
To test whether activated OT-1 cell–mediated killing of SIINFEKL-B16 cells in a packed cell pellet–type assay depends on OT-1 cell concentration, we cosedimented varying numbers of activated OT-1 cells with 104 SIINFEKL-pulsed or nonpulsed B16 cells in wells of a 96-well plate, incubated them for 4 h at 37°C, and assayed the number of clonogenic B16 cells remaining. Virtually no unpulsed B16 cells were killed (Fig. 4 A). In contrast, at OT-1/B16 cell ratios between 0.1:1 and 5:1, corresponding to OT-1 cell concentrations of 4 × 107 and 1.5 × 109/ml, SIINFEKL-B16 cell killing increased in proportion to both OT-1 cells/SIINFEKL-B16 ratio and OT-1 cell concentration. However, at OT-1/B16 cell ratios of ≥5:1 there was no proportionality between the OT-1/B16 ratio and OT-1 cell–mediated killing of B16 cells. At these high effector/target cell ratios, a 10-fold increase in the OT-1/B16 cell ratio (Fig. 4 A) produced an insignificant increase in B16 cytolysis (i.e., 58% killing at 5:1 and 62% killing at 50:1 [Fig. 4 A]) and only a 3.1-fold increase in OT-1 cell concentration.
Figure 4.
OT-1 cell concentration determines the efficiency of killing of SIINFEKL peptide–pulsed B16 cells in packed cell pellet–type assays. (A) 104 SIINFEKL-pulsed (♦) or nonpulsed (■) B16 cells were mixed with 10–5 × 105 activated OT-1 cells in 200 µl OT-1 medium in round-bottom wells of a 96-well plate. Plates were centrifuged at 50 g for 5 min to pellet the cells and incubated at 37°C for 4 h. Cells in each well were dissociated with trypsin-EDTA, and their viability was measured by clonogenic assay. Data shown represent mean ± SEM of n = 3 experiments performed in duplicate. (B) Efficiency of OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels (▴) versus packed pellet–type assays (♦). Data for collagen-fibrin gels were obtained from Table S1. Data for packed pellet–type assays were obtained from A.
It was possible that differences in density and deformability of B16 cells versus OT-1 cells could result in an inhomogeneous distribution of these cells in a cell pellet and contribute to the plateau effect observed in Fig. 4 A. To test this possibility, we cosedimented activated OT-1 and SIINFEKL-B16 cells at 10:1, 50:1, and 100:1 ratios of OT-1/B16 cells, fixed the pellets with formalin, bisected them parallel to the axis of sedimentation, sectioned them, stained the sections with hematoxylin/eosin, and observed the distribution of OT-1 and B16 cells in the pellet by light microscopy. Under all conditions tested, the OT-1 and B16 cells were randomly and uniformly distributed throughout the pellet (unpublished data). Thus, the observed plateau in killing is not a result of inhomogeneous distribution of the two cell types.
It was also possible that at very high OT-1 cell concentrations these cells saturate the B16 cells’ MHC-I–SIINFEKL binding sites for OT-1 T cell receptors or cluster around the B16 cells and sterically hinder contact with them. Although we cannot formally exclude the former explanation, we examined the latter. Given diameters of 7 and 17.4 µm for OT-1 cells and B16 cells (Ochalek et al., 1988), respectively, and hexagonal packing of the OT-1 cells on the surfaces of B16 cells, we calculated that ∼48 OT-1 cells could bind simultaneously to each SIINFEKL-B16 cell. This is approximately ninefold in excess of the 5:1 ratio of OT-1/B16 cells that produces near maximal killing of SIINFEKL-B16 cells in packed cell pellets (Fig. 4 A). Therefore, it is unlikely that at very high OT-1 cell concentrations, such as those which occur in packed cell pellet–type assays, access to each target cell’s surface limits effector cell cytolytic activity.
These experiments reveal two major differences between OT-1 cell killing of target cells in packed cell pellet–type assays (Fig. 4) and in collagen-fibrin gels (Fig. 2 and Table S1). First, in packed cell pellet–type assays (Fig. 4) there was no detectable killing of SIINFEKL-B16 cells at OT-1 cell concentrations ≤4 × 106/ml. In contrast, OT-1 cells at 106 and 107/ml killed ∼62 and 98%, respectively, of nongrowing (Table S1) or growing (Fig. 2) SIINFEKL-B16 cells in 24 h in collagen-fibrin gels. Thus, packed cell pellet–type assays may underestimate the cytocidal activity of CD8+ T cell populations by 70–90%. Second, packed cell pellet–type assays are 4,500–5,500-fold less sensitive than collagen-fibrin gel assays (Fig. 4 B).
To further examine the relationship between OT-1 cell concentration and B16 cell killing, we cosedimented a mixture containing 2 × 104, 4 × 104, or 105 SIINFEKL-B16 cells, 105 activated OT-1 cells, and sufficient naive wild-type C57BL/6 splenocytes to bring the total volume of cell pellets to ∼263 nl (Table S3). Under these conditions, the ratio of OT-1/SIINFEKL-B16 cells rose from 1:1 to 5:1 but the OT-1 cell concentration remained constant at ∼3.8 × 108 OT-1 cells/ml. At all three ratios, the percentage of SIINFEKL-B16 cells killed was 18%. This result is consistent with those reported in Fig. 2 and Table S1 and confirms that OT-1 cell concentration determines the efficiency of OT-1 cell killing of SIINFEKL-B16 cells in packed cell pellet–type assays.
OT-1 cells kill SIINFEKL-B16 cells in spheroids as efficiently as single SIINFEKL-B16 cells
Melanoma cells, like many other tumor cells, grow in nests or clusters in vivo. B16 cells spontaneously form spheroids when cultured in suspension (Sutherland, 1988; Kuwashima et al., 1993) or in collagen-fibrin gels for more than 3 d (Fig. 1 B). To assess whether the multilayering of B16 cells that occurs in spheroids, or in the matrix proteins which the B16 cells produce (Dewever et al., 2007), affects the sensitivity of these cells to OT-1 cell–mediated killing, we pulsed intact spheroids (∼100 B16 cells/spheroid) with SIINFEKL peptide, coincubated ∼103 SIINFEKL-spheroids or ∼105 SIINFEKL-B16 cells dissociated from these spheroids with 106 activated OT-1 cells for 24 and 48 h in collagen-fibrin gels, and measured the number of clonogenic B16 cells remaining. OT-1 cells killed ∼24% more (not significant) spheroid-derived single SIINFEKL-B16 cells than SIINFEKL-B16 cells in spheroids at 24 h (Fig. S3). At 48 h, OT-1 cells killed roughly equal percentages of B16 cells dissociated from spheroids (85%) and B16 cells in spheroids (80%). Light microscopy of sections of gels containing similar concentrations of OT-1 cells and SIINFEKL-B16 spheroids showed OT-1 cells clustered around and invading spheroids. Similarly, Joseph-Pietras et al. (2006) noted human monocyte invasion of B16 cell spheroids when the two cell types were co-cultured for 38–62 h. Thus, there was no evidence that multilayering of B16 cells in spheroids, or the matrix proteins associated with them (Dewever et al., 2007), affected OT-1 cell penetration of spheroids or their capacity to kill SIINFEKL-B16 cells in spheroids.
