Abstract
In the absence of IL-10, colonic inflammation ensues, which is characterized by high levels of IL-17. Here, we demonstrate a direct correlation between ICOS expression and IL-17 production in cIELs. IL-10−/− mice had increased numbers of cIELs and greater colon weight. Although the CD69 early activation antigen was expressed on cIELs from normal and IL-10−/− mice, ICOS was expressed only on cIELs from IL-10−/− mice. IL-17-producing cells in IL-10−/− mice consisted of CD4+ and CD8+ cIELs; however, CD4+ cells were the predominant IL-17-producing cell population. Culture of cIELs from IL-10−/− mice with IL-23 resulted in an increase in ICOS and IL-17 expression, whereas IL-10 suppressed expression of ICOS and IL-17. This occurred in primary cultures and recall stimulation experiments. The ICOS ligand B7RP-1 was up-regulated on colonic epithelial cells and on a population of large granular leukocytes during inflammation. Culture of cIELs with B7RP-1+ DCs enhanced IL-17A production from normal cIELs but failed to do so using cIELs from ICOS−/− mice. In vivo treatment of IL-10−/− mice with antibody to ICOS resulted in a significant reduction in colonic pathology. These findings implicate ICOS as an activational signal of Th17 cells during chronic intestinal inflammation, and they suggest that under some conditions, control of ICOS expression may help to suppress chronic intestinal inflammation.
Keywords: cytokines, inflammation, knockout, mucosa, rodent
Introduction
IL-10 is a 37-kDa homodimer cytokine with broad, immunosuppressive effects [1]. IL-10−/− mice develop Th1-like colonic inflammation and extensive cytokine dysregulation, which model aspects of IBD [2,3,4]. IL-17 is a 35-kDa homodimer with strong, proinflammatory activity [5]. A subset of peripheral T cells has been described that is dedicated to the production of IL-17, consisting of CD4+ (Th17) and CD8+ (Tc17) IL-17-secreting T cells. IL-23, with IL-6, IL-21, and TGF-β, has been shown to drive the differentiation of Th17 cells [6,7,8]. Although the IL-23/IL-17 pathway plays a role in the host immune response during acute infection [9], aberrant expression of IL-17 has been implicated in a number of autoimmune diseases and chronic inflammatory conditions [10,11,12,13,14,15]. IL-17 expression can be inhibited by IL-23p19 blockade [16], and expression of the IL-23R has been linked to Crohn’s disease and ulcerative colitis [17]. A definitive role of Th17 cells in the development of colitis is evident from studies in retinoid-related orphan receptor-γt mice, which lack the transcriptional factor necessary for differentiation of Th17 cells and fail to develop colitis in an adoptive transfer model [18].
ICOS has also been implicated in colonic inflammation [19]. As a member of the CD28/CTLA4 family of coactivational surface receptors, ICOS is important for T cell activation and function [20,21,22]. Whereas CTLA4 down-regulates T cell responses, CD28 and ICOS initiate and promote T cell activation. Spleen cells isolated from ICOS−/− mice produce significantly less IL-17 in comparison with normal animals [23]. Conversely, ICOS stimulation of naïve, splenic T cells from normal mice increases IL-17 production [23, 24]. ICOS hyperexpression is observed in patients with IBD [19].
The goal of the present study was twofold. First, whereas several studies have examined the role of lamina propria lymphocytes in colonic inflammation [25, 26], less is known about the involvement of cIELs in the local inflammatory response. However, because of their strategic location in the intestinal mucosa, cIELs may be a contributing factor to the inflammatory response of IBD. Second, we were interested in examining the relationship between ICOS and IL-10 in the regulation of IL-17 synthesis. Our findings indicate that activation of cIELs through ICOS results in a stronger IL-17 response and that IL-10 ameliorates that response in part by modulating ICOS expression.
MATERIALS AND METHODS
Animals
BALB/c, C57BL/6, and IL-10−/− BALB/c mice were purchased from Harlan (Indianapolis, IN, USA) or from The Jackson Laboratory (Bar Harbor, ME, USA). ICOS−/− mice were purchased from The Jackson Laboratory. Animals were used according to the University of Texas Health Science Center at Houston Committee on Animal Welfare and Use (Houston, TX, USA).
Cell isolation and culture
Isolation of cIELs was done as reported previously [15]. For intracellular IL-17 and IFN-γ staining of cIELs from IL-10−/− and BALB/c mice, 0.5 × 106 cells/ml were cultured overnight in RPMI-1640 media supplemented with 10% FBS, penicillin/streptomycin, and 2 μM L-glutamine in 6- or 24-well plates at 37°C with 5% CO2. The next day, 0.66 μl/ml (2 μM) BD GolgiStop™ (Monensin, BD Biosciences, San Diego, CA, USA) was added to the cultures for 5 h prior to the intracellular staining.
