Abstract
The presence of IL-12 during antigen stimulation instructs naive CD8+ T cells for long-term effector responses, but their mechanisms of collaboration are not understood completely. Herein, we report that CD8+ T cells (OT-I T cells) stimulated with antigen for a longer duration show enhanced sensitization to IL-12 as a result of Erk1/2-dependent, increased Ets-1 phosphorylation and subsequent increases in IL-12Rβ2 expression. Correspondingly, naive OT-I T cells stimulated by antigen for a longer duration in the presence of IL-12, irrespective of frequency of APCs, show robust effector maturation and mount long-term antigen-recall responses upon adoptive transfer. These results identify the role of antigen strength-dependent Erk1/2 activation for Ets-1-mediated collaboration with IL-12 in CD8+ T cells.
Keywords: IFN-γ, CTL
Introduction
A brief period of antigen stimulation is sufficient to trigger activation and proliferation of CD8+ T cells, but longer periods of antigen stimulation are required for optimal effector maturation and survival [1,2,3]. Although several in vitro and in vivo studies have demonstrated that the amount of antigen stimulation (the frequency of APCs and duration of antigen stimulation) can influence antigen-specific T cell responses [4,5,6], the precise mechanisms underpinning these outcomes were not elucidated completely. By the use of infectious disease models, the influence of amount of antigen on T cell responses has been amply demonstrated [7, 8]. However, as a result of the inherent inability to control variables such as the replication rate of the infectious agent, elicitation of inflammatory/anti-inflammatory cascades, and involvement of several cell types, the information obtained from these studies is not suitable for discerning the relative contribution of antigen strength in regulating collaborations with inflammatory cytokines.
The presence of IL-12 during antigen stimulation programs naïve CD8+ T cells for robust type 1 effector differentiation [9, 10]. However, its role in promoting memory CD8+ T cell responses is somewhat unclear, as several contradictory effects of IL-12 on memory cell fate determination have been reported [11,12,13,14]. The ability of IL-12 to skew naive CD4+ and CD8+ T cells toward the type 1 phenotype has been shown to require STAT4- and STAT1-mediated T-bet expression [15, 16], but the mechanisms that guide collaboration between antigen stimulation and IL-12 are not well understood. Previously, it has been reported that naive CD8+ T cells fail to respond to IL-12 unless they are subjected to prolonged exposure to antigen [10]. The lack of understanding of mechanisms that regulate antigen stimulation-induced IL-12 sensitivity remains a hurdle for optimizing the use of antigen plus adjuvant for efficacious CD8+ T cell responses.
The MAPKs, including the ERK, JNK/stress-activated protein kinase, and p38 MAPK, have been shown to play a role in TCR- and B7.1-induced T cell activation [17, 18]. The activation of the ERK pathway is necessary for CD8+ T cell proliferation and survival [19]. In contrast, p38 and JNK pathways are activated by cellular stress signals and have functions in tolerance and differentiation [20]. Upon activation, the Erk1/2 proteins regulate expression of various transcription factors such as Ets-1, c-myc, and c-Jun by translocating to the nucleus [21]. It has been noted that Ets-1, a prototypic member of the Ets family of transcription factors, plays an important role in CD4+ T cell effector differentiation, but Ets-1 can also act as a negative regulator of Th17 differentiation [22, 23]. The role of Erk1/2 and/or Ets-1 in regulating an antigen-activated CD8+ T cell response remains relatively uncharacterized.
Herein, we have used an in vitro approach used previously to demonstrate the impact of antigen dose and duration on naïve CD8+ T cell responses [2, 3]. By altering the dose of cognate antigen and the duration of antigen stimulation (1–20 h) in the presence or absence of IL-12, we report that the amount of antigen stimulation governs a naïve CD8+ T cell response to IL-12 by regulating IL-12Rβ2 expression. The amount of antigen stimulation affects Ets-1 phosphorylation by Erk1/2 signaling, which in turn, controls IL-12Rβ2 expression on CD8+ T cells. The heightened collaboration between antigen and IL-12 signals promotes CD8+ T cell effector maturation and empowers them for long-term immune responses. These results identify the mechanisms that mediate collaboration between the amount of antigen stimulation and IL-12, which can be exploited for optimization of antigen-specific CD8+ T cell responses.