OT-1 cells kill SIINFEKL-B16 cells at a constant exponential rate and produce sterilizing immunity in collagen-fibrin gels
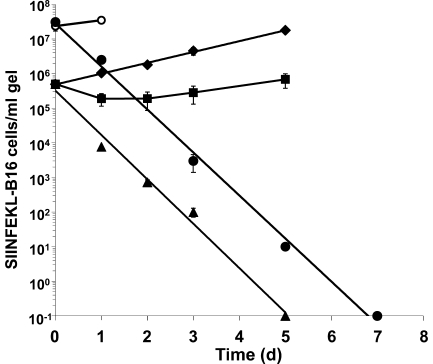
The most stringent test of the efficacy of cellular immunotherapy of tumors is eradication of all tumor cells. Therefore, we examined whether OT-1 cells generate sterilizing immunity in vitro. Co-incubation of 106/ml activated OT-1 cells with 5 × 105/ml SIINFEKL-B16 cells in collagen-fibrin gels for 3 d resulted in recovery of 92% fewer B16 cells than gels containing B16 cells alone (Fig. 5). The B16 cells resumed growth thereafter, surpassing the initial inoculum of 5 × 105 B16 cells/ml of gel by day 5. In contrast, 107 activated OT-1 cells/ml coincubated with 5 × 105 SIINFEKL-B16 cells/ml in collagen-fibrin gels killed the B16 cells at a constant exponential rate (∼99.9%/d) for 5 d, killing 100% of the B16 inoculum over this period (Fig. 5). Under these conditions, 100% of B16 cells were killed. Gels harvested on days 6 and 7 also yielded no viable B16 cells (unpublished data).
Figure 5.
OT-1 cells affect sterilizing immunity versus SIINFEKL-B16 cells in collagen-fibrin gels. Collagen-fibrin gels containing 5 × 105 SIINFEKL-B16 cells/ml of gel alone (♦) or with 106 (■) or 107 (▴)/ml OT-1 cells were incubated at 37°C for up to 5 d. Gels were lysed on the day indicated and assayed for viable B16 cells as described in Materials and methods. Data shown represent mean ± SEM of n = 3 experiments performed in duplicate. Collagen-fibrin gels containing 2 × 107 SIINFEKL-B16 cells/ml of gel in medium containing 20 U IL-2/ml of gel alone (○) or with 107 (•)/ml OT-1cells were incubated at 37°C for up to 7 d, lysed, and assayed for viable B16 cells as described in Materials and methods. Shown are the results of a representative experiment performed in duplicate.
Human and mouse melanomas are reported to contain 107–108 tumor cells/ml or /g of tumor (Stephens and Peacock, 1978; Hemstreet et al., 1980; Daugherty et al., 1981; Whiteside et al., 1986). To determine whether activated OT-1 cells are capable of eradicating SIINFEKL-B16 cells growing in collagen-fibrin gels at concentrations in this range, we coincubated 2 × 107 SIINFEKL-B16 cells/ml and 107 OT-1 cells/ml collagen-fibrin gel for 7 d. (B16 cells at >2 × 107/ml lysed the collagen-fibrin gels and could not be tested.) To assure that the OT-1 cells remained viable and active throughout this period, we supplemented the medium with IL-2. No viable B16 cells were recovered on day 7 (Fig. 5).
We draw three conclusions from these experiments. First, the finding that OT-1 cells kill SIINFEKL-B16 cells at a constant exponential rate (∼99.3–99.9%/d) for 5–7 d (Fig. 5) provides independent confirmation that killing of SIINFEKL-B16 cells depends strictly on OT-1 cell concentration (Fig. 5). Were this not so, the killing rate would change as the target cell concentration declined. Second, it indicates that the concentration of cytolytically active effector cells remained constant for 5 d in the absence of added IL-2 and for 7 d in its presence. Were this not so, the rate of target cell killing would change as the concentration of effector cells declined. Third, it demonstrates that 107 OT-1 cells/ml produce sterilizing immunity versus 2 × 107 SIINFEKL-B16 cells/ml, a concentration of melanoma cells within the range found in human melanomas in vivo (Stephens and Peacock, 1978; Hemstreet et al., 1980; Daugherty et al., 1981; Whiteside et al., 1986).
Does bt /b0 = e−kpt + gt (Eq. 1) describe OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels?
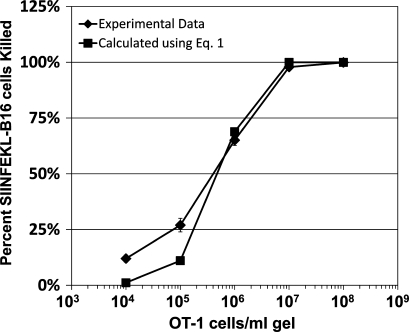
The experiments reported in Fig. 2, and in experiments which were not depicted, indicate that the CTC required to control growth of SIINFEKL-B16 cells in collagen-fibrin gels is 3–4 × 105 OT-1 cells/ml of gel. By definition (Li et al., 2002, 2004), the CTC = g/k (Eq. 2). We calculate that g, the growth rate of SIINFEKL-B16 cells in collagen-fibrin gels (Figs. 1 and 2), is 2.84 × 10−4 per min. Using this value of g and the experimentally determined value for the critical OT-1 cell concentration in collagen-fibrin gels of 3.5 × 105 OT-1 cells/ml, we solved Eq. 2, for k, the rate constant for effector cell killing of target cells, and obtained a value of 8.1 × 10−10 ml/OT-1 cell/min. We also calculated k using Eq. 1 (Li et al., 2004 and Materials and methods) and values of bt, b0, g, and t from experiments described in Figs. 2 and 6, and obtained a mean value for k of 8.19 × 10−10 ml/OT-1 cell/min. Substituting k = 8.1 × 10−10 ml/OT-1 cell/min into Eq. 1, we compared the experimentally determined and calculated values for OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels (Fig. 6). The Pearson correlation coefficient for the curves described by these datasets (Fig. 6) is 0.994 (P = 0.000483; R2 = 0.989), which indicates that Eq. 1 accurately describes OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels.
Figure 6.
Comparison of experimentally derived versus calculated values for OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels. Experimental data ±SEM are from Fig. 2 and Table S1. Calculated values were determined using Eq. 1 (Li et al., 2004), and k = 8.1 × 10−10 ml/OT-1 cell/min. Pearson’s correlation between experimental and calculated values = 0.994 (P = 0.00048).
Does Eq. 1 describe CD8+ T cell cytolytic activity in vivo?
Regoes et al. (2007) derived an equation similar to Eq. 1 to model killing of lymphocytic choriomeningitis virus (LCMV)–infected splenocytes by LCMV-specific CD8+ T cells in mouse spleen in vivo. Using Eq. 1 and the findings of Regoes et al. (2007) that LCMV-immune mouse spleen contains ∼5 × 106 LCMV-specific CD8+ T cells and that these T cells kill 99.994% of LCMV peptide–pulsed target cells in 240 min, we calculated k for LCMV-specific CD8+ T cell killing. It is 6.4 × 10−10 ml/LCMV-specific CD8+ T cell/min, a value very similar to that observed for OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels. Note that Regoes et al. (2007) used nongrowing splenocytes as target cells in their experiments. Therefore, g = 0. For this reason, we could not calculate a CTC for LCMV-specific CD8+ T cell–mediated killing of LCMV peptide-pulsed splenocytes. Thus, Eq.1 describes CD8+ T cell killing of cognate antigen-expressing target cells in vitro and in vivo.
