Abstract
In human basophils from different subjects, maximum IgE-mediated histamine release and the level of syk protein expression correlate well. It is not clear when in the basophil’s lifetime the set-point for syk expression is reached or how expression levels are determined for a given individual. An examination of syk expression in peripheral blood eosinophils, neutrophils, monocytes, B and T cells, DCs, and NK cells showed that with the exception of T cells, basophils were unique in expressing low levels of syk. No correlations were observed between syk expression in basophils and other types of leukocytes, suggesting a unique mechanism of regulation for basophils. The expression level of syk in CD34+ progenitors was ∼11-fold higher than in peripheral blood basophils, and it remained at this level during maturation of the cells in IL-3 to a cell with characteristics of peripheral blood basophils. Down-regulation of syk expression in the culture-derived basophils was induced by culturing under conditions of chronic aggregation of FcεRI. Syk was down-regulated to peripheral blood basophil levels in 50% of the cells. Despite the chronic aggregation of FcεRI, the cells retained the same expression of FcεRI, histamine content, and morphological staining of granules as cells not experiencing chronic aggregation. These results suggest that chronic stimulation through FcεRI during basophil maturation might be a mechanism for down-regulating syk expression, while retaining other characteristics associated with mature peripheral blood basophils.
Keywords: development, adaptive immunity, Fc receptors
Introduction
For human basophils, the maximum histamine release that can be obtained when stimulating through the high-affinity IgE receptor (FcεRI) varies among individuals and is by and large a relatively stable characteristic of an individual’s basophils [1,2,3]. Previous studies of human basophils have shown that the level of syk, a critical early signaling tyrosine kinase, largely determines the extent of histamine release [4,5,6], but the exact mechanism by which the natural variation in syk arises is largely unknown.
Currently, there are three known mechanisms to modulate the natural levels of syk in peripheral blood basophils: incubation with IL-3 [4, 6,7,8], IgE-dependent stimulation [6, 9,10,11,12,13], and non-IgE-dependent stimulation [9]. There is also a recent study that demonstrates a relationship between the expression levels of mRNA for syk and syk protein levels, which suggests that there are some pretranslational regulatory mechanisms. As such, some of these mechanisms of syk expression regulation might be shared with other leukocytes. A limited study of syk expression in B cells, eosinophils, and neutrophils indicated similar levels of syk expression in two groups of subjects: releaser and nonreleasers [4], and syk expression in basophils was not similar. However, there have been no prior broad studies of the relationship between syk expression in human basophils and other circulating leukocytes. In the broader population, syk expression in basophils may not be uniquely variable, and there may be correlated levels of syk expression among other leukocyte types, particularly in those that also express the high-affinity IgER, such as pDCs or monocytes. In addition, there have been no studies examining the relationship between CD34+ progenitors and the circulating leukocytes that they ultimately generate. Finally, the current models for generating basophils from these CD34+ progenitors have not been examined for their relative syk expression, and these may prove useful models to explore regulation of syk expression in basophils as it occurs during maturation.
Despite significant differences in syk expression, peripheral blood basophils generally retain a common phenotype: the presence of high-affinity IgER, whose expression level is sensitive to the presence of circulating IgE; 1–1.5 pg histamine/cell; and morphological staining for granules such as that observed with Alcian blue staining. Therefore, any proposed explanation for the diversity of syk expression would not be expected to alter these basic phenotypic characteristics. The current studies also explored control of syk expression during basophil maturation from CD34+ progenitors, a previously unexplored period of time for syk regulation.