To determine the effects of rIL-23 and rIL-10 on cIELs from IL-10−/− mice, 10 ng/ml mouse rIL-23 (eBioscience, San Diego, CA), 50 ng/ml mouse rIL-10 (BD Biosciences), or PBS (media control) was added to the cIELs to overnight cultures as detailed above.
For the ELISA supernatants, cIELs were cultured for 24 h and 48 h in supplemented RPMI 1640 under Th17-inducing conditions [5 ng/ml TGF-β, 10 ng/ml rIL-23, 20 ng/ml IL-6 (all eBioscience), 50 ng/ml PMA, 750 ng/ml ionomycin (all Sigma-Aldrich, St. Louis, MO, USA), and 1 μg/ml anti-CD3ε antibody (BD Biosciences)] in the presence or absence of irradiated bone marrow DCs, which were generated in vitro using published protocols [27]. Bone marrow cells isolated from mouse femurs were cultured at 2 × 106 cells/ml in supplemented RPMI 1640 containing mouse rGM-CSF (eBioscience). The bone marrow cells were fed every 2–3 days for up to 11 days. Lightly adhered cells were harvested and exposed to 3000 rad irradiation from a 137Cs Gammacell 1000 irradiator (Atomic Energy of Canada, Nordion, Ontario, Canada). DCs were seeded into cIEL cultures at a ratio of 1:10.
Histopathology
Mice were killed, and the mid-portions of the proximal and distal colon were harvested, fixed in 10% buffered formalin, and processed for paraffin embedding. Representative tissue sections (3 μM) were made from different regions of colon and stained with H&E. The degree of inflammation and related histologic changes was graded microscopically on cross-sections of the colon using a validated scoring system as described in our previously published paper. This histopathologic scoring system consisted of score 0: no signs of inflammation; score 1: very low level of leukocytic infiltration in the lamina propria; score 2: low level of leukocytic infiltration in the lamina propria; score 3: moderate level of leukocytic infiltrate in the lamina propria with occasional crypt abscess; score 4: high levels of leukocytic in the lamina propria, crypt abscess, high vascular density, loss of goblet cells, and thickening of the colon wall; and score 5: transmural leukocytic infiltration, loss of goblet cells, high vascular density, and thickening of the colon wall. An experienced, board-certified pathologist performed histopathologic grading in a blinded manner.
In vivo treatment of mice with anti-ICOS antibody
Two cohorts of four IL-10−/− mice were used; each group was injected daily for 10 days with 10 μg purified anti-ICOS antibody (clone 7E.17G9) or purified rat IgG2b control antibody (clone RTK4530, BioLegend, San Diego, CA, USA). Mice were killed on the day after the final injection, and colonic tissues were prepared for histopathological analyses.
Cell staining and flow cytometry
Antibodies used were PE-anti-CD3 and purified NA/LE anti-CD3, PE- and FITC-anti-CD4, PE- and FITC-anti-CD8α (BD Biosciences), PE-anti-ICOS, AlexaFluor-647-IL-17A, FITC-anti-IFN-γ, biotin-B7RP-1, PE-anti-CD45 LCA, PE-anti-G8.8, PE-anti-CD69, unlabeled anti-CD16/32, biotinylated rat IgG2a, and streptavidin-allophycocyanin (all eBioscience). Cell-surface and intracellular staining was done as reported previously [15, 28, 29]. Stained cells were analyzed using a FACSCalibur flow cytometer with CellQuest software (BD Biosciences).
RNA isolation and qRT-PCR
Total RNA was isolated from the cIELs using the RNeasy Protect Minikit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. A SYBR Green qRT-PCR was used to analyze mRNA expression of the Il17A, Icos, and GAPDH genes using the iScript One-Step RT-PCR kit with SYBR Green (BioRad, Hercules, CA, USA), according to the manufacturer’s instructions. Primers were purchased from IDT Technologies (Coralville, IA, USA); IL-17 forward: 5′-ccccagctcgaagatagcatcagtgtatt-3′, reverse: 5′-cccaagcttgtttgcgcgtcctgatca-3′; ICOS forward: 5′-cccaagcttgagcagtcattgagaggc-3′, reverse: 5′-cccaagcttagtgctcaaaagtgtcag-3′; and GAPDH forward: 5′-agaacatcatccctgcatcc-3′, reverse: 5′-agccgtattcattgtcatacc-3′. Relative gene expression was calculated using the 2–ΔΔct method of Livak and Schmittgen [30] after normalizing to GAPDH.
ELISA
Mouse IL-17A protein levels were quantified using a commercially available mouse IL-17A ELISA kit (eBioscience), according to the manufacturer’s instructions. Cell-free supernatants were collected from cIELs cultured for the indicated time periods and conditions.
Statistical analyses
Calculations of Pearson correlation coefficients and comparisons of mean values for statistical significance using a two-tailed t-test were done with Microsoft Excel software.