Comparison of the rates at which OT-1 cells kill SIINFEKL-B16 cells in collagen-fibrin gels in vitro and ova peptide–expressing B16 cells in tumors in vivo
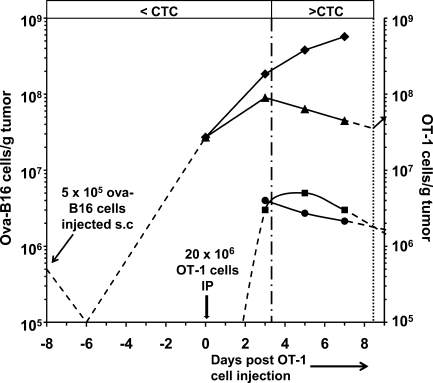
Petersen et al. (2006) studied the effect of i.p. administration of in vitro–activated OT-1 cells on growth/regression of established (8 d old, 90.5 mm3 mean vol) ova-B16 cell tumors. They reported the mean volume of these ova-B16 tumors in control (untreated) and in OT-1 cell–treated mice on days 3, 5, and 7 after OT-1 cell administration (Fig. 7). B16 tumors contain ∼3 × 105 B16 cells/mm3 (Stephens and Peacock, 1978). We used this value to calculate the number of ova-B16 cells in tumors of control and OT-1 cell–treated mice (Fig. 7 and Table S4). Petersen et al. (2006) also reported the intratumoral concentration of OT-1 cells/g of tumor on days 3, 5, and 7. We have used these values, and those for ova-B16 tumor growth and regression, to calculate k and CTC (Fig. 7 and Table S4).
Figure 7.
Relationship between ova-B16 tumor growth/regression and intratumoral OT-1 cell concentration in OT-1 cell–treated ova-B16 tumor–bearing mice. Data are obtained from Figs. 2 and 4 in Petersen et al. (2006) and were calculated as described in Table S4. C57BL/6 mice were inoculated with 5 × 105 ova-B16 cells subcutaneously on day −8, and with 20 × 106 in vitro–activated OT-1 cells i.p. on day 0. Intratumoral concentration is shown of OT-1 cells (■) from Fig. 2 (Petersen et al., 2006). Also shown are the number of ova-B16 cells in tumors of control mice (♦) and in tumors of mice that received 20 × 106 in vitro–activated OT-1 cells on day 0 (▴) calculated from Fig. 4 of Petersen et al. (2006) as described in (Table S4). CTC (•) was calculated as described in Li et al. (2004) and in the Materials and methods (Table S4). Dashed lines represent extrapolated trends based on findings reported in Petersen et al. (2006) and Stephens and Peacock (1978). The vertical dashed-dotted line indicates the point in time at which the intratumoral OT-1 concentration exceeds the CTC. The vertical dotted line indicates the estimated point in time at which the intratumoral OT-1 concentration falls below the CTC, thereby permitting resumption of tumor growth (arrow).
The growth rate of ova-B16 cell tumors slowed between the day of OT-1 cell administration (day 0) and day 3 (Fig. 7). However, tumor regression, as measured by a reduction in tumor volume and, therefore, in the total number of ova-B16 cells in these tumors, did not begin until day 3. It then proceeded at a constant rate through day 7. Note that the intratumoral OT-1 cell concentration averaged 1.5 × 106/g of tumor between days 0 and 3, was 3 × 106/g of tumor on day 3, peaked at 5 × 106/g of tumor on day 5, and declined to 3 × 106/g by day 7. Inspection of Fig. 7 shows that the CTC, the OT-1 concentration at which the rate of killing of ova-B16 cells exactly matched the rate of ova-B16 cell growth, was ∼3 × 106 OT-1 cells/g of tumor and that so long as the intratumoral OT-1 concentration remained above the CTC (i.e., 3 × 106 OT-1 cells/g of tumor), tumors regressed. Fig. 7 also shows that when the intratumoral OT-1 cell concentration declines to less than the CTC (i.e., on days 8 or 9), tumor growth will resume. Indeed, this is consistent with the observations of Petersen et al. (2006).
To determine whether the CTC calculated using values of g and k obtained using Eq. 1 fits the findings of Petersen et al. (2006; Fig. 7), we used their data (Table S4) for g, t, and intratumoral OT-1 cell concentration for each of the three time intervals (days 0–3, 3–5, and 5–7) described in the previous paragraph to calculate the CTC. We obtained mean values for g and k of 2.7 × 10−4/min and 8.9 × 10−11 ml/OT-1 cell/min, respectively, for the three time intervals, yielding a mean CTC (g/k) of 2.93 × 106 OT-1 cells/g ova-B16 tumor. The values of g for ova-B16 cell growth in control mice (Table S4) were within the range of values we obtained for growth of B16 cells in collagen-fibrin gels in vitro (Fig. 1) and those reported by Li et al. (1984) for B16 cell growth in tumors in vivo. In contrast, the values of k and CTC for OT-1 cell killing of ova-B16 cells in vivo were approximately ninefold smaller and eightfold larger, respectively, than comparable values for OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels in vitro, indicating that OT-1 cells kill ova-B16 cells less efficiently in tumors in vivo than in collagen-fibrin gels in vitro.
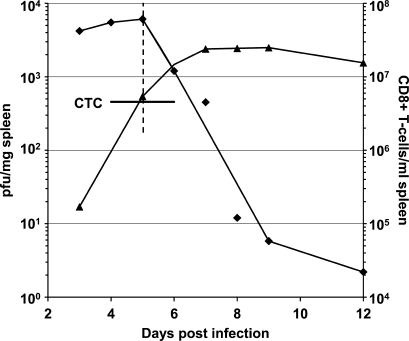
We made similar calculations using data from the study by Lukacher et al. (1999) of polyoma virus antigen–specific CD8+ T cell–mediated killing of polyoma virus–infected splenocytes in spleens of immunocompetent mice. We found mean values of k and CTC of 5.7 × 10−11 ml/polyoma virus antigen–specific CD8+ T cell/min and 4.8 × 106 polyoma virus antigen–specific CD8+ T cells/ml, respectively (Fig. 8 and Table S5). The value of k for polyoma virus antigen–specific CD8+ T cell–mediated killing of polyoma virus–infected splenocytes is ∼14-fold smaller than that calculated for OT-1 cell killing of ova-B16 cells in collagen-fibrin gels in vitro and for LCMV antigen–specific CD8+ T cell killing of LCMV peptide–pulsed splenocytes in spleen (Regoes et al., 2007).
Figure 8.
Effect of polyoma antigen–specific CD8+ T cells on polyoma virus growth in splenocytes from mice inoculated subcutaneously on day 0 with 2 × 106 PFU of virus. Data are obtained from Fig. 2 and Table I in Lukacher et al. (1999). Polyoma virus concentration (♦, PFU/mg of spleen). Intrasplenic concentration of polyoma antigen–specific CD8+ T cells/ml of spleen (▴) was calculated as reported in Table S5. k and CTC were calculated using Eqs. 1 and 2 (Li et al., 2004), respectively, as described in Table S5 and in Materials and methods.