MATERIALS AND METHODS
Reagents
The following were purchased: PIPES, BSA, EDTA, D-glucose, and erythrosin B (Sigma-Aldrich, St. Louis, MO, USA); crystallized HSA (Miles Laboratories, Elkhart, IN, USA); FCS (BioWhittaker, Walkersville, MD, USA); Percoll (Pharmacia, Piscataway, NJ, USA); Dulbecco’s PBS, StemPro-34 serum-free medium with nutrient supplement, L-glutamine, and penicillin/streptomycin (Gibco/Invitrogen, Carlsbad, CA, USA); rhIL-3 (BioSource, Camarillo, CA, USA); anti-syk mAb (4D10, Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-fMLP-R mAb (350418, R&D Systems, Minneapolis, MN, USA); anti-c-kit/CD117 (A3C6E2) PE-conjugated and anti-BDCA-2/CD303 (AC144) FITC-conjugated mAb (Miltenyi Biotec; Auburn, CA, USA); anti-CD14 (2D-15C) mAb (Chemicon, Victoria, Australia); anti-IL-3R/CD123 (9F5) PE-conjugated, anti-CD3 (SK7) FITC-conjugated, anti-CD19 (HIB19) FITC-conjugated, and anti-CD56 (NCAM16.2) PE-conjugated mAb and mIgG1 (MOPC-21) FITC-conjugated and mIgG2b (27-35) PE-conjugated isotype controls (BD Biosciences, San Jose, CA, USA); mIgG1 PE-conjugated and purified mIgG2a isotype controls (Caltag/Invitrogen, Carlsbad, CA, USA); purified mIgG1 isotype control (Zymed Laboratories/Invitrogen, Carlsbad, CA, USA); anti-CD9 (ALB6) FITC-conjugated and anti-CD16 (3G8) PE-conjugated mAb (Beckman Coulter, Fullteron, CA, USA); anti-mIgG1-Alexa647, anti-mIgG2a-Alexa647, and anti-mIgG2a- and anti-mIgG2b-Alexa488 secondary antibodies (Molecular Probes/Invitrogen, Carlsbad, CA, USA); and anti-hIgE monomeric IgM (6061P; Hybridoma Reagent Lab, Baltimore, MD, USA). The anti-FcεRIα antibody 22E7 was a gift from Hoffman-LaRoche (Nutley, NJ, USA). The anti-gp120 peptide-specific hIgE SE44 was a gift from Tanox Inc. (Houston, TX, USA). The polyclonal goat anti-hIgE antibody was prepared as described [14]. RBL-SX38 cells (RBL cells transfected with hFcεRIαβγ) were developed and provided by Dr. Jean-Pierre Kinet (Harvard University, Cambridge, MA, USA).
PAG buffer consisted of 25 mM PIPES, 110 mM NaCl, 5 mM KCl, 0.1% glucose, and 0.003% HSA. Labeling with antibodies for flow cytometry was conducted in PAG with 1 mM EDTA containing 0.25% BSA in place of 0.003% HSA.
Alcian blue staining, histamine content, and histamine release
Basophils were detected by Alcian blue staining [15]. For progenitor cultures, only intact cells were analyzed for Alcian blue staining. Intact cells (20,000–40,000) were lysed in 1.6% perchloric acid and analyzed for histamine content by automated fluorimetry [16]. Histamine content is given as “pg/blue cell,” where the number of blue cells = (fraction blue×intact cell count). This measurement was found to be noisy, most likely as a result of the greater difficulty clearly identifying cells as Alcian blue-positive in the CD34-derived cultures.
At Day 21, a portion of cells was resuspended in PAG supplemented with 1 mM CaCl2 and 1 mM MgCl2 and stimulated with the anti-FcεRI antibody 22E7 (1:100,000 final) in the presence of a goat anti-mouse F(ab′)2 secondary antibody (10 μg/ml; 22E7 antibody induces release without a secondary antibody, but release is considerably weaker than release with secondary antibody) or 100 nM fMLP peptide for 45 min. Supernatants were analyzed for histamine content. Total cellular histamine content was obtained by a 1.6% perchloric acid lysis.
Analysis of surface markers by flow cytometry
All analysis was performed on a BD FACSCalibur flow cytometer. For surface marker comparisons, purified (>99%) peripheral blood basophils were obtained from residual cells of normal donors undergoing leukapheresis, as described previously [17]. IL-3R expression was measured on basophils during the measurement of syk in leukocytes (see below). Dr. Peter Peachell (University of Sheffield, UK) provided Percoll-enriched human lung mast cells. FcεRI and fMLP-R expression levels were detected using the anti-FcεRI antibody 22E7 and the anti-fMLP-R antibody and an appropriate conjugated secondary antibody. For the expression of c-kit, cells were stained with an anti-c-kit PE-conjugated antibody, then fixed, and stored in PBS with 5% FCS until analyzed.