RESULTS
IL-17 and ICOS are up-regulated in cIELs of IL-10−/− mice with colonic pathology
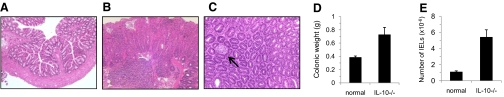
Colon tissue sections were examined microscopically, and the degree of inflammation and related histopathologic changes were graded using a scoring system reported previously by our laboratory [15] and as described in Materials and Methods. IL-10−/− mice used in this study exhibited variable degrees of colitis with the histopathologic scores of 2–4 (Fig. 1B). Colonic sections from IL-10−/− mice with high histopathologic scores frequently had evidence of crypt abscesses (Fig. 1C). In contrast, pathologic changes in colonic sections of age-matched control BALB/c mice were minimal (score ≤1.0; Fig. 1A). IL-10−/− mice with colonic inflammation had significantly greater colon weight (Fig. 1D) and significantly more cIELs (Fig. 1E) than age-matched, normal BALB/c mice.
Figure 1.
Colonic tissue sections. Colonic tissue sections from (A) a normal BALB/c mouse and (B) an IL-10−/− mouse with grade 3 histopathology. (C) Presence of crypt abscess (arrow) in colonic tissue section from an IL-10−/− mouse with intestinal pathology. (D) Colonic weight and (E) numbers of IELs from normal BALB/c mice and age-matched IL-10−/− mice with intestinal pathology; mean values ± sem of 18 mice.
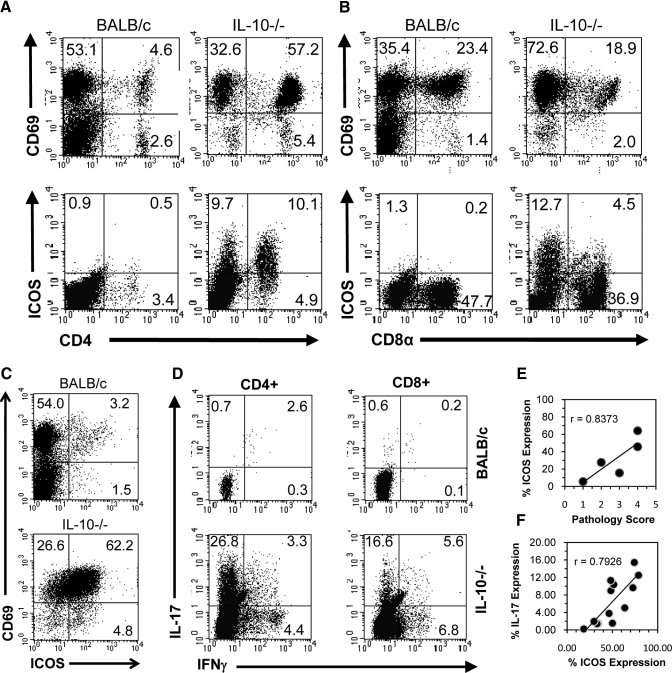
To examine the relationship between IL-17 and ICOS in IL-10−/− mice, cIELs were isolated and stained for intracellular IL-17 expression and surface expression of ICOS in conjunction with CD4 and CD8α. ICOS was expressed on CD4+ and CD8α+ cIELs from IL-10−/− mice, although it was not expressed on cIELs from normal mice (Fig. 2, A and B). Although cIELs from normal mice expressed the CD69 early activation antigen, they did not express ICOS (Fig. 2, A and B). The percent of CD4+ CD69+ T cells increased in IL-10−/− mice compared with normal BALB/c mice, suggesting that there was an influx of activated CD4+ T cells; in contrast, the percent of CD8α+ CD69+ cells remained similar in IL-10−/− and BALB/c mice (Fig. 2, A and B). Overall, the majority of cIELs in IL-10−/− mice expressed ICOS and CD69 (Fig. 2C). In IL-10−/− mice with histopathologic scores >1.0, CD4+ and CD8α+ cIELs were IL-17-producing cells, although the CD4+ subset was the predominant source of IL-17 (Fig. 2D). By comparison, <1% of cIELs in age-matched, normal BALB/c mice expressed IL-17 (Fig. 2D). These finding were consistent in multiple cIEL isolates from IL-10−/− and BALB/c mice. Based on Pearson correlation analyses, there was a strong positive correlation (r=0.8373) between cIEL ICOS expression and colonic histopathology (Fig. 2E), indicating that ICOS expression increased with disease severity. Similarly, there was a strong positive correlation (r=0.7926) between cIEL ICOS expression and IL-17 production (Fig. 2F). These data indicate that the absence of IL-10 results in the dysregulation of IL-17 and ICOS expression.
Figure 2.