MATERIALS AND METHODS
Mice and reagents
B6 (National Cancer Institute, Rockville, MD, USA), OT-I, and OT-I/PL mice were housed and used as per Roswell Park Cancer Institute-Institutional Animal Care and Use Committee (Buffalo, NY, USA) guidelines. The Ets-1-deficient mice (B6×129Sv) were kindly provided by Lee Ann Garrett-Sinha (State University of New York, Buffalo, NY, USA). The murine rIL-12 was a gift from Wyeth, Inc. (Cambridge, MA, USA).
Antibodies and flow cytometry
All antibodies used for flow cytometry were purchased from BD PharMingen (San Jose, CA, USA) except anti-IL-7Rα (eBioscience, San Diego, CA, USA), antiphosphorylated STAT4 (Zymed Lab, San Francisco, CA, USA), antiphosphorylated ERK1/2 (E10; Cell Signaling Technology, Beverly, MA, USA), and antiphosphorylated Ets-1 (pT38) polyclonal antibody (Invitrogen, Carlsbad, CA, USA). ICS for IFN-γ, phosphorylated STAT4, and phosphorylated ERK1/2 was performed as described [24, 25].
OT-I cell activation
CD8+ T cells were purified from lymph nodes and spleens of OT-I/Rag−/− mice by negative selection as described previously [11]. Two adherent cell lines, namely BOK (expressing H-2Kb, OVAp, and B7.1) and MEC II (expressing H-2Kb), kindly provided by Dr. Stephen P. Schoenberger (La Jolla Institute for Allergy and Immunology, San Diego, CA, USA), were used as APCs to stimulate naïve OT-I cells as described previously [2, 3]. To vary the frequency of APCs, the BOK and MEC II cells were mixed at a ratio of 1:5 (16.7% APC, low dose) or 1:0 (100% APCs, high dose), keeping the final cell density at 1 × 105 cells/ml, and seeded in 24-well culture plates overnight. At 1 h or 20 h poststimulation, nonadherent OT-I cells were harvested and transferred to new wells containing media only. OT-I cells were evaluated at indicated time-points. In some experiments, naïve OT-I T cells were labeled with CFSE (Molecular Probes, Eugene, OR, USA) as described previously [26].
Evaluation of percent cell recovery and apoptosis
Cell viability was assessed by trypan blue dye exclusion analysis by microscopic evaluation. The ratio between cells, added and harvested 72 h after culture, was multiplied by 100 for determining percent recovery.
Cytotoxicity assay
The cytolytic ability of OT-I cells was analyzed by using a standard 4-h chromium release assay as described previously [27]. 51Cr-labeled EL4 cells pulsed with 10 nM SIINFEKL peptide served as the antigenic target cells, whereas EL4 cells pulsed with an irrelevant peptide were used as control.
RT-PCR
This was carried out as described previously [11]. Primers were: murine IL-12Rβ2, 5′-TCC ATT CCC GGA GCA AAG-3′ and 5′-GGC TGC TTA TTG GAT GTG AGT TT-3′; IFN-γ, 5′-TAC TGC CAC GGC ACA GTC ATT GAA-3′ and 5′-CCC CCA ATC CTC CAC AAG ACC-3′; granzyme B, 5′-AAG GAC AAC ACT CTT GAC GCT G-3′ and 5′-CTT GAC TTC ATG TCC CCC GAT-3′; perforin, 5′-TCC AAG GTA GCC AAT TTT GCA-3′ and 5′-TCT GAG CGC CTT TTT GAA GTC-3′; β-actin, 5′-ACG GCC AGG TCA TCA CTA TTG-3′ and 5′-CAA CGT CAC ACT TCA TGA TGG A-3′. The results shown were obtained after 30 cycles of amplification.
Evaluation of antigen-specific CD8+ T cells in vivo responses
Activated CD8+/Thy1.1+ T cells (2×106) were adoptively transferred (i.v.), and recipient mice were immunized with OVA (5 μg) in IFA on Day 66 and the T cell responses evaluated 4 days later. The total number of CD8α+/Thy1.1+ of adoptively transferred cells was calculated by multiplying the total lymphocyte count by the percentage of CD8α+/Thy1.1+ T cells gate. Ex vivo IFN-γ staining and in vivo CTL assays were performed as described [26].
Statistical analysis
For statistical analysis, the unpaired Student’s t-test was applied. Significance was set at P < 0.05.