Analysis of syk protein expression by intracellular flow cytometry
The measurement of syk protein expression by flow cytometry using the anti-syk antibody 4D10 has been described previously and validated with respect to standard Western blotting [9, 18]. This method has been used on impure as well as purified cell fractions with equivalent results. Syk protein expression is reported as normalized net MFI, the difference between 4D10 and isotype-labeled cells corrected for instrument variability using CaliBRITE allophycocyanin calibration beads (BD Biosciences). To examine the relationship between FcεRI and syk expression, the cells were labeled after fixation with CRA-1 antibody (a mIgG2b anti-hFcεRIα mAb, eBioscience, San Diego, CA, USA). After one wash and permeabilization (as described above), the cells were labeled with 4D10 (a mIgG2a) antibody. Secondary antibodies were an anti-mIgG2b-Alexa488 and an anti-mIgG2a-Alexa647.
Quantitative real-time PCR for syk mRNA expression
Total RNA was extracted from 0.25 × 106 intact cells using a HighPure RNA isolation kit (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturer’s instructions. Integrity of the samples was measured on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA); the RIN ranged from 6.6 to 8.3. A pilot survey established that a RIN >4 did not alter the measurement of syk mRNA. Samples were analyzed using a TaqMan primer/probe set (Applied Biosystems, Foster City, CA, USA) with primers to the human syk cDNA sequence [19]. The forward PCR primer was 5′-AGCAGAAGCAAATGTCATGCA-3′, and the reverse PCR primer was 5′-CCTCGCATATCCCGATCATC-3′. The probe sequence was 5′-CAGCTGGACAACCCGTACATCGTGC-3′. Samples (1/14th of the total RNA) were run in duplicate and analyzed by a one-step PCR protocol, which included a 30-min reverse-transcription step and 40 cycles of replication. Syk expression is given relative to a 50-ng sample of Jurkat total RNA (Stratagene, La Jolla, CA, USA) that was included on each PCR plate to normalize samples run on different plates. The average efficiency per cycle of replication was equal to 1.95 ± 0.04 based on twofold serial dilutions of Jurkat RNA.
Differentiation of basophils from CD34+ progenitors
Frozen G-CSF-mobilized peripheral blood CD34+ progenitors were purchased from the lab of Dr. Shelly Heimfeld (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). To generalize syk-level measurements in progenitors, CD34+ obtained from bone marrow and cord blood was purchased from Stem Cell Technologies (Canada). After thawing, cells were resuspended at a cell density of 0.3–0.7 × 106 cells/ml in StemPro serum-free medium (Gibco/Invitrogen), which contained the provided nutrient supplement, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 ng/ml rhIL-3 (“complete StemPro-34”). Cells were kept at 37°C with 5% CO2. Cell counts and viability were checked each week by erythrocin B staining, and freshly prepared media were added so that the total volume was doubled.
Stimulation of differentiating basophils with anti-IgE
For experiments in which basophils were grown in the presence of a chronic aggregating stimulus, thawed CD34+ progenitors were resuspended in complete StemPro-34 media and split into four flasks. Control cells received media alone. The anti-gp120 peptide-specific hIgE SE44 was added to the other three flasks at a final concentration of 500 ng/ml (2.6 nM). Two of the flasks also received the anti-hIgE IgM antibody 6061P at two different IgE:anti-IgE ratios: 42 ng/ml (0.26 nM) or 210 ng/ml (1.3 nM) for a 10:1 and 2:1 ratio of IgE:anti-IgE, respectively. The concentrations of each reagent were kept constant throughout the culture, starting from Day 0. Previous studies have shown that IgE is stable in culture for at least 1 week [20], but the stability of the IgE:anti-IgE conjugate in these cultures is not known. FcεRI and syk expression were measured at Day 21.
Stimulation of CD34-derived basophils with anti-IgE
CD34-derived basophils, grown in the absence of IgE or anti-IgE, were sensitized with the hIgE SE44 (5 μg/ml in PAG) for 1 h on ice, washed with PAG, and resuspended in complete StemPro-34 media. One-half of the cells received media alone, and the other half received anti-hIgE antibody 6061P at a final concentration of 0.5 μg/ml. Cells were incubated at 37°C for 18 h and then analyzed for syk expression.
Analysis of syk expression in peripheral blood leukocytes
To analyze syk protein expression simultaneously in peripheral blood cells, a small amount of blood (10 ml) was obtained by venipuncture from donors at the Johns Hopkins Asthma and Allergy Center (Baltimore, MD, USA). Recruitment was quasi-random; there were no specific exclusion criteria other than those specified by good practice for blood drawing. The mean age was 44 (25–60), and 56% were female. With respect to atopy, subjects were simply asked if they had any known allergies after the study was complete; 25% reported having allergies to common antigens (animal danders, aeroallergens, insects). A medication history was not obtained.