As observed for OT-1 cell killing of ova-B16 cells in vivo, elimination of polyoma virus–infected cells did not begin until the concentration of polyoma antigen–specific CD8+ T cells was at or above the critical concentration of 4.8 × 106 polyoma virus antigen–specific CD8+ T cells/ml or /g of spleen (Fig. 7 and Table S5). Moreover, in contrast to the situation described after administration of OT-1 cells to ova-B16 cell tumor-bearing mice (Fig. 7), the concentration of polyoma virus antigen–specific CD8+ T cells/ml or /g of spleen remained well above the CTC for many days after the concentration of polyoma virus had fallen to undetectable levels. Lukacher et al. (1999) reported that the persistence of polyoma virus antigen–specific CD8+ T cells above CTC resulted in sterilizing immunity versus polyoma virus.
We draw five conclusions from these findings. First, Eq. 1 accurately models killing of antigen-expressing tumor cells and virus-infected splenocytes by cognate antigen–specific CD8+ T cells. Second, the critical concentration concept is applicable to CD8+ T cell–mediated control of tumor cell growth and viral infection in vivo (Fig. 7 and Fig. 8) Third, by use of Eq. 1 it is possible to calculate and compare the efficiency (k) with which antigen-specific CD8+ T cells kill cognate antigen–expressing target cells in vitro and in various tissue compartments in vivo. Fourth, by use of Eq. 2 it is possible to calculate the critical concentration of antigen-specific CD8+ T cells under all conditions examined in vivo and in vitro. Fifth, by determining the rate of tumor regression, or the rate of clearance of virus-infected cells, and the time period during which the intratumoral or intralesional concentration of tumor or viral antigen-specific CD8+ T cells remains above the CTC, it is possible to predict whether the tumor or virus infection will be eradicated or recrudesce.
DISCUSSION
These results enable us to define three principles that, in combination with Eqs. 1 and 2, provide a quantitative framework for describing CD8+ T cell–mediated host defense versus viral and intracellular bacterial pathogens and for cellular immunotherapy. First, the concentration of activated antigen-specific CD8+ T cells determines the efficiency with which these cells kill cognate antigen-expressing target cells under all conditions examined. Second, Eq.1 (Li et al., 2004) describes CD8+ T cell killing of cognate antigen-expressing target cells in vitro and in vivo. Third, a critical concentration of antigen-specific CD8+ T cells is required to control growth of cognate antigen–expressing transformed cells in collagen gels in vitro and in mouse subcutaneous tissue in vivo, and of polyoma virus in mouse splenocytes in vivo.
Collagen-fibrin gels
Melanomas originate in the epidermis but invade and grow in the dermis before spreading to other organs. The concentrations of collagen I and fibrinogen used to form the gels used in these experiments are in the range of those prevailing in carcinomas in rodent dermis and in normal human dermis (Grabowska, 1959; Dvorak et al., 1992; Dewever et al., 2007). The concentration of serum proteins in the medium bathing these gels is ∼20% of that found in extracellular fluids from human skin (Takeda and Chen, 1967; Le et al., 1998; Svedman et al., 2002). Thus, the gels in which activated OT-1 and SIINFEKL-B16 cells were co-cultured contain similar concentrations of collagen and fibrin, but lower concentrations of serum proteins, than those found in tumors, in rodent dermis, and in human dermis in vivo.
Clonogenic assay
In combination with the collagen-fibrin gel system described in this paper, the clonogenic assay used to measure the number of viable B16 melanoma cells is 4,500–5,500-fold more sensitive than the packed cell pellet–type assays generally used to measure CD8+ T cell cytocidal activity in vitro (Fig. 4 B). These methods enable us to distinguish 99.99 versus 99.9999% tumor cell killing and sterilizing immunity. Using this system, we demonstrate that at a concentration of ≥107/ml of gel, OT-1 cells produce sterilizing immunity versus 2 × 107/ml SIINFEKL-B16 melanoma cells in 7 d (Fig. 5), a concentration of B16 cells which is at the lower range of tumor cells in human melanomas in vivo (Whiteside et al., 1986). We are certain that all clonogenic B16 cells were killed because the clonogenic assay used is capable of detecting a single clonogenic B16 cell, and because cultures harvested on subsequent days (Fig. 5) yielded no viable B16 cells. To our knowledge, this is the first identification of the concentration of antigen-specific CD8+ T cells required to kill 100% of tumor cells growing at a concentration similar to that found in tumors. As Blattman and Greenberg (2006) note, in cancer therapy, the difference between mostly dead and all dead is a life and death distinction.
Relationship between antigen-specific CD8+ T cell killing of target cells expressing a cognate antigen in collagen-fibrin gels in vitro and in melanomas in vivo
The value of k for OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels (8.1 × 10−10 ml/OT-1 cell/min) is very similar to that for LCMV antigen-specific CD8+ T cell killing of LCMV peptide–pulsed splenocytes in mouse spleen in vivo (6.4 × 10−10 ml/LCMV antigen-specific CD8+ T cell/min). In contrast, the values of k for OT-1 cell killing of ova-B16 cells in melanomas in vivo (∼8.9 × 10−11 ml/OT-1 cell/min) and for polyoma virus antigen–specific CD8+ T cell killing of polyoma virus–infected splenocytes in spleen in vivo (∼5.7 × 10−11 ml/polyoma virus–specific CD8+ T cell/min) are ∼9- and 14-fold smaller than those for OT-1 cell killing of SIINFEKL-B16 cells in collagen-fibrin gels. In the case of OT-1 cells, these differences are particularly striking because in both the in vitro (Fig. 2) and in vivo (Fig. 7 and Table S4) situations, the OT-1 cells were activated in vitro under similar conditions. This ∼9- to 14-fold difference in k values also is matched by a ∼10-fold difference in CTC. What accounts for these large differences in k and CTC in collagen-fibrin gels versus in tumors and in polyoma virus–infected spleen in vivo?
In the case of OT-1 cell killing of ova-B16 cells in tumors and of polyoma antigen–specific CD8+ T cell killing of polyoma virus–infected splenocytes in spleen, immunomodulatory activities elicited by the tumor and by viral infection may have altered the tissue environment, thereby reducing the killing efficiency of the antigen-specific CD8+ T cells. In the case of tumors (Whiteside et al., 1986; Bronte and Mocellin, 2009) and of chronic viral infections (Virgin et al., 2009), such immunomodulatory activities are well known. To our knowledge, there is no corresponding information about the extent to which the intrasplenic environment is modified by acute viral infection (e.g., polyoma virus [Fig. 8]). Nonetheless, it is likely that immunomodulatory factors (e.g., T cell suppressive factors) account for the ∼9- to 14-fold difference in CTC observed in collagen-fibrin gels in vitro versus tumors and acute intrasplenic viral infections in vivo, respectively.
Spleen and tumors contain macrophages, dendritic cells, regulatory and effector T cells, fibroblasts, and endothelial cells, most of which have the capacity to produce proinflammatory (e.g., TNF and IL-12) and antiinflammatory (e.g., TGF-β and IL-10) substances and extracellular matrix components. In contrast, collagen-fibrin gels lack these stromal cells and their soluble and insoluble (e.g., matrix proteins) secretory products. This is a positive attribute of these gels in the sense that it enables investigators to compare the cytolytic activity of antigen-specific CD8+ T cells versus cognate antigen–expressing target cells in a three-dimensional matrix devoid of stromal cells and stromal cell secretory products with the cytolytic activity of the same CD8+ T cells versus the same target cells expressing the same antigens in tissues containing stromal cells and their secretory products. Investigators also can form gels containing antigen-specific CD8+ T cells, peptide-pulsed target cells, and one or more of these stromal cells and/or secretory products and assess their effects, individually and collectively, on the cytolytic activity of CD8+ T cells. By these means, it may be possible to replicate in vitro the pro- and antiinflammatory environments of infected, inflamed, and tumor-bearing tissues. Thus, what might at first be perceived as a negative attribute of collagen-fibrin gels could be one of their greatest assets.