RESULTS AND DISCUSSION
The amount of antigen stimulation regulates IL-12Rβ2 expression and IL-12-mediated CD8+ T cell effector differentiation
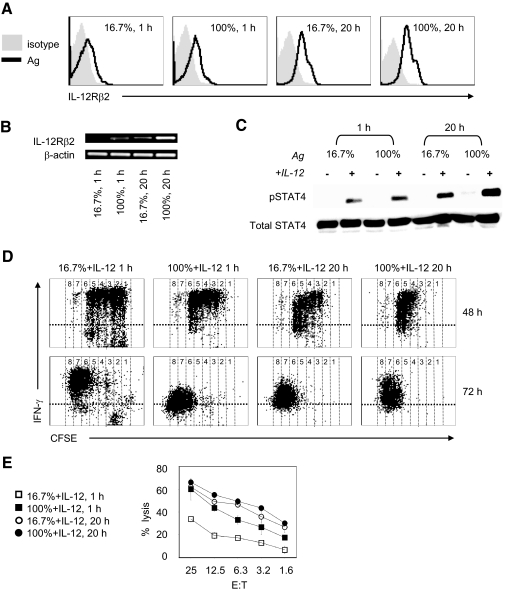
The lower sensitivity of naïve versus antigen-experienced CD8+ T cells for IL-12 is thought to be a result of the relatively higher expression of IL-12Rβ2 on antigen-experienced T cells [28]. However, the mechanism by which the amount of antigen stimulation impacts the ability of CD8+ T cells to respond to IL-12 is uncharacterized. We first evaluated the expression of IL-12Rβ2 on CD8+ T cells stimulated with various amounts of antigen. Naïve OT-I cells cultured with high amounts of antigen [BOK (SIINFEKL):MEC II (no SIINFEKL)=1:0, 100%] or low amounts of antigen (BOK:MEC II=1:5, 16.7%) for 1 h or 20 h were transferred into empty wells and maintained without further antigen stimulation. The examination of levels of IL-12Rβ2 mRNA (RT-PCR) and protein (flow cytometry) in OT-I cells showed less IL-12Rβ2 expression on OT-I cells receiving 1 h and/or low-dose antigen (16.7%) as compared with high-dose antigen (100%) and/or 20 h antigen stimulation (Fig. 1, A and B). The highest expression of the IL-12Rβ2 protein was observed in OT-I cells stimulated with 100% antigen presenting for 20 h (Fig. 1A). The amount of antigen stimulation also increased IL-12Rβ2 mRNA (Fig. 1B), suggesting a transcriptional mode of action, but further studies are required for confirmation. Indeed, stimulation with high amounts of antigen increases IL-12Rβ2 expression and induced higher levels of STAT4 phosphorylation when treated with IL-12 (2 ng/ml; Fig. 1C). Notably, duration of stimulation rather than antigen dose affected IL-12-mediated STAT4 phosphorylation induction significantly (Fig. 1C; 1 h vs. 20 h at 16.7% and 100%), thus implicating the importance for the duration of antigen stimulation in rendering CD8+ T cells sensitive to IL-12.
Figure 1.
High amount of antigen stimulation increases expression of IL-12Rβ2 and promotes IL-12-mediated CD8+ effector. Naive OT-I T cells stimulated with varying ratios of antigen-expressing fibroblasts (BOK) versus nonantigen-expressing fibroblasts (MEC II); 1:5 (16.7%) or 1:0 (100%) for 1 h or 20 h. (A) Surface expression of IL-12Rβ2 on CD8+ gated OT-I T cells at 48 h. (B) RT-PCR of IL-12β2 in CD8+ selected OT-I T cells at 24 h. The β-actin mRNA serves as an internal control. (C) OT-I T cells were stimulated for 1 h or 20 h with 16.7% or 100% ratio of BOK/MEC II cells in the presence or absence of IL-12 (2 ng/ml). Cells were harvested and lysed and protein subjected to Western blot analyzed for STAT4 [phosphorylation (pSTAT4) and total] with specific antibodies, respectively. (D and E) Naive CFSE-labeled OT-I CD8+ T cells stimulated with 16.7% or 100% ratio of BOK/MEC II cells for 1 h or 20 h in the presence of IL-12 (2 ng/ml) were subjected to ICS for IFN-γ and flow cytometry analysis at 48 h and 72 h (D). The OT-I cells were harvested at 72 h and evaluated for cytolylic ability in a standard 4-h chromium release assay (E). The results are representative of three independent experiments.