Blood samples were partially enriched using a two-step Percoll (53%/62%) gradient [17]. After separation, cells at the first interface (containing monocytes and pDCs) and the second interface (containing lymphoyctes and basophils) were washed separately, resuspended in PAG, and kept on ice until further use. RBCs were removed from the pellet containing neutrophils and eosinophils by hypotonic lysis. Each cell fraction had >90% viability.
Approximately 0.5 × 106 total cells from the appropriate cell fraction were taken for each cell type and analyzed for syk expression. The appropriate gating antibody was included for each cell type. In preliminary experiments, a separate aliquot of cells was stained with an appropriate isotype control to determine which cells were positive for a given cell marker. Expression of surface markers was generally stable between donors, so these controls were eliminated from further experiments. The following phenotypes were used to identify each cell type: pDCs, IL-3R+/BDCA-2+; monocytes, CD14+; basophils, IL-3R+, BDCA-2neg; T cells, CD3+; B cells, CD19+; NK cells, CD56hi/CD3neg; eosinophils, CD9+; neutrophils, CD16+. In some cases, forward-/side-scatter was also used to isolate the cell type before gating on surface marker expression.
Statistics
Where comparisons were made, mean and sem are shown. CV (sd/mean) is noted for population distributions. Correlation coefficients, Pearson R, were calculated for the syk expression levels in leukocytes. As 28 comparisons were made in the leukocyte study, a Bonferroni correction was applied; α = 0.05/28 = 0.0018 as the threshold for statistical significance.
RESULTS
Relationship of syk expression in CD34+ progenitors and leukocytes
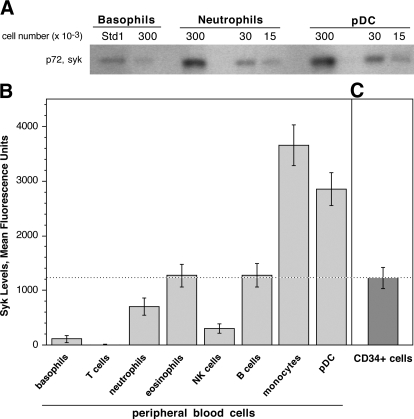
Syk has been implicated in a wide variety of signal transduction cascades in most cells of the immune system. However, syk has not been measured quantitatively or comprehensively in circulating leukocytes. Syk was measured in the predominant subsets of leukocytes to determine whether there was a correlation between syk levels in basophils and other leukocytes, whether the variation in syk expression observed in basophils could be found in other leukocytes, and whether there was a unique relationship between progenitors and the syk levels in peripheral blood basophils. Syk protein was measured simultaneously in the peripheral blood leukocytes of 16 donors using a flow cytometric method. Although we have extensively validated the flow cytometric method of measuring syk in basophils [9, 18], the method was validated further for other types of leukocytes. In pilot studies, the relative levels of syk measured by flow cytometry were compared with those measured by Western blotting of purified pDCs, basophils, and neutrophils and were found to be the same as those assessed by flow cytometry (Fig. 1A).
Figure 1.
Expression of syk in peripheral blood leukocytes and CD34+ progenitors. (A) Western blot of syk expression in lysates of basophils, neutrophils, and pDCs. The cell number equivalents added to each lane are indicated. The notation “Std1” is a pool of purified basophil lysates that is used to standardize Western blots. (B) Peripheral blood leukocytes from 16 donors were screened for their expression of syk by intracellular flow cytometry after partial enrichment of the cells using sedimentation gradient centrifugation and labeling with the appropriate gating antibodies. Syk protein expression is given as the average normalized MFI ± se. The dotted line represents the level of syk found in the CD34+ progenitors. (C) Measurements of syk protein expression in CD34+ progenitors (n=9).