Implications of these findings for cellular immunotherapy of cancer
The findings reported here have obvious relevance for cellular immunotherapy of melanoma and perhaps other tumors. Cellular immunotherapy produces objective melanoma regression in >50% of patients yet cures a very small percentage of them (Dudley et al., 2002; Dudley and Rosenberg, 2003). This paper suggests at least one reason for this disconnect between response and cure: it is the failure to deliver and maintain a sufficient intratumoral concentration of tumor antigen–specific CD8+ T cells/ml of tumor for a sufficient time period to kill 100% of clonogenic tumor cells (Figs. 5 and 7). The studies reported in this paper provide quantitative information regarding these parameters. They show that a concentration of ≥107 activated OT-1 cells/ml is required to kill 100% of SIINFEKL-B16 cells/ml collagen-fibrin gel in 7 d (Fig. 5). In these gels, target cell cytolysis depends only on the CD8+ T cell concentration (Figs. 2, 5, and 7). Accordingly, assuming melanomas contain 3 × 108 melanoma cells/ml or /g of tumor (Stephens and Peacock, 1978), and that melanoma antigen–specific CD8+ T cells kill melanoma cells in vivo at the same rate as the OT-1 cells used in the experiments shown in Fig. 5, delivery and maintenance of ≥107 melanoma antigen-specific CD8+ T cells/g melanoma for ≥8 d should result in eradication of the melanoma. However, our analysis of the findings of Petersen et al. (2006; Fig. 7 and Table S4) shows that OT-1 cells kill ova-B16 cells at an approximately ninefold lower rate (k) and have an approximately eightfold higher CTC in tumors in vivo than in collagen-fibrin gels. Under these circumstances, delivery and maintenance of ≥108 melanoma antigen-specific CD8+ T cells/ml or /g melanoma for ≥8 d would be required to effect tumor eradication. This is a very high threshold and one that does not bode well for success in cellular immunotherapy of cancer. Nonetheless, in a small number of cases cellular immunotherapy has been demonstrated to be curative (Dudley et al., 2002), suggesting that in vivo there are other factors that, under certain circumstances, contribute to the efficacy of antigen-specific CD8+ T cells. We describe four of these factors in the subsequent paragraph.
First, our calculations regarding the concentration of tumor antigen–specific CD8+ T cells that must accumulate in a tumor bed to effect tumor eradication assumes that these CD8+ T cells are the sole cytolytic effectors. However, the presence of activated tumor antigen–specific CD8+ T cells within the tumor bed may promote accumulation and tumor cell killing by other cytolytic effector cells (e.g., NK cells and activated macrophages). Under these conditions, CD8+ T cells may be only one of several cytolytically active effector cells at work within the tumor. Therefore, our calculations may underestimate the total cytocidal activity of all effector leukocytes that assemble in a tumor in response to signals initiated by tumor antigen-specific CD8+ T cells and or tumor cell cytolysis. Second, our calculations assume that tumor cell killing by clones of CD8+ T cells whose TCRs recognize different tumor antigens is merely additive. It is possible it is multiplicative. If so, the intratumoral presence of 3 × 106 CD8+ T cells versus putative tumor antigen A, 3 × 106 CD8+ T cells versus putative tumor antigen B, and 3 × 106 CD8+ T cells versus putative tumor antigen C could result in a 27-fold increase in tumor cell killing. Third, our calculations assume that all CD8+ T cells kill with the same efficiency as OT-1 cells. CD8+ T cells vary widely in their killing efficiencies (Stuge, et al., 2004). Accordingly, it may be possible to produce CD8+ T cell populations that kill at a higher rate than that observed for OT-1 cells. Indeed, we have identified OT-1 cell activation protocols that more than double their efficiency in killing SIINFEKL-B16 cells in collagen-fibrin gels (unpublished data). Fourth, we have assumed that the tumor vasculature of all melanomas is equally efficient in delivering CD8+ T cells to the tumor parenchyma. Buckanovich et al. (2008) have shown that the efficiency of this process can be increased by >10-fold. For all of the reasons stated here, it is important to determine the efficiency of entry of ex vivo–expanded CD8+ T cells into tumor beds, their residence time in the tumor bed, and their cytolytic activity under both ideal (e.g., collagen-fibrin gels) and in vivo conditions.
The studies reported in this paper identify three parameters that appear to be especially important for understanding the therapeutic potential of cellular immunotherapy. The first is the concentration of all cytolytic effector cells delivered to, and active within, tumors. The second is the killing efficiencies (k) of all effector cells, singly and in combination, within the tumor bed. The third is the length of time each of these effector cells remains cytolytically active within the tumor bed. Information about these parameters will enable investigators to assess whether the high frequency of objective tumor regression, but low frequency of cure, observed in melanoma patients treated with ex vivo–activated CD8+ T cells is a consequence of delivery of an insufficient number of tumor antigen–specific CD8+ T cells to tumors to raise the intratumoral concentration to 30-fold above the CTC (Fig. 5), or of deleterious effects of the tumor environment on the viability and/or effector functions of CD8+ T cells. Studies in progress show that the collagen-fibrin gel system described in this paper is also useful for assessing killing by cloned tumor antigen–specific human CD8+ T cells of human melanoma cells expressing a cognate antigen (unpublished data).
Other applications of Eq. 1
Our findings with respect to CD8+ T cell killing of nongrowing B16 cells (Table S1) may be relevant to studying and understanding host defense versus viruses and intracellular bacterial pathogens and rejection of organ transplants. With respect to microbial pathogens, it will be useful to determine the CTC for antigen-specific CD8+ T cells to control infection in various tissues. Assuming OT-1 cells are representative of the majority of antigen-specific CD8+ T cells, the results reported in this paper and by Snyder et al. (2003) that only 2–4% of these cells are cytolytically active suggest that viral and bacterial antigen–specific CD8+ T cells control infections largely by secreting IFN-ɣ and TNF and that CD8+ T cells use their cytolytic effector activity more sparingly than previously assumed.
With respect to the effects of host CD8+ T cells on allogeneic transplants, we suggest that such transplants remain functional so long as they contain sufficient stem cells to replace differentiated cells at a rate equal to the rate of differentiated cell killing by CD8+ T cells. However, if CD8+ T cells kill the organ’s differentiated cells at a faster rate than can be replaced by the organ’s stem cells, or if CD8+ T cells attack the organ’s stem cells themselves, the organ is likely to fail. The collagen-fibrin gel system described here may be useful for investigating these possibilities in vitro.
MATERIALS AND METHODS
Materials.
Human fibrinogen was obtained from American Diagnostica Inc., collagen I, cell culture inserts and tissue culture plates from BD, SIINFEKL peptide (OVA 257–264) from American Peptide Company, CFSE from Invitrogen, and recombinant mouse IL-2 from Millipore. All other reagents were purchased from Sigma-Aldrich. All experiments using mice were Institutional Review Board approved by Columbia University.
Cells.