To test whether CD8+ T cells rendered sensitive to IL-12 by high amounts of antigen stimulation would also augment IL-12-mediated effector differentiation, we next evaluated IFN-γ production and CTL activity of OT-I T cells stimulated with varying amounts of antigen in the absence or presence of IL-12 (2 ng/ml). As expected, the addition of IL-12 promoted robust differentiation of OT-I into IFN-γ-producing (Fig. 1D) cytolytic cells (Fig. 1E), in comparison with antigen only (data not shown). Notably, IL-12 combines with weak stimulation (1 h, 16.7%) to produce considerably less IFN-γ and cytolysis than with strong stimulation (20 h, 100%; Fig. 1, D and E). The addition of IL-12 to naïve OT-I cells failed to produce IFN-γ or cytolysis (data not shown), confirming the critical requirement for antigen signals for IL-12-mediated robust CD8+ effector maturation. The increase in CD8+ effector maturation achieved with strong antigen stimulation paralleled the ability of the amount of stimulation to regulate IL-12Rβ2 expression at mRNA and protein levels. Moreover, the increases in receptor expression also produced enhanced, IL-12-mediated STAT4 phosphorylation. These results confirm that the amount of antigen stimulation regulates OT-I cell responsiveness to IL-12 and suggest the potential role of increased IL-12Rβ2 expression in the observed sensitivity.
The Erk1/2 phosphorylation induced by antigen signal “tunes” for IL-12-mediated robust CD8+ T cell differentiation
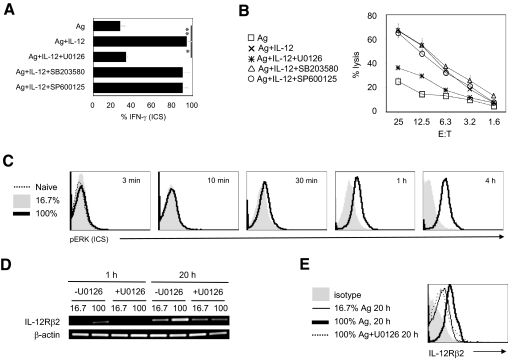
The engagement of the TCR by cognate antigen in the context of MHC class I activates several signaling cascades including the MAPK [29]. To investigate whether MAPK pathways are required by CD8+ T cells to bridge antigen and IL-12 signals, we pharmacologically inhibited the MAPK pathways (MEK) Erk1/2, p38, and JNK (with U0126, SB203580, and SP600125, respectively) and tested the ability of antigen plus IL-12 to produce OT-I effector maturation. Interestingly, the IL-12-mediated production of IFN-γ and cytotoxicity function was not reduced by inhibition of p38 and JNK pathways, but IL-12-mediated effector maturation was reduced significantly by inhibiting MEK1-mediated Erk1/2 phosphorylation (Fig. 2, A and B). To test whether the amount of antigen stimulation regulates Erk1/2 phosphorylation in OT-I cells, we monitored Erk1/2 phosphorylation in OT-I cells stimulated with varying amounts of antigen stimulation (Fig. 2C). As expected, weak antigen stimulation can induce detectable Erk1/2 phosphorylation by 4 h, which was augmented considerably in OT-I cells that received a high dose of antigen. Moreover, the induced Erk1/2 activation was persistent with stronger antigen stimulation (Fig. 2C; 30 min–4 h). These results demonstrate that the amount of antigen stimulation affects the kinetics as well as the extent of Erk1/2 phosphorylation, which was regulated for IL-12-mediated CD8+ T cell effector maturation.
Figure 2.
The activation of ERK is required for CD8+ effector maturation and the expression of IL-12Rβ2 in CD8+ T cells. (A and B) Naive OT-I T cells preincubated 1 h with U0126 (10 μM), SB203580 (10 μM), or SP600125 (10 μM) were cultured with BOK cells for an additional 20 h in the presence or absence of IL-12 (2 ng/ml). OT-I T cells were transferred, rested, and then harvested at 72 h. The percent of IFN-γ+CD8+ T cells was determined by ICS and flow cytometry analysis at 72 h (A). The cytolylic ability was evaluated in a standard 4-h chromium release assay (B). (C) Intracellular phospho-ERK (pERK) staining of CD8+ OT-I T cells stimulated with 16.7% or 100% ratio of APCs for indicated times. (D and E) Purified, naïve OT-I CD8+ T cells that had been incubated for 1 h with U0126 (10 μM) or DMSO were cultured for the indicated condition. (D) RT-PCR analysis of IL-12β2 mRNA at 1 h or 20 h after stimulation. The level of β-actin mRNA was used as an internal control. (E) Surface expression of IL-12Rβ2 on CD8+ gated OT-I T cells at 48 h. *, P < 0.05; **, P < 0.01. The results are representative of three independent experiments.