Percent CD69 and ICOS expression. (A) Percent CD69 and ICOS expression between CD4+ and (B) CD8α+ cIELs in fresh isolates from BALB/c and IL-10−/− mice. (C) Note that ICOS is expressed on few cIELs in normal mice despite the expression of CD69 but is up-regulated in IL-10−/− mice. (D) IL-17+ and IFN-γ+ cells among CD4+ and CD8α+ cIELs in fresh isolates from normal BALB/c and IL-10−/− mice. Pearson correlation analysis of (E) ICOS expression versus pathology score and (F) IL-17 expression versus ICOS expression in cIELs from IL-10−/− mice with intestinal pathology. Data are representative of findings from five to 12 mice.
ICOS+ cIELs in IL-10−/− mice are the primary source of IL-17, and IL-17 and ICOS expression is under negative regulation by IL-10
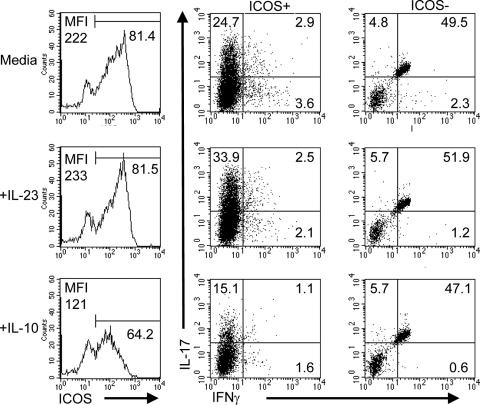
cIELs from IL-10−/− mice were cultured for 24 h with medium alone, medium plus rIL-23, or medium plus rIL-10. Cells were collected and stained for expression of ICOS, IL-17, and IFN-γ. IL-17-producing cells were ICOS+ cells (Fig. 3A). Moreover, culture with rIL-23 resulted in an increase in expression of ICOS and IL-17 in comparison with cIELs cultured in medium alone, whereas culture with rIL-10 suppressed ICOS expression and IL-17 synthesis (Fig. 3A). These findings were consistent in multiple cultures of cIELs from IL-10−/− mice (Fig. 3, B–D). The reduction in ICOS and IL-17 expression following exposure to rIL-10 was confirmed at the transcriptional level (Fig. 3E).
Figure 3.
ICOS and IL-17 expression are up-regulated by IL-23 and suppressed by IL-10. cIELs from IL-10−/− mice were cultured in the absence of CD3 stimulation for 24 h in the presence of media, media plus rIL-23, or media plus rIL-10. (A) rIL-23 increased the percent of IL-17-producing cells and the (C) MFI of ICOS expression. IL-10 supplementation resulted in (A and B) a decrease in ICOS expression and (C) a decrease in the MFI of ICOS expression. (D) rIL-23 supplementation resulted in an overall increase in the percent of ICOS+, IL-17+ cells, whereas IL-10 supplementation resulted in a decrease in the percent of ICOS+, IL-17+ cells. (E) Levels of ICOS and IL-17 gene expression were lower in cIELs from IL-10−/− mice following culture with rIL-10. Data are derived from three to five independent experiments.
To determine whether the effects of IL-23 and IL-10 exposure on cIELs from IL-10−/− mice were retained following recall stimulation, cIELs were cultured for 24 h with anti-CD3 antibody with medium alone, medium plus rIL-23, or medium plus rIL-10. Recall stimulation in the presence of rIL-23 increased the percent of IL-17+ cells in the ICOS+ population, whereas rIL-10 exposure decreased the percent of IL-17+ cells (Fig. 4). Additionally, the MFI of ICOS expression was increased by exposure to rIL-23 and decreased by rIL-10 exposure relative to cells cultured in medium alone (Fig. 4). The high percentage of IL-17+ IFN-γ+-positive cells in the ICOS– population may be a result of autofluorescence of those cells.
Figure 4.
The effects of IL-23 and IL-10 exposure on cIELs from IL-10−/− mice were retained following recall stimulation. For recall stimulation, cIELs from IL-10−/− mice stimulated in vitro for 24 h with 1 μg/ml plate-bound anti-CD3 antibody in the presence of medium, medium plus rIL-23, or medium plus rIL-10. Recall stimulation in the presence of rIL-23 increased the percent of IL-17+ cells in the ICOS+ population, whereas IL-10 exposure decreased the percent of IL-17+ cells. Similarly, the MFI of ICOS expression was increased by exposure to rIL-23 and decreased by IL-10 exposure relative to cells cultured in medium alone. The high percentage of IL-17+ IFN-γ+-positive cells in the ICOS– population may be a result of autofluorescence of dead cells. Data are representative of two independent experiments.