The level of syk in other peripheral blood leukocytes was markedly higher than peripheral blood basophils, with the exception of the peripheral blood T cell population that had a small but measurable level of protein (Fig. 1B). The variation in the expression of syk among the 16 donors was highest for the basophils, based on CV = 0.582, which is consistent with previous basophil survey studies [6]. In contrast, all of the other cell types displayed narrow distributions (see Table 1) using ANOVA to test for equal variance for the types of leukocytes; basophil variance differs from other cells (P<0.0001), NK cells differ from monocytes and pDC, but all other pair-wise comparisons are not different. There were no correlations between the expression of syk in basophils and any of the other leukocytes, suggesting that basophils have a unique mechanism to establish expression levels in the terminally differentiated cell. It was noted that there was a modest correlation coefficient for syk expression between pDCs and eosinophils (R=0.604; P=0.0133), between monocytes and eosinophils (R=0.614; P=0.0114), and between pDCs and monocytes (R=0.486; P=0.0564). However, with a Bonferroni correction for multiple comparisons, these P values are not considered significant.
TABLE 1.
Correlation of Syk in Leukocytes
| CV | Monocytes | Basophils | T cells | B cells | NK cells | Eosinophils | Neutrophils | |
|---|---|---|---|---|---|---|---|---|
| pDCs | 0.106 | 0.486 | −0.450 | −0.358 | 0.285 | −0.185 | 0.604 | 0.123 |
| Monocytes | 0.102 | −0.077 | −0.003 | 0.331 | 0.112 | 0.614 | 0.402 | |
| Basophils | 0.582 | 0.004 | 0.058 | 0.436 | −0.063 | −0.094 | ||
| T cells | −0.506 | −0.210 | −0.424 | 0.211 | ||||
| B cells | 0.165 | 0.177 | 0.412 | 0.074 | ||||
| NK cells | 0.283 | 0.250 | −0.228 | |||||
| Eosinophils | 0.165 | 0.201 | ||||||
| Neutrophils | 0.228 |
Column 1, labeled CV, is the CV for each cell type examined in the 16-subject survey. The remainder of the table indicates the pairwise Pearson R correlations among the syk expression levels found for the different types of leukocytes. Numerals in bold have P values <0.05 for the particular comparison, but with a Bonferroni correction for these 28 comparisons (α=0.0018), they are not statistically significant.
The amount of syk was ∼11-fold higher in CD34+ progenitors than in peripheral blood basophils but similar or less than syk expression in other types of leukocytes (Fig. 1C). Similar levels were found for cord blood and bone marrow CD34+ cells (data not shown). There was little variation in expression between the donor sources of CD34+ cells (CV=0.13).
Phenotypic characteristics of culture-derived basophils
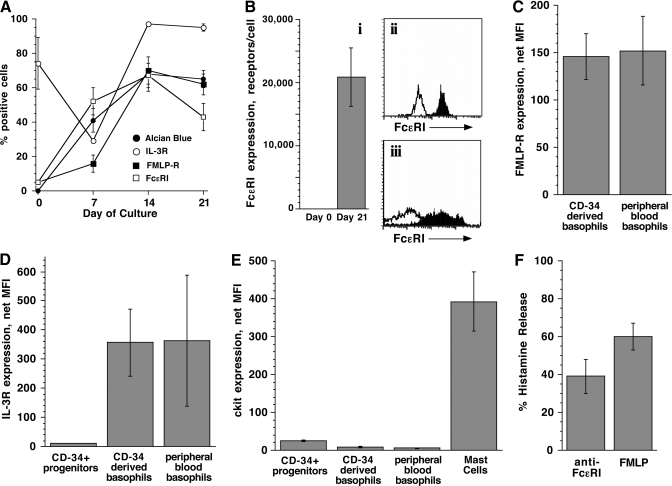
A commonly used model of human basophil maturation is to culture CD34+ progenitors in IL-3 for 3 weeks [21,22,23]. This model was tested for its expression of syk during the transition to basophil-like cells. To characterize this model system, the expression of basophil surface markers (FcεRI, fMLP-R, IL-3R), Alcian blue staining, histamine content, and functional responses was monitored throughout the culture to establish the phenotype of the cells and compare it with that of peripheral blood basophils. Progenitor cells from five different donors were used for these experiments. Each experiment represents the use of cells from one particular donor, and n represents the number of different 3-week cultures in which the marker was measured. Thus, the markers were measured more than once/person in some cases. At Day 21, the cultures were a mixture of viable cells, intact cells that were dead or dying based on erythrosin B staining, and cellular debris. For surface-marker measurements by flow cytometry, only viable cells were analyzed using propidium iodide to gate out dead cells and debris where possible. In a subset of the experiments, full time-courses of marker expression were obtained to assess the number of cells staining positive and the absolute expression levels of the marker.