Based on these observations (Figs. 1 and 2), we predicted that Erk1/2-mediated IL-12Rβ2 expression facilitated collaboration between antigen signaling and IL-12 signaling. Indeed, inhibition of Erk1/2 phosphorylation by U0126 treatment decreased IL-12Rβ2 mRNA as well as IL-12Rβ2 protein induced by a high amount of antigen stimulation (Fig. 2, D and E). Thus, suggesting the specific role for the antigen-induced MEK pathway in collaborations with IL-12 generated signal for CD8+ T cell effector maturation. Although, these observations are in contrast with findings demonstrating that neither Erk1 nor Erk2 is required for CD8+ T cell effector maturation [19], it is envisaged that results obtained by the use of pharmacological inhibitors such as U0126 may be a result of its ability to block Erk1 and Erk2 signaling, whereas the genetic loss of a single Erk component was insufficient, perhaps as a result of compensatory effects.
The Erk1/2-mediated Ets-1 activation is required for CD8+ T cell effector differentiation and survival
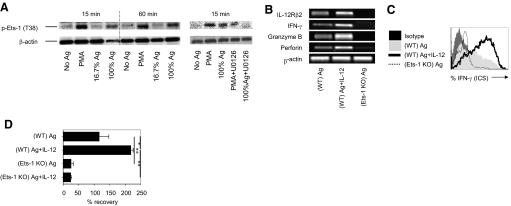
Based on a reported role of Erk1/2 signaling in regulating transcription factor Ets-1 activity (phosphorylation on T38) [22, 30], we hypothesized that the amount of antigen stimulation affects IL-12Rβ2 expression by controlling Erk1/2-mediated Ets-1 activation. To test this, naive CD8+ T cells were stimulated with various amounts of antigen for 15 min or 60 min and analyzed for phosphorylated Ets-1 (pT38) by Western blot analysis. As a positive control, naive CD8+ T cells were also stimulated with PMA, which has been shown to activate Ets-1. The activation of CD8+ T cells with PMA or antigen induced rapid phosphorylation of Ets-1 (pT38) in an antigen dose-dependent manner (Fig. 3 A, left panel). The level of Ets-1 phosphorylation was decreased after blocking the Erk1/2 signaling pathway by treatment with U0126 (Fig. 3A, right panel), thus indicating that the amount of antigen stimulation regulates Erk1/2-induced Ets-1 activation in CD8+ T cells.
Figure 3.
Ets-1 activation is required for CD8+ T cells effector function and survival. (A) Purified, naïve OT-I CD8+ T cells were stimulated with PMA (20 μM) or low or high frequency of APCs, with or without U0126 (10 μM). Cells were lysed after 15 min or 60 min. Induction of Ets-1 phosphorylation (p-Ets-1) was detected by Western blot analysis. (B–D) CD8+ T cells derived from wild-type (WT) and Ets-1-deficient [knockout (KO)] mice were stimulated with anti-CD3 mAb (1 μg/ml) and anti-CD28 mAb (0.5 μg/ml). (B) RT-PCR analysis of indicated genes at 48 h. (C) ICS and flow cytometry analysis for IFN-γ at 72 h. (D) Percent cell recovery at 72 h. *, P < 0.05; **, P < 0.01. The results are representative of three independent experiments.