B16 melanoma cells (vol. = 2,757 µm3 assuming spherical diameter = 17.4 µm [Ochalek, et al., 1988]; H2-Kb [Hu and Lesney, 1964]), were maintained as monolayer cultures in RPMI 1640 medium supplemented with 10% FBS and 50 µM β-ME (OT-1 growth medium) at 37°C in a 95% air/5% CO2 humidified atmosphere, detached by incubation at 37°C for 5 min in PBS containing 5 mM EDTA, pelleted by centrifugation (400 g for 10 min), and resuspended at 106 cells/ml in OT-1 growth medium. Where indicated, B16 cells were incubated in suspension at 106 cells/ml in RPMI medium containing 1 µM SIINFEKL peptide (ovalbumin residues 257–264) for 2 h at 37°C, washed three times in PBS, and resuspended in OT-1 growth medium. B16 spheroids were sedimented at 1 g through a cushion of FBS to separate them from single B16 cells. The purified spheroids were pulsed with 1 µM SIINFEKL for 2 h and a measured aliquot of this preparation was disaggregated by trituration in EDTA/0.05% trypsin buffer, releasing >95% of cells from the spheroids. The number of released B16 cells was assessed by counting cells in a hemocytometer.
OT-1 CD8+ T cells, harvested from spleens of C57BL/6 mice, express a transgene encoding a TCR that specifically recognizes SIINFEKL peptide bound to MHC-I H-2kb (Hogquist et al., 1994). Activated OT-1 T cells were generated by incubation of 5 × 106/ml OT-1 SIINFEKL-pulsed mouse splenocytes in vitro for 5–7 d in the presence of IL-2 (Moore et al., 1988; Curtsinger et al., 1998). In brief, an OT-1 mouse spleen was homogenized, the released cells were pelleted and resuspended in 5 ml ACK buffer (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM EDTA) for 1 min to lyse red blood cells, and the splenocytes were pelleted, washed, resuspended at 5 × 106 cells/ml in OT-1 growth medium containing 0.75 µg/ml SIINFEKL peptide, and incubated at 37°C in a 95% air/5% CO2 humidified atmosphere. On days 3 and 5, 25 ml of fresh OT-1 growth medium containing 10 U/ml of mouse recombinant IL-2 was added to the cultures. On day 7, the cells were harvested and OT-1 cells were purified by centrifugation at 400 g for 30 min at room temperature over a Histopaque gradient (density = 1.083). Cells isolated by this method were incubated with PE-labeled anti-CD8 or FITC-labeled anti-Vβ5 monoclonal antibodies, washed, and assayed by FACS (BD). Over 90% were CD8+ and Vβ5+.
Formation of collagen-I–fibrin gels containing B16 and OT-1 cells.
Each well of a 48-well tissue culture plate was filled sequentially with 5 µl PBS containing 0.1 U thrombin, 100 µl PBS containing 1 mg/ml of human fibrinogen, 1 mg/ml of rat tail collagen I, 10% FBS, and 103–2 × 106 SIINFEKL peptide-pulsed B16 cells (SIINFEKL-B16 cells), with or without 103–106 OT-1 cells. The plates were incubated for 15 min at 37°C in a 95% air/5% CO2 humidified atmosphere to allow the fibrin to gel. In some early experiments, 10 µl of 10−7 Md-phenylalanyl-l-propyl-l-arginine chloromethyl ketone (PPACK) was added to the top of each gel to inhibit thrombin and the plates were incubated at 37°C for another 15 min to allow the collagen to gel. (Subsequent experiments indicated that this PPACK step was unnecessary and it was omitted.) Gels were overlaid with 0.5 ml OT-1 growth medium and incubated at 37°C in a 95% air/5% CO2 humidified atmosphere. These gels are 0.1 ml in volume and ∼1,500 µm in height. In preliminary experiments, we found that B16 cells incubated in gels composed of fibrin alone dissolved these gels in 3–4 d. Addition of collagen I maintained gel integrity for 7–10 d.
Clonogenic assay for B16 cells.
SIINFEKL-B16 cells, with or without OT-1 cells, were incubated at 37°C in collagen-fibrin gels in wells in a 48-well plate. At indicated times, medium overlying each gel was removed and gels were dissolved by overlaying each gel with 100 µl PBS containing 2.5 mg/ml collagenase type 1A for 20 min at 37°C, followed by further addition of 100 µl PBS containing 2.5 mg/ml trypsin for another 20 min at 37°C. The resulting solution containing cells and dissolved gel components was diluted 10–1,000-fold (depending on the initial B16 cell concentration in the gel) in OT-1 growth medium and 100-µl aliquots of the final dilution were plated in each of two 60 × 15-mm tissue culture dishes containing 2 ml OT-1 growth medium. The dishes were incubated at 37°C for 7 d in a 95% air/5% CO2 humidified atmosphere to allow B16 cells to form macroscopic colonies, washed with PBS, treated with 2 ml of 3.7% formaldehyde in PBS for 15 min to fix the B16 cells, washed again with PBS, incubated for 2 h in 2 ml of 4% wt/vol methylene blue in H2O at room temperature, washed with distilled water to remove excess methylene blue, dried, and the blue-stained colonies counted manually as previously described (Freedman et al., 1984). Control experiments showed that B16 cells had a plating efficiency of ∼60% regardless of whether they were released from plastic tissue culture plates with EDTA or from collagen-fibrin gels with collagenase and trypsin and/or plated in β-ME–containing medium. Thus, the number of B16 cells reported as placed into fibrin-collagen I gels is ∼1.66-fold the number of cells recovered from them.
Cell sizes and volumes.
Activated OT-1 cells are ∼7 µm in diameter as measured by phase-contrast microcopy of unfixed cells. Packed cell volumes of B16 cells, activated OT-1 cells, and naive splenocytes from wild-type C57BL/6 spleen were determined by centrifuging 104 B16 cells, 104 OT-1 cells, or 104 splenocytes in 20 µl OT-1 medium for 2 min at 200 g in 25-µl glass micropipettes (sealed at one end). The volumes of each cell type, calculated by dividing the packed cell volume by the number of cells in the pipette, were 2.5 × 10−3 nl/B16 cell (4 × 108 B16 cells/ml), 1.7 × 10−4 nl/activated OT-1 cell (5.9 × 109 OT-1 cells/ml), and ∼2.2 × 10−4 nl/splenocytes (4.5 × 109 splenocytes/ml).
Packed cell pellet–type assays.
Wells of a 96-well round-bottom tissue culture plate were each filled with 0.2 ml OT-1 medium containing 104 B16 cells, pulsed previously with 10−6 M SIINKFEKL peptide, and the number of activated OT-1 cells required to produce the ratios of OT-1/B16 cells indicated in the figures. The plate was centrifuged at 1,000 rpm (∼200 g) for 5 min to pellet cells and then incubated at 37°C in a humidified incubator for 4 h. The medium was removed and the pellet was disaggregated by incubation in 0.1 ml of PBS containing 5 mM EDTA for 5 min at 37°C. The solution was diluted 100-fold in OT-1 growth medium and 0.1-ml aliquots of the final dilution were plated for colony formation as described in Clonogenic assay for B16. In some experiments, naive splenocytes (filler cells) from wild-type C57BL/6 mice were added to vary the ratio of OT-1 to B16 cells while keeping total cell volume constant.
CFSE labeling of OT-1 cells.