CD34+ progenitors did not express any measurable FcεRI and did not stain with Alcian blue on Day 0. By Day 21, the majority of cells was Alcian blue+ (65±6%, n=8) and FcεRI+ (70±5%, n=10) and expressed an average of 20,800 ± 4700 FcεRI molecules (Fig. 2B, left panel, range 3000–48,000). In fact, Alcian blue staining and FcεRI expression were observed in approximately half of the cells by Day 7 (41±7% and 52±8%, respectively), which demonstrates early differentiation of the cells to the basophil lineage (Fig. 2A). The appearance of FcεRI by Day 7 is consistent with the detection of FcεRIα transcripts after 1 week of culture [25]. Kinetically, FcεRI expression at Days 7 and 14, relative to Day 21, was 0.76 ± 0.4 and 1.24 ± 0.48 (n=2). The median histamine content of the CD34-derived basophils at Day 21 was 1.2 pg histamine/blue cell (n=9; mean was 1.57±1.07), compared with 1.3 pg/cell in peripheral blood (see below). It was also noted that the expression of FcεRI on CD34-derived cells was broader than on peripheral blood basophils, although the distribution was still unimodal (Fig. 2B, right panels).
Figure 2.
Phenotypic characterization of CD34-derived basophils. Progenitor cells from five different donors were used for these experiments. (A) Time-course for the appearance of basophil markers as assessed by flow cytometry and Alcian blue staining, represented as the fraction of cells positive for a particular marker; Alcian blue (n=8), FcεRI (n=2), fMLP-R (n=3), and IL-3R (n=2). (B) Expression of FcεRI on CD34-derived basophils (n=10), expressed as the number of receptors (i) and comparison of FcεRI expression on peripheral blood basophils (ii) versus CD34-derived basophils (iii). Solid, Anti-FcεRI; outline, isotype control. The number of FcεRI receptors was calculated based on previous calibration of the flow data to the amount of IgE that could be eluted from the peripheral blood basophils [24]. (C) Expression of fMLP-R on CD34-derived basophils (n=4) versus peripheral blood basophils (n=3). (D) Expression of IL-3R on CD34+ progenitors (n=4) versus CD34-derived basophils (n=4) and peripheral blood basophils (n=15). (E) Expression of c-kit on CD34-derived basophils (n=2) versus CD34+ progenitors (n=3), peripheral blood basophils (n=3), and human lung mast cells (n=4). (F) Histamine release of CD34-derived basophils in response to an optimal concentration of anti-FcεRI antibody (n=4) and fMLP peptide (n=3).
To confirm that the FcεRI+/Alcian blue+ cells at Day 21 were basophil-like, three additional surface markers were examined. Expression of fMLP-R lagged behind that of FcεRI; only 16 ± 6% of the cells were positive at Day 7, but the percentage of cells expressing this marker reached that of FcεRI by Days 14 and 21 (Fig. 2A). The absolute expression level for fMLP-R also lagged at Day 7 and was two- to threefold lower than the expression on Day 21 (data not shown). As shown in Figure 2C, fMLP-R expression (given as MFI) on CD34-derived basophils at Day 21 was similar to the expression on peripheral blood basophils (147±21 MFI vs. 155±38 MFI). Expression of IL-3R was measured on fixed cells during the analysis of syk protein expression (see Materials and Methods). The CD34+ progenitors were 73 ± 15% positive for a small level of IL-3R at Day 0 (Fig. 2, A and D). In two time-courses measured, the percentage of cells expressing IL-3R dropped to 30 ± 1% at Day 7, and the absolute expression level of the receptor increased ∼7.5-fold from Day 0. The fraction of cells expressing IL-3R also reached a plateau at 98 ± 1% and 96 ± 3% between Days 14 and 21 (Fig. 2A), and the final level of receptor expression was the same as peripheral blood basophils (317±94 MFI vs. 363±223 MFI; Fig. 2D). Interestingly, expression of fMLP-R and in particular, IL-3R was bimodal in some cultures at one or more time-points (more often for IL-3R than fMLP-R and mainly at Days 7 and 14, but it was also observed at Day 21 for IL-3R). As IL-3R was measured simultaneously with syk, it was possible to assess how syk expression tracks with IL-3R under some conditions (see below).