To test directly whether Ets-1 activation was required for antigen strength-dependent IL-12Rβ2 expression and CD8+ effector maturation, we analyzed mRNA levels for IL-12Rβ2 and effector-related genes (IFN-γ, granzyme B, and perforin) in wild-type and Ets-1 knockout CD8+ T cells. Indeed, IL-12 enhanced antigen-induced expression of IL-12Rβ2 and effector-related mRNA (Fig. 3B). The antigen-induced steady-state mRNA for IL-12Rβ2 and effector-related genes (IFN-γ, granzyme B, and perforin) was decreased significantly in purified CD8+ T cells derived from Ets-1-deficient mice (Fig. 3B). Importantly, IFN-γ production and cell survival enhanced by IL-12 addition were reduced considerably in Ets-1 knockout CD8+ T cells (Fig. 3, C and D), therefore supporting the notion that antigen-induced Erk1/2 alters Ets-1 transcriptional activity, which sensitizes CD8+ T cells for IL-12-mediated effector differentiation and survival. These novel observations extend the role of Ets-1 in CD8+ T cell effector maturation and provide mechanistic insights for collaboration between the amount of antigen stimulation and IL-12 for CD8+ effector functions. Although, the validity of this pathway in CD4+ T cells is uncertain, the use of this information is likely to facilitate integration of signal 1 and signal 3 for CD8+ T cell-mediated therapeutic benefits.
The amount of antigen stimulation tunes CD8+ T cells for an IL-12 response
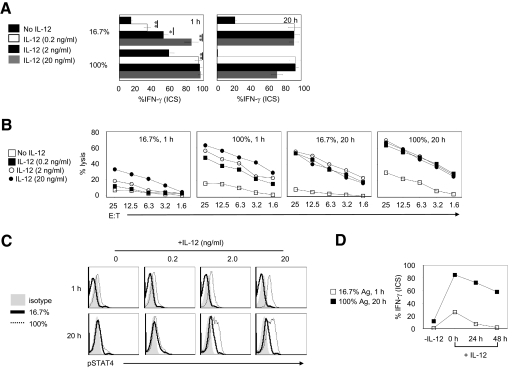
The ability of a higher amount of antigen stimulation to enhance Erk1/2/Ets-1-dependent IL-12Rβ2 expression suggests that the amount of antigen stimulation tunes CD8+ T cells for IL-12-mediated effector maturation. To test this and determine whether the “tuning” was dose- and time-sensitive, we first stimulated OT-I T cells with 16.7% or 100% antigen presenting for 1 h or 20 h in the presence of varying doses of IL-12 (0.2–20 ng/ml) and evaluated their effector maturation phenotype. As shown in Figure 4A, OT-I T cells stimulated with weak antigen (16.7%; 1 h) required higher doses of IL-12 (20 ng/ml) to produce moderate levels of IFN-γ as well as CTL activity. This effect was overcome by extending the duration of low-dose antigen stimulation (Fig. 4, A and B); however, persistent stimulation with a higher dose produced considerably higher effector maturation as evidenced by increased CTL activity (Fig. 4B; 16.7% 20 h vs. 100% 20 h). As expected, OT-I cells stimulated with strong stimulation show increased STAT4 phosphorylation in response to IL-12 addition in an antigen dose-dependent manner (Fig. 4C). These observations indicate that higher amounts of antigen stimulation increase CD8+ T cell sensitivity for IL-12 in an antigen strength-dependent manner. Furthermore, when the addition of IL-12 to OT-I cells stimulated with varying amounts of antigen was delayed after antigen stimulation, the high amount of antigen-stimulated OT-I cells was able to integrate IL-12 signals, as determined by effector maturation (Fig. 4D), thus pointing to the ability of the amount of antigen stimulation to impart plasticity for integrating antigen and IL-12 signals to program effector CD8+ T cell maturation, which has profound implications for immunotherapy.
Figure 4.
The amount of antigen stimulation tunes CD8+ T cells for IL-12-conditioned effector maturation. Naive OT-I T cells cultured in various conditions were transferred into new wells containing media alone or IL-12 (0, 0.2, 2, and 20 ng/ml). (A) The OT-I cells were evaluated for IFN-γ production by ICS and flow cytometry analysis at 72 h. (B) The OT-I cells were evaluated for cytolylic ability in a standard 4-h chromium release assay at 72 h. (C) ICS for phospho-STAT4 at 48 h. (D) IL-12 (2 ng/ml) was added at 0, 24, and 48 h after OT-I T cells transfer. OT-I T cells were harvested and evaluated for IFN-γ production by ICS and flow cytometry analysis at 72 h.*, P < 0.05; **, P < 0.01. Experiments shown were repeated three times with identical outcomes.