106–107 OT-1 cells were incubated in 1 ml PBS containing 0.1% glucose, 0.1% BSA (PBS-G-BSA), and 10 µM CFSE at 37°C for 15 min, washed three times with 1 ml OT-1 medium, resuspended at a concentration of 106 cells/ml in OT-1 medium, and co-embedded in collagen-fibrin gels with B16 cells at the indicated concentrations. Gels were overlaid with 1 ml OT-1 medium ± 100 U/ml IL-2 and incubated at 37°C in a 95% air/5% CO2 humidified atmosphere. 24, 48, and 72 h later, the gels were digested with collagenase and trypsin and the released cells were washed with PBS-G-BSA and divided into two aliquots. The cells in the first aliquot were incubated with 1.5 µM propidium iodide in PBS-G-BSA for 10 min, washed with PBS-G-BSA, and the number and fluorescence of viable CFSE-labeled OT-1 cells was assessed with a FACSCalibur (BD). The second aliquot was assayed as described in Clonogenic assay for B16.
Frozen sections.
Collagen-fibrin gels containing SIINFEKL-B16 cells were formed, as described in Formation of collagen-I–fibrin gels…, in 8-µm pore cell culture inserts seated in wells containing 1 ml OT-1 medium in 24-well tissue culture plates (BD) and overlaid with 0.5 ml OT-1 medium, and they were incubated at 37°C in a 95% air/5% CO2 atmosphere. 24, 72 and 120 h after forming the gels, the medium overlying the gels was removed and the inserts were placed in wells containing PBS at room temperature for 15 min to wash away serum proteins, fixed with 10% neutral-buffered formalin for 1 h at room temperature, washed with PBS, overlaid with 0.5 ml of 5% gelatin in PBS at 37°C, and incubated at this temperature for 20 min to allow the gelatin solution to permeate the collagen-fibrin gels. The inserts then were placed at 4°C for 30 min to allow gelatin to gel. Gelatin-impregnated gels were mechanically released from inserts using a scalpel, bisected vertically, and incubated in 30% sucrose overnight at 4°C. Each half gel was placed in a cryomold and the cryomold was filled with OCT embedding medium and placed in a bath of 2-methylbutane and dry ice. 10-µm-thick frozen sections were cut parallel to the vertically bisected face of the gels, and the sections were stained with hematoxylin/eosin and placed on glass slides for light microscopy.
Calculations.
The value of k (the experimentally determined target cell killing constant) was calculated as previously described (Li et al., 2004) using Eq. 1 (bt/b0 = e−kpt+gt). bt = experimentally determined target cell concentration per milliliter of gel or tumor at time t (in minutes) of co-incubation of antigen-expressing target cells with antigen-specific CD8+ T cells in collagen-fibrin gels or inoculation of mice with antigen-specific CD8+ T cells. b0 = initial target cell concentration per ml gel or tumor. The CD8+ CTC was calculated using Eq. 2 (Li et al., 2004; CTC = g/k). g = the experimentally determined rate of B16 cell growth in vitro or in vivo or of increase in polyoma virus–infected target cells, calculated using the relationship ln bt/b0 = g × t (min). We estimated mouse spleen volume and wet weight for LCMV infected mice to be 0.15 ml and 0.15 g, respectively, and 0.1 ml and 0.1 g, respectively, for polyoma virus–infected mice. B16 melanoma tumors contain 3 × 108 B16 cells/ml or /g of tumor (Stephens and Peacock, 1978).
Statistics.
Unless otherwise indicated, all experiments were performed at least three times in duplicate. Data are reported as the mean ± SEM for the number of experiments indicated.
Online supplemental material.
Fig. S1 shows the OT-1 SIINFEKL peptide concentration required for optimal killing of B16 cells in collagen-fibrin gels. Fig. S2 shows the killing of SIINFEKL-B16 cells in spheroids versus single SIINFEKL-B16 cells by OT-1 cells. Fig. S3 shows that CMA inhibits OT-1 cell killing of SIINFEKL-B16 cells. Table S1 shows that activated OT-1 cells kill growing and nongrowing SIINFEKL-pulsed B16 cells with approximately equal efficiency. Table S2 shows that the addition of naive spleen cells had no effect on killing efficiency of OT-1 cells in collagen-fibrin gels. Table S3 shows that OT-1 cell concentration determines the efficiency of killing of SIINFEKL-B16 cells in packed-cell-pellet type assays. Table S4 shows OT-1 cell killing of ova-B16 cells in 8-d-old tumors in vivo. Table S5 shows polyoma virus antigen–specific CD8+ T cell killing of polyoma virus–infected splenocytes in mouse spleen in vivo. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091279/DC1.
Acknowledgments
We thank Drs. Rustom Antia and Rafi Ahmed of Emory University for data from Regoes et al. (2007. Proc. Natl. Acad. Sci. USA. doi:10.1073/pnas.0508830104) and Dr. Raul Rabadan for assistance with statistics and calculations.
This study was supported by National Institutes of Health grants AI20516 to S.C. Silverstein and CA94037 to R. Clynes.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CMA
- concanamycin A
- CNC
- critical neutrophil concentration
- CTC
- critical OT-1 T-cell concentration
- LCMV
- lymphocytic choriomeningitis virus
References
- Agger R., Petersen M.S., Petersen C.C., Hansen S.B., Stødkilde-Jørgensen H., Skands U., Blankenstein T., Andersen T.E., Hulgaard E.F., Jørgensen J.T., et al. 2007. T cell homing to tumors detected by 3D-coordinated positron emission tomography and magnetic resonance imaging. J. Immunother. 30:29–39 10.1097/01.cji.0000211326.38149.7e [DOI] [PubMed] [Google Scholar]
- Baramova E.N., Coucke P., Leprince P., De Pauw-Gillet M.C., Bassleer R., Foidart J.M. 1994. Evaluation of matrix metalloproteinases and serine proteases activities in three B16 melanoma cell lines with distinct tumorigenic potential. Anticancer Res. 14:841–846 [PubMed] [Google Scholar]
- Blattman J.N., Greenberg P.D. 2006. PD-1 blockade: rescue from a near-death experience. Nat. Immunol. 7:227–228 10.1038/ni0306-227 [DOI] [PubMed] [Google Scholar]
- Bronte V., Mocellin S. 2009. Suppressive influences in the immune response to cancer. J. Immunother. 32:1–11 10.1097/CJI.0b013e3181837276 [DOI] [PubMed] [Google Scholar]
- Brunner K.T., Mauel J., Cerottini J.C., Chapuis B. 1968. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 14:181–196 [PMC free article] [PubMed] [Google Scholar]
- Buckanovich R.J., Facciabene A., Kim S., Benencia F., Sasaroli D., Balint K., Katsaros D., O’Brien-Jenkins A., Gimotty P.A., Coukos G. 2008. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 14:28–36 10.1038/nm1699 [DOI] [PubMed] [Google Scholar]
- Cerottini J.C., Engers H.D., Macdonald H.R., Brunner T. 1974. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J. Exp. Med. 140:703–717 10.1084/jem.140.3.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J.M., Lins D.C., Mescher M.F. 1998. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C-) to TCR/CD8 signaling in response to antigen. J. Immunol. 160:3236–3243 [PubMed] [Google Scholar]
- Daugherty D.F., Pretlow T.P., Peacock L.M., Pitts A.M., Mitchell C.E., Pretlow T.G., II 1981. Separation and characterization of the neoplastic and stromal elements of the R3230AC mammary adenocarcinoma. Cancer Res. 41:5064–5069 [PubMed] [Google Scholar]