The B7RP-1 molecule displays a different pattern of distribution on leukocytes and enterocytes in the colon of IL-10−/− and normal mice, and exposure to B7RP-1-bearing cells increases IL-17 production by cIELs
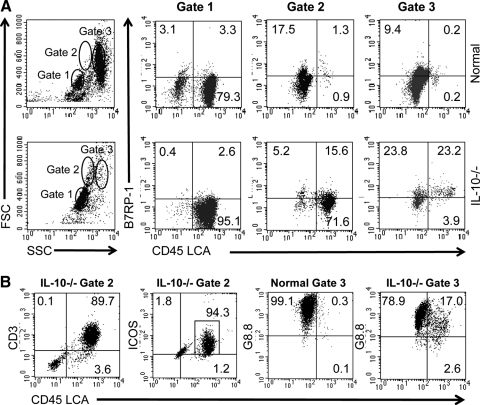
The B7RP-1 ICOS ligand is expressed on APCs and epithelial cells [28]. As B7RP-1 provides a stimulatory signal for activation through ICOS, we examined B7RP-1 expression on colonic leukocytes and enterocytes that were discriminated by CD45 into LCA+ hematopoietic cells and LCA– nonhematopoietic cells. Three cell populations were defined by flow cytometry, according to physical characteristics (Fig. 5A). In normal and IL-10−/− mice, cells in Gate 1 were 80–95% LCA+ cells with physical properties of cIELs. Cells in Gate 2 differed significantly in normal and IL-10−/− mice in that nearly all of these cells in normal mice were nonleukocytes, most likely enterocytes, whereas cells in Gate 2 from IL-10−/− mice were predominantly leukocytes. The latter cells, which were most likely macrophages and/or DCs, were larger and more granular than cIELs. Cells in Gate 3 were predominantly LCA– nonleukocytes in both groups; however, a significant percent, approximately one-fourth, included LCA+ leukocytes in IL-10−/− mice. The latter was only present in isolates from IL-10−/− mice.
Figure 5.
The B7RP-1 ICOS ligand is up-regulated on nonhematopoietic cells and leukocytes in the intestinal epithelium of IL-10−/− mice. (A) Freshly isolated cells consisting of cIELs and enterocytes from the colonic epithelium of normal BALB/c and IL-10−/− mice were stained for expression of CD45 LCA and B7RP-1. Cells were defined as hematopoietic cells based on CD45 LCA expression or nonhematopoietic cells based on a lack of CD45 LCA expression. Three populations (Gates 1, 2, and 3) in cell isolates from each animal type were defined by properties of forward or side scatter (FSC/SSC, respectively) by flow cytometry. Two notable differences were observed in normal versus IL-10−/− mice. First, there was a large percent of large granular LCA+ cells in the Gate 2 population of IL-10−/− mice that did not exist in normal animals. Second, approximately one-half of all of the cells in the Gate 3 population of IL-10−/− mice was B7RP-1+ cells, which consisted of roughly equivalent percentages of hematopoietic and nonhematopoietic cells. To characterize the cells in Gate 2, freshly isolated cIELs from IL-10−/− mice were stained for CD3 and ICOS expression. (B) The majority of those cells included CD3+, ICOS+, and LCA+ cells, indicating that they were activated T cells. Additionally, cells from normal and IL-10−/− mice were stained using the G8.8 antibody that reacts with Ep-CAM on epithelial cells and some APCs [31, 32]. Cells from normal mice in Gate 3 were almost entirely G8.8+, LCA– epithelial cells, whereas some cells in Gate 3 from IL-10−/− mice were G8.8+, LCA+ leukocytes that were probably macrophages or DCs. This implies that there is a loss of epithelial cell homeostasis in IL-10−/− with colonic pathology. Data are representative of two to three independent experiments.
B7RP-1 expression also differed notably in IL-10−/− and normal mice in that the B7RP-1 antigen in Gates 2 and 3 in normal mice was expressed principally on LCA– cells (Fig. 5A), whereas B7RP-1 in IL-10−/− mice was expressed on LCA– and LCA+ cells in Gates 2 and 3. B7RP-1 expression on colonic cells in Gate 3 from IL-10−/− mice was distributed equally between nonleukocytes and large granular leukocytes (Fig. 5A, Gates 2 and 3). Based on control staining using an isotype control antibody, the fluorescence in Gate 3 of cIELs from IL-10−/− mice was not a result of autofluorescence (data not shown). Thus, in colonic inflammation, there is an influx of B7RP-1+ leukocytes into the epithelium.
cIELs from IL-10−/− mice were stained for CD3 and ICOS expression to characterize the cells in Gate 2. The majority of those cells included CD3+, ICOS+, LCA+ cells (Fig. 5B), indicating that they were activated T cells. Additionally, cells from normal and IL-10−/− mice were stained with the G8.8 antibody that reacts with Ep-CAM on epithelial cells and some APCs [31, 32]. Cells from normal mice in Gate 3 were almost entirely G8.8+ epithelial cells, whereas although the majority of cells in Gate 3 from IL-10−/− mice included G8.8+ and LCA– cells some G8.8+ and LCA+ cells were present (Fig. 5B). This suggests that there is an influx of macrophages and/or DCs in the epithelium of IL-10−/− mice that is associated with a loss of epithelial cell homeostasis.