The amount of antigen stimulation and IL-12 conditions CD8+ T cells for long-term recall responses
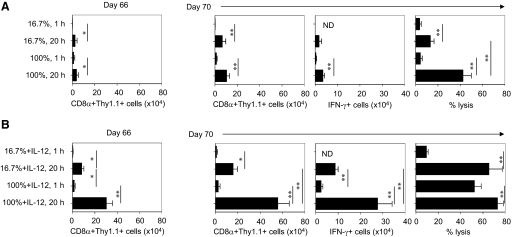
As antigen dose and duration of antigen stimulation regulated OT-I responsiveness to IL-12 in vitro, we next tested whether the amount of antigen stimulation, with or without IL-12, affected long-term CD8+ antigen-recall responses. We enumerated OT-I cells (CD8α+Thy1.1+), which were stimulated with Ag/B7.1, in the presence or absence of IL-12 after adoptive transfer on Day 66. Only few OT-I cells were detected in recipients of weak antigen-stimulated OT-I cells (16.7%, 1 h), and significantly increased numbers of OT-I cells were detected in host receiving stronger antigen stimulation (100%, 20 h; Fig. 5A, left panel). However, the highest number of OT-I cells was found in host receiving IL-12-conditioned, stronger antigen-stimulated OT-I cells (100%+IL-12, 20 h; Fig. 5B, left panel), thus demonstrating that a high amount of antigen stimulation collaborates with IL-12 signaling for CD8+ T cell persistence. We then tested the ability of these cells to mount a secondary antigen-specific response by evaluating the OT-I numbers and effector functions 4 days after immunization on Day 66. There was a significant increase in OT-I cell numbers detected in host receiving OT-I cells with a longer duration of stimulation but no significant difference in OT-I cell numbers detected in host receiving a short duration of stimulation (16.7% 1 h or 100% 1 h) before and after antigen challenge (Fig. 5A, Day 70, right panel). In recipients of IL-12-conditioned, stronger antigen-stimulated OT-I cells (100%+IL-12, 20 h), the OT-I cell number was increased dramatically compared with the other recipients (Fig. 5B, Day 70, right panel). Moreover, in those mice that received IL-12-conditioned, stronger antigen-stimulated OT-I cells, more IFN-γ production and cytolytic ability were detected (Fig. 5B, Day 70, right panel). Surprisingly, a long duration of stimulation by itself induced considerable CTL maturation in the absence of significant IFN-γ production (Fig. 5A, Day 70, right panel). The OT-I cells stimulated for longer duration demonstrated greater persistence and antigen-recall abilities at Days 66–70 after adoptive transfer, thus suggesting that the duration of antigen stimulation has a profound impact on the OT-I cell in the presence or absence of IL-12 for long-term immune responses. The presence of IL-12 augments the response of OT-I detected after antigen rechallenge, particularly the frequency of IFN-γ-producing OT-I T cells and their ability to produce CTL activity (Fig. 5B), lending support to the notion that CD8+ T cells stimulated with a high amount of stimulation in the presence of IL-12 may undergo programmed differentiation that enables them with the ability to persist and to generate long-term immune responses. This observation is consistent with previous studies that show that longer duration of antigen stimulation induces sustained clonal explanation and effector maturation of CD8+ T cells in vivo [2, 3]. As the in vivo observations are in agreement with the ability of amount of stimulation to enhance CD8+ T cell persistence, we submit that sustenance of antigen stimulation to the CD8+ T cell is crucial for achieving the durable immune response, and thus, new immunotherapy strategies should focus on long-term persistence to enhance efficacy of CD8+ T cell adoptive therapy. However, it should be noted that in certain disease conditions such as cancer, the tumor antigen-specific CD8+ T cells can exhibit an “anergic” or “unresponsive” state, and thus, the ex vivo conditions required for their activation and differentiation may be different.
Figure 5.
High amount of antigen and IL-12 is required for CD8+ T cells long-term antigen-recall response. Naïve OT-I/PL cells stimulated with 16.7% or 100% ratio of BOK/MECII cells with or without IL-12 were harvested at 72 h and adoptively transferred (2×106 cells) into naïve B6 recipients. The recipients were challenged with 5 μg OVA in IFA on Day 66, and secondary CD8+ T cell responses were measured 4 days after the challenge. (A) The absolute number of adoptively transferred antigen conditioned OT-I T cells on Day 66 (left panel). The absolute number of OT-I cells, the absolute numbers of IFN-γ-secreting OT-I T cells, and the in vivo antigen-specific cytolysis after challenge in recipients received antigen-conditioned OT-I cells (right panel). (B) The absolute number of adoptively transferred antigen plus IL-12 conditioned OT-I T cells on Day 66 (left panel). The absolute number of OT-I cells, the absolute numbers of IFN-γ-secreting OT-I T cells, and the in vivo antigen-specific cytolysis after challenge in recipients received antigen plus IL-12-conditioned OT-I cells (right panel). Representative data of two independent experiments are shown. ND, Not determined. *, P < 0.05; **, P < 0.01.