- Dewever J., Frérart F., Bouzin C., Baudelet C., Ansiaux R., Sonveaux P., Gallez B., Dessy C., Feron O. 2007. Caveolin-1 is critical for the maturation of tumor blood vessels through the regulation of both endothelial tube formation and mural cell recruitment. Am. J. Pathol. 171:1619–1628 10.2353/ajpath.2007.060968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski M.J., Reome J.B., Dutton R.W. 2001. Immunopotentiating role of IFN-gamma in early and late stages of type 1 CD8 effector cell-mediated tumor rejection. Clin. Immunol. 98:70–84 10.1006/clim.2000.4945 [DOI] [PubMed] [Google Scholar]
- Dudley M.E., Rosenberg S.A. 2003. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat. Rev. Cancer. 3:666–675 10.1038/nrc1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J., Topalian S.L., Sherry R., Restifo N.P., Hubicki A.M., et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298:850–854 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H.F., Nagy J.A., Berse B., Brown L.F., Yeo K.T., Yeo T.K., Dvorak A.M., van de Water L., Sioussat T.M., Senger D.R. 1992. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann. N. Y. Acad. Sci. 667:101–111 10.1111/j.1749-6632.1992.tb51603.x [DOI] [PubMed] [Google Scholar]
- Freedman V.H., Gorrell T.E., Nathan C.F., Copeland C.S., Silverstein S.C. 1984. Bacillus Calmette-Guérin–activated murine macrophages kill syngeneic melanoma cells under strict anaerobic conditions. J. Exp. Med. 160:94–107 10.1084/jem.160.1.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska M. 1959. Collagen content of normal connective tissue, of tissue surrounding a tumour and of growing rat sarcoma. Nature. 183:1186–1187 10.1038/1831186a0 [DOI] [PubMed] [Google Scholar]
- Hemstreet G.P., III, Enoch P.G., Pretlow T.G., II 1980. Tissue disaggregation of human renal cell carcinoma with further isopyknic and isokinetic gradient purification. Cancer Res. 40:1043–1049 [PubMed] [Google Scholar]
- Hofmann U.B., Houben R., Bröcker E.-B., Becker J.C. 2005. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 87:307–314 10.1016/j.biochi.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27 10.1016/0092-8674(94)90169-4 [DOI] [PubMed] [Google Scholar]
- Hu F., Lesney P.F. 1964. The isolation and cytology of two pigment cell strains from B-16 mouse melanomas. Cancer Res. 24:1634–1643 [PubMed] [Google Scholar]
- Huang S.-C., Ho C.-T., Lin-Shiau S.-Y., Lin J.-K. 2005. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem. Pharmacol. 69:221–232 10.1016/j.bcp.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Joseph-Pietras D., Carlier A., Madoulet C., Albert P. 2006. Anti-tumoural activity of peripheral blood mononuclear cells against melanoma cells: discrepant in-vitro and in-vivo effects. Melanoma Res. 16:325–333 10.1097/01.cmr.0000205016.31235.a9 [DOI] [PubMed] [Google Scholar]
- Kataoka T., Shinohara N., Takayama H., Takaku K., Kondo S., Yonehara S., Nagai K. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678–3686 [PubMed] [Google Scholar]
- Kuwashima Y., Yamada T., Saio M., Takami T. 1993. Growth characteristics of murine B16 melanoma multicellular spheroids: a model for invasion and effects of doxorubicin treatments. Anticancer Res. 13:1215–1217 [PubMed] [Google Scholar]
- Le D.T., Borgs P., Toneff T.W., Witte M.H., Rapaport S.I. 1998. Hemostatic factors in rabbit limb lymph: relationship to mechanisms regulating extravascular coagulation. Am. J. Physiol. 274:H769–H776 [DOI] [PubMed] [Google Scholar]
- Li X.H., Paulus G., Atassi G., Buyssens N. 1984. Growth response of B16 melanoma to in vivo treatment with 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) at the initial stage after tumor transplantation. Am. J. Pathol. 115:403–411 [PMC free article] [PubMed] [Google Scholar]
- Li Y., Karlin A., Loike J.D., Silverstein S.C. 2002. A critical concentration of neutrophils is required for effective bacterial killing in suspension. Proc. Natl. Acad. Sci. USA. 99:8289–8294 10.1073/pnas.122244799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Karlin A., Loike J.D., Silverstein S.C. 2004. Determination of the critical concentration of neutrophils required to block bacterial growth in tissues. J. Exp. Med. 200:613–622 10.1084/jem.20040725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A.E., Moser J.M., Hadley A., Altman J.D. 1999. Visualization of polyoma virus-specific CD8+ T cells in vivo during infection and tumor rejection. J. Immunol. 163:3369–3378 [PubMed] [Google Scholar]
- Martz E. 1975. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. I. Resolution and characterization. J. Immunol. 115:261–267 [PubMed] [Google Scholar]
- Moore M.W., Carbone F.R., Bevan M.J. 1988. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 54:777–785 10.1016/S0092-8674(88)91043-4 [DOI] [PubMed] [Google Scholar]
- Ochalek T., Nordt F.J., Tullberg K., Burger M.M. 1988. Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res. 48:5124–5128 [PubMed] [Google Scholar]
- Petersen C.C., Petersen M.S., Agger R., Hokland M.E. 2006. Accumulation in tumor tissue of adoptively transferred T cells: A comparison between intravenous and intraperitoneal injection. J. Immunother. 29:241–249 10.1097/01.cji.0000203078.97493.c3 [DOI] [PubMed] [Google Scholar]
- Regoes R.R., Barber D.L., Ahmed R., Antia R. 2007. Estimation of the rate of killing by cytotoxic T lymphocytes in vivo. Proc. Natl. Acad. Sci. USA. 104:1599–1603 10.1073/pnas.0508830104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.E., Bowers W.J., Livingstone A.M., Lee F.E., Federoff H.J., Mosmann T.R. 2003. Measuring the frequency of mouse and human cytotoxic T cells by the Lysispot assay: independent regulation of cytokine secretion and short-term killing. Nat. Med. 9:231–235 10.1038/nm821 [DOI] [PubMed] [Google Scholar]
- Stephens T.C., Peacock J.H. 1978. Cell yield and cell survival following chemotherapy of the B16 melanoma. Br. J. Cancer. 38:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuge T.B., Holmes S.P., Saharan S., Tuettenberg A., Roederer M., Weber J.S., Lee P.P. 2004. Diversity and recognition efficiency of T cell responses to cancer. PLoS Med. 1:e28 10.1371/journal.pmed.0010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R.M. 1988. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 240:177–184 10.1126/science.2451290 [DOI] [PubMed] [Google Scholar]
- Svedman C., Yu B.B., Ryan T.J., Svensson H. 2002. Plasma proteins in a standardised skin mini-erosion (I): permeability changes as a function of time. BMC Dermatol. 2:3 10.1186/1471-5945-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Chen A.Y. 1967. Studies of the metabolism and distribution of fibrinogen in patients with hemophilia A. J. Clin. Invest. 46:1979–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W., Wherry E.J., Ahmed R. 2009. Redefining chronic viral infection. Cell. 138:30–50 10.1016/j.cell.2009.06.036 [DOI] [PubMed] [Google Scholar]
- Whiteside T.L., Miescher S., MacDonald H.R., Von Fliedner V. 1986. Separation of tumor-infiltrating lymphocytes from tumor cells in human solid tumors. A comparison between velocity sedimentation and discontinuous density gradients. J. Immunol. Methods. 90:221–233 10.1016/0022-1759(86)90079-7 [DOI] [PubMed] [Google Scholar]