ICOS stimulation of cIELs results in higher levels of IL-17A production, and treatment of IL-10−/− mice with anti-ICOS antibody reduces colonic pathology
To understand how B7RP-1+ leukocytes might contribute to IL-17 production, cIELs from normal BALB/c mice were cultured with or without bone marrow-derived DCs in the presence of an IL-17-inducing cocktail as described in Materials and Methods. Bone marrow DCs were confirmed by flow cytometry to express the B7RP-1 molecule (Fig. 6A). cIELs cultured with B7RP-1-bearing DCs in the presence of an IL-17-inducing cocktail (media plus DCs) produced significantly more IL-17A than cells cultured with the IL-17 cocktail alone (media); IL-17A was not produced by DCs cultured by themselves with the IL-17-inducing cocktail (DCs plus media; Fig. 6B).
Figure 6.
IL-17 expression and pathology are regulated by ICOS signaling. (A) Bone marrow DCs express the B7RP-1 ICOS ligand. (B) cIELs from normal BALB/c mice cultured with IL-17-inducing cocktail in the presence of DCs (media plus DCs) produced more IL-17A as determined by ELISA than cIELs cultured with IL-17-producing cocktail alone (media). No IL-17A was produced by DCs by themselves when cultured with IL-17-inducing cocktail (DCs plus media); data are mean values of three replicate samples. (C) cIELs cultured in media plus DCs resulted in a statistically significant increase in IL-17A production. Culture of cIELs from ICOS−/− mice with B7RP-1+ DCs failed to result in elevated levels of IL-17A production. Data for A–C are representative of two to three independent experiments. (D) IL-10−/− mice treated for 10 days with 10 μg anti-ICOS antibody (n=4) have statistically significant, lower pathology scores compared with mice treated with 10 μg isotype control Ig (n=4).
To confirm that the increased IL-17 production following stimulation was associated with ICOS signaling, cIELs from ICOS−/− mice and from normal mice were cultured in the presence of the IL-17-inducing cocktail, with or without syngeneic C57BL/6 DCs. Normal cIELs had a statistically significant increase in IL-17A production in the presence of DCs, whereas cIELs from ICOS−/− mice cultured with syngeneic DCs failed to elicit higher IL-17A production (Fig. 6C). In vivo treatment of IL-10−/− mice with anti-ICOS antibody resulted in a statistically significant reduction in colonic pathology (Fig. 6D). As determined by intracellular staining for IL-17, treatment of IL-10−/− mice with anti-ICOS antibody did not alter the number of IL-17+ cells (data not shown), possibly indicating that ICOS-independent pathways exist for IL-17 expression, as suggested by others [9, 33]. Thus, the presence of B7RP-1 on professional APCs such as DCs contributes to the propagation of IL-17 within the colonic epithelium, and stimulation of ICOS via B7RP-1 results in greater IL-17 synthesis by cIELs.
DISCUSSION
The goal of this study was to examine the role of cIELs in colonic inflammation with specific emphasis on the causative factors that determine IL-17 expression. The findings reported here extend our understanding of how IL-17-producing cells contribute to colonic pathology. Although several studies have focused on the contribution of CD4+ IL-17-secreting T cells to pathology [13, 19, 33], and CD8α+ T cells have been shown to contribute to pathology in other models of colitis [34, 35], the present study demonstrates that CD4+ and CD8α+ cIELs are IL-17-producing cells in IL-10−/− mice.
Spleen cells in ICOS−/− mice produce significantly less IL-17 than their wild-type counterparts [23]. ICOS hyperexpression occurs in patients with IBD [19]. In adoptive transfer models of colitis, treatment of ICOS+/+ mice with anti-ICOS antibody has been shown to prevent colitis [33]. Control of inflammation by CD4+ CD25+ Tregs required CD28 but not ICOS signaling [36]. Although IL-17 production can proceed in the absence of ICOS expression [9], and IL-17 can be produced in ICOS−/− mice [33], the observation in the present study, that colonic pathology increases as the percent of ICOS-expressing cells increases and that there was a strong positive correlation between IL-17-producing cells and ICOS expression, points to a link between ICOS expression and IL-17 output. That association was supported further by the findings that stimulation of cIELs using B7RP-1-bearing cells increased IL-17 synthesis, as the B7RP-1 molecule was up-regulated on leukocytes in IL-10−/− with colonic inflammation, and from experiments demonstrating suppression of colonic pathology in IL-10−/− mice treated with antibody to ICOS. Altogether, these findings indicate that ICOS is a contributing factor in enhancing IL-17 production. Interestingly, IL-27 has been shown recently to drive the differentiation of IL-10-producing Tr1 Tregs through a process that required ICOS signaling in conjunction with the c-Maf transcription factor and IL-21 [37], thus pointing to a complex role for ICOS in IL-17 regulation.