The receptor for stem cell factor c-kit is expressed on hematopoietic progenitors [26]. Although mature mast cells are c-kit+, basophils only express a low level of this marker [27]. Expression of this cell-surface marker was low on CD34-derived basophils at levels similar to peripheral blood basophils (10±4 MFI vs. 8±1 MFI, respectively) and almost 40-fold lower than the expression of c-kit on human lung mast cells (394±76 MFI; Fig. 2E). CD34+ progenitors had approximately threefold more c-kit than the basophils (31±1 MFI).
The analysis of surface markers suggests that the majority of cells is FcεRI+/fMLP-R+/IL-3Rhigh/c-kitlow by Day 21. Furthermore, these cells are functionally responsive, releasing histamine upon stimulation through FcεRI or fMLP-R (Fig. 2F). Thus, by these markers, the CD34-derived basophils are phenotypically similar to peripheral blood basophils. The predominant morphology is a cell with variably sparse granules and bi-lobed/kidney bean-shaped nuclei, consistent with the morphology of peripheral blood basophils, but the cultures are heterogeneous with respect to cell size and the degree of staining with Alcian blue.
Cell division
Progenitors were labeled with 1 μM CFSE in PBS containing 5% FCS at Day 0, and the number of divisions in the viable cell population (determined by propidium iodide staining) was assessed on Day 7. In a separate culture, CFSE was added on Day 7 and the number of divisions assessed on Day 14. In both cases, flow cytometry identified four to five populations with different levels of CFSE (as analyzed by FlowJo), suggesting that four to five divisions occurred between Days 0 and 7 and Days 7 and 14, for a total of eight to 10 divisions (data not shown).
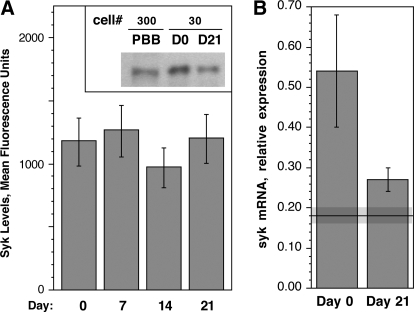
Expression of syk in CD34-derived basophils
In the standardized flow cytometric procedure [9, 18], the average level of syk protein in peripheral blood basophils corresponds to a MFI of 110 ± 16 (see Fig. 1A). However, syk expression during the maturation of CD34 progenitors to the basophil-like cells discussed above did not change throughout culture (Fig. 3A, 1092±47 MFI; P=0.23; paired t-test) with little variance between preparations.
Figure 3.
Syk expression in CD34+ cells cultured in IL-3 for 3 weeks. (A) Weekly measurements of syk protein expression as measured by intracellular flow cytometry of CD34+ cells cultured in IL-3 (n=4). The inset in A is a Western blot showing syk expression in peripheral blood basophils (PBB), CD34+ progenitors (D0), and Day 21 (D21)-cultured, derived basophils. The number of cell equivalents (×10−3) loaded into each lane is designated by “cell #”. (B) Syk mRNA expression as measured by quantitative real-time PCR (n=5). Syk mRNA levels are expressed relative to the expression of syk in a Jurkat RNA total sample, which was used to normalize samples run on separate PCR plates, and represent mRNA levels in intact cells (which includes intact but nonviable cells). The solid line and gray region around the line represent the average ± sem level of mRNA in peripheral blood basophils (0.18±0.02 units).
The levels of syk mRNA were measured by quantitative real-time PCR in the Day-0 and Day-21 samples (Fig. 3B). The difference between Day 0 and Day 21 mRNA levels was marginally significant (0.55±0.13 vs. 0.26±0.04; P=0.0494 by paired t-test). The level of syk mRNA in peripheral blood basophils was 0.18 ± 0.02. This method could not distinguish between the basophils at Day 21, which were phenotypically closer to maturity, and the immature basophils or nonbasophils, which are in these asynchronous cultures. Relative to Day 0 CD34+ progenitors, peripheral blood basophils show about one-third the level of syk mRNA. This raised the question of whether the syk mRNA was a relatively stable species in mature basophils. In separate experiments, purified peripheral blood basophils were incubated with and without actinomycin D and syk mRNA levels measured for a period of 4 h. In the presence of actinomycin D, mRNA for syk decayed with a half-life of 2 h (data not shown), suggesting a dynamic turnover.