Collectively, our study has provided new insights into mechanisms regulating collaboration between the amount of antigen stimulation and IL-12 (dose and kinetic integration). Moreover, by demonstrating the impact of this collaboration on CD8+ T cells effector maturation and long-term responses, we have identified new targets for therapeutic modulation.
AUTHORSHIP
Q. L. designed/conducted experiments, analyzed data, and wrote the manuscript; C. E. assisted with experiments; K. O. contributed discussion and interpretation of results; and P. A. S. directed the project, designed experiments, interpreted results, and helped write the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from National Institutes of Health-National Cancer Institute (R01 CA104645) and Alliance Foundation of Roswell Park (P. A. S.).
Footnotes
Abbreviations: B6=C57BL/6 mice, ICS=intracellular staining
References
- Kaech S M, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stipdonk M J, Lemmens E E, Schoenberger S P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- Van Stipdonk M J, Hardenberg G, Bijker M S, Lemmens E E, Droin N M, Green D R, Schoenberger S P. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Benvenuti F, Lagaudriere-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, Lantz O, Amigorena S. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- Prlic M, Hernandez-Hoyos G, Bevan M J. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storni T, Ruedl C, Renner W A, Bachmann M F. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J Immunol. 2003;171:795–801. doi: 10.4049/jimmunol.171.2.795. [DOI] [PubMed] [Google Scholar]
- Quigley M, Huang X, Yang Y. Extent of stimulation controls the formation of memory CD8 T cells. J Immunol. 2007;179:5768–5777. doi: 10.4049/jimmunol.179.9.5768. [DOI] [PubMed] [Google Scholar]
- Curtsinger J M, Lins D C, Mescher M F. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J M, Johnson C M, Mescher M F. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- Li Q, Eppolito C, Odunsi K, Shrikant P A. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- Chang J, Cho J H, Lee S W, Choi S Y, Ha S J, Sung Y C. IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol. 2004;172:2818–2826. doi: 10.4049/jimmunol.172.5.2818. [DOI] [PubMed] [Google Scholar]
- Pearce E L, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Keppler S J, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur J Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- Afkarian M, Sedy J R, Yang J, Jacobson N G, Cereb N, Yang S Y, Murphy T L, Murphy K M. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Thieu V T, Yu Q, Chang H C, Yeh N, Nguyen E T, Sehra S, Kaplan M H. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Ashwell J D. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- D'Souza W N, Chang C F, Fischer A M, Li M, Hedrick S M. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman L S, Lee J T, Shelton J G, Navolanic P M, Blalock W L, Franklin R A, McCubrey J A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Grenningloh R, Miaw S C, Moisan J, Graves B J, Ho I C. Role of Ets-1 phosphorylation in the effector function of Th cells. Eur J Immunol. 2008;38:1700–1705. doi: 10.1002/eji.200738112. [DOI] [PubMed] [Google Scholar]
- Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho I C. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel G, Frucht D M, Fleisher T A, Holland S M. Detection of intracellular phosphorylated STAT-4 by flow cytometry. Clin Immunol. 2001;100:270–276. doi: 10.1006/clim.2001.5078. [DOI] [PubMed] [Google Scholar]
- McNeil L K, Starr T K, Hogquist K A. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci USA. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Segal B H, Eppolito C, Li Q, Dennis C G, Youn R, Shrikant P A. Aspergillus fumigatus extract differentially regulates antigen-specific CD4+ and CD8+ T cell responses to promote host immunity. J Leukoc Biol. 2006;80:529–537. doi: 10.1189/jlb.0106026. [DOI] [PubMed] [Google Scholar]
- Shrikant P, Khoruts A, Mescher M F. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL)-12R β 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberola-Ila J, Forbush K A, Seger R, Krebs E G, Perlmutter R M. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- Yang B S, Hauser C A, Henkel G, Colman M S, Van Beveren C, Stacey K J, Hume D A, Maki R A, Ostrowski M C. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]