CD69 is regarded as an early activation antigen of T cells. Its functional role remains controversial, however, and it may be linked to leukocyte retention in peripheral compartments [38,39,40]. In mice, CD69 is expressed on small and large intestinal IELs [41,42,43,44,45], probably reflecting the fact that IELs display some features of activated T cells. The present study demonstrates that despite the presence of CD69 on cIELs in healthy mice, ICOS is not normally expressed. ICOS up-regulation on cIELs thus appears to be a reliable indicator of activation. This is consistent with a model of IEL activation described by our laboratory, in which most IELs under normal homeostatic conditions are held in a stage of partial activation but are poised to move into a higher level of activation that is characterized by the expression of additional function-associated activation molecules, including ICOS [46, 47]. The findings reported here support that hypothesis, and they identify IL-10 as a regulatory element that governs that transition.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants DK035566 and DE015355.
Footnotes
Abbreviations: cIEL=colonic intraepithelial lymphocyte, DC=dendritic cell, Ep-CAM=epithelial cell adhesion molecule, IBD=inflammatory bowel disease, IL-10−/−=IL-10-deficient, LCA=leukocyte common antigen, MFI= mean fluorescence intensity, qRT-PCR=quantitative RT-PCR, Treg=T regulatory cell
References
- Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Donnelly R P, Dickensheets H, Finbloom D S. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I J, Blumberg R S. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Rouvier E, Luciani M F, Mattei M G, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- Veldhoen M, Hocking R J, Atkins C J, Locksley R M, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom T B, Oukka M, Kuchroo V K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang X O, Martinez G, Zhang Y, Panopoulos A D, Ma L, Schluns K, Tian Q, Watowich S S, Jetten A M, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Mangan P R, Harrington L E, O'Quinn D B, Helms W S, Bullard D C, Elson C O, Hatton R D, Wahl S M, Schoeb T R, Weaver C T. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Chen Z, O'Shea J J. Regulation of IL-17 production in human lymphocytes. Cytokine. 2008;41:71–78. doi: 10.1016/j.cyto.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenders M I, Joosten L A, van den Berg W B. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumor necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis. 2006;65:iii29–iii33. doi: 10.1136/ard.2006.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang X O, Chang S H, Nurieva R, Wang Y H, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenders M I, Lubberts E, van de Loo F A, Oppers-Walgreen B, van den Bersselaar L, Helsen M M, Kolls J K, Di Padova F E, Joosten L A, van den Berg W B. Interleukin-17 acts independently of TNF-α under arthritic conditions. J Immunol. 2006;176:6262–6269. doi: 10.4049/jimmunol.176.10.6262. [DOI] [PubMed] [Google Scholar]
- Montufar-Solis D, Schaefer J, Hicks M J, Klein J R. Massive but selective cytokine dysregulation in the colon of IL-10−/− mice revealed by multiplex analysis. Int Immunol. 2008;20:141–154. doi: 10.1093/intimm/dxm126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg M C, Cua D J, McKenzie B S, Powrie F, Maloy K J. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R H, Taylor K D, Brant S R, Rioux J D, Silverberg M S, Daly M J, Steinhart A H, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta L W, Kistner E O, Schumm L P, Lee A T, Gregersen P K, Barmada M M, Rotter J I, Nicolae D L, Cho J H. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M, Becker C, Ivanov I I, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy A J, Valenzuela D M, Yancopoulos G D, Becher B, Littman D R, Neurath M F. RORγ-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Sato T, Kanai T, Watanabe M, Sakuraba A, Okamoto S, Nakai T, Okazawa A, Inoue N, Totsuka T, Yamazaki M, Kroczek R A, Fukushima T, Ishii H, Hibi T. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology. 2004;126:829–839. doi: 10.1053/j.gastro.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich A M, Beier K C, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek R A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Dong C, Juedes A E, Temann U A, Shresta S, Allison J P, Ruddle N H, Flavell R A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- McAdam A J, Chang T T, Lumelsky A E, Greenfield E A, Boussiotis V A, Duke-Cohan J S, Chernova T, Malenkovich N, Jabs C, Kuchroo V K, Ling V, Collins M, Sharpe A H, Freeman G J. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- Nurieva R I, Treuting P, Duong J, Flavell R A, Dong C. Inducible costimulator is essential for collagen-induced arthritis. J Clin Invest. 2003;111:701–706. doi: 10.1172/JCI17321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nurieva R I. Regulation of immune and autoimmune responses by ICOS-B7h interaction. Clin Immunol. 2005;115:19–25. doi: 10.1016/j.clim.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Ivanov I I, McKenzie B S, Zhou L, Tadokoro C E, Lepelley A, Lafaille J J, Cua D J, Littman D R. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Elliott D E, Metwali A, Leung J, Setiawan T, Blum A M, Ince M N, Bazzone L E, Stadecker M J, Urban J F, Jr, Weinstock J V. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M B, Kukutsch N, Ogilvie A L, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Montufar-Solis D, Garza T, Teng B B, Klein J R. Upregulation of ICOS on CD43+ CD4+ murine small intestinal intraepithelial lymphocytes during acute reovirus infection. Biochem Biophys Res Commun. 2006;342:782–790. doi: 10.1016/j.bbrc.2006.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montufar-Solis D, Wang H C, Klein J R. Stimulatory and costimulatory effects of IL-18 directed to different small intestinal CD43 T cell subsets. J Leukoc Biol. 2007;82:1166–1173. doi: 10.1189/jlb.0207108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nelson A J, Dunn R J, Peach R, Aruffo A, Farr A G. The murine homolog of human Ep-CAM, a homotypic adhesion molecule, is expressed by thymocytes and thymic epithelial cells. Eur J Immunol. 1996;26:401–408. doi: 10.1002/eji.1830260220. [DOI] [PubMed] [Google Scholar]
- Farr A, Nelson A, Truex J, Hosier S. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J Histochem Cytochem. 1991;39:645–653. doi: 10.1177/39.5.2016514. [DOI] [PubMed] [Google Scholar]
- Totsuka T, Kanai T, Iiyama R, Uraushihara K, Yamazaki M, Okamoto R, Hibi T, Tezuka K, Azuma M, Akiba H, Yagita H, Okumura K, Watanabe M. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124:410–421. doi: 10.1053/gast.2003.50050. [DOI] [PubMed] [Google Scholar]
- Nancey S, Holvoet S, Graber I, Joubert G, Philippe D, Martin S, Nicolas J F, Desreumaux P, Flourie B, Kaiserlian D. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131:485–496. doi: 10.1053/j.gastro.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, Ishigame H, Iwakura Y, Kitamura H, Nishimura T. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong Y P, Rietdijk S T, Faubion W A, Abadia-Molina A C, Clarke K, Mizoguchi E, Tian J, Delaney T, Manning S, Gutierrez-Ramos J C, Bhan A K, Coyle A J, Terhorst C. Blocking inducible co-stimulator in the absence of CD28 impairs Th1 and CD25+ regulatory T cells in murine colitis. Int Immunol. 2004;16:205–213. doi: 10.1093/intimm/dxh019. [DOI] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu S M, Lai C Y, Madan R, Sharpe A H, Karp C L, Miaw S C, Ho I C, Kuchroo V K. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Kasprowicz D J, Yamashita M, Schubert L A, Gillard G, Kimura M, Didierlaurent A, Koseki H, Ziegler S F. The generation of mature, single-positive thymocytes in vivo is dysregulated by CD69 blockade or overexpression. J Immunol. 2002;168:87–94. doi: 10.4049/jimmunol.168.1.87. [DOI] [PubMed] [Google Scholar]
- Rosen H, Sanna M G, Cahalan S M, Gonzalez-Cabrera P J. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Feng C, Woodside K J, Vance B A, El-Khoury D, Canelles M, Lee J, Gress R, Fowlkes B J, Shores E W, Love P E. A potential role for CD69 in thymocyte emigration. Int Immunol. 2002;14:535–544. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- Aranda R, Sydora B C, McAllister P L, Binder S W, Yang H Y, Targan S R, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- Bagriacik E U, Okabe M, Klein J R. Origins of intestinal intraepithelial lymphocytes: direct evidence for a thymus-derived γ δ T cell component. Immunol Lett. 2000;75:77–83. doi: 10.1016/s0165-2478(00)00275-3. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991;147:1746–1751. [PubMed] [Google Scholar]
- Bagriacik E U, Armstrong M D, Okabe M, Klein J R. Differential expression of CD43 isoforms on murine T cells and their relationship to acute intestinal graft versus host disease: studies using enhanced-green fluorescent protein transgenic mice. Int Immunol. 1999;11:1651–1662. doi: 10.1093/intimm/11.10.1651. [DOI] [PubMed] [Google Scholar]
- Ibraghimov A R, Lynch R G. Heterogeneity and biased T cell receptor α/β repertoire of mucosal CD8+ cells from murine large intestine: implications for functional state. J Exp Med. 1994;180:433–444. doi: 10.1084/jem.180.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J R. T-cell activation in the curious world of the intestinal intraepithelial lymphocyte. Immunol Res. 2004;30:327–337. doi: 10.1385/IR:30:3:327. [DOI] [PubMed] [Google Scholar]
- Montufar-Solis D, Garza T, Klein J R. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]