Down-regulation of syk in basophils grown in the presence of a chronic aggregating stimulus
CD34-derived basophils grown in IL-3 alone did not down-regulate their syk to peripheral blood basophil levels. Previous studies have demonstrated that syk expression can be down-regulated by aggregation of FcεRI [10, 11, 13]. For reasons to be discussed, we hypothesized that if the cells were grown in the presence of a stimulus capable of chronically aggregating FcεRI during maturation, such as an IgE and anti-IgE pair, the high levels of syk observed in the progenitor cells and CD34-derived basophils could be down-regulated without altering other phenotypic characteristics that describe basophils.
The IgE chosen was a monoclonal rhIgE that has been shown previously not to induce detectable signaling upon binding to FcεRI [28]. The concentration (500 ng/ml) was sufficient to allow modest FcεRI up-regulation [29]. In some cultures, a monomeric IgM anti-hIgE antibody was also included. IgG mAb were avoided as a result of their ability to interact with the low-affinity IgGR, FcγRIIb [28] (which led to the avoidance of anti-receptor antibodies). To establish chronic receptor aggregation without disturbing the cells during culture, a fixed ratio of IgE and anti-IgE was used for the entire 3 weeks. To estimate a ratio that would generate stimulation in these cultures, RBL-SX38 cells expressing hFcεRI were stimulated with several different ratios of IgE:anti-IgE. A 2:1 ratio of IgE:anti-IgE induced the greatest release of histamine. A 10:1 ratio of IgE:anti-IgE did not induce histamine release from the RBL-SX38 cells but was also included in the CD34-derived basophil experiment, as prior studies of the loss of syk in peripheral blood basophils show that syk loss can be induced with a weak stimulus that does not induce histamine release [6, 13].
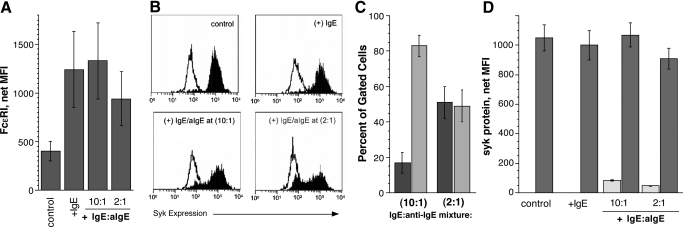
Binding of IgE to FcεRI prevents the removal of the receptor from the cell surface, thus allowing the cell to accumulate more FcεRI [29]. Cells cultured in the presence of IgE showed approximately threefold higher average expression of FcεRI at Day 21 (Fig. 4A). FcεRI expression on the cells grown in the presence of IgE:anti-IgE was not statistically different from those grown in IgE alone, indicating no significant loss of the receptor over the 3-week culture.
Figure 4.
Down-regulation of syk in CD34-derived basophils cultured in the presence of a chronic aggregating stimulus. CD34+ progenitors were cultured in the presence of 500 ng/ml IgE (2.6 nM) or IgE plus anti-IgE (aIgE) at a 10:1 or 2:1 molar ratio (42 ng/ml and 210 ng/ml, respectively) for the entire 3-week culture. Expression of FcεRI and syk in the treated cells was compared with cells grown without any IgE or aggregating stimulus (control) at Day 21 of cultures. (A) FcεRI expression. (B) Representative flow cytometry experiment in which syk levels (solid) are measured relative to the isotype control (outline). (C) Percentage of cells in the high versus low syk populations observed for cells grown in the presence of a chronic aggregating stimulus (n=4 for 10:1 ratio; n=3 for 2:1 ratio). The light bars represent cells that fall in the low syk expression analysis “gate” and the dark bars, cells in the high syk expression gate. (D) Average syk levels (MFI) in the high and low syk populations. The light bars represent the mean syk expression levels in cells “gated” for low syk expression and dark bars, mean expression levels for cells gated for high expression.
The presence of IgE alone did not alter the levels of syk at Day 21 in the CD34-derived basophils compared with the control cells, and there was a unimodal distribution of syk in these cells (Fig. 4B). From the flow cytometric analysis, there was no correlation between syk and FcεRI expression at the single-cell level in cells incubated with or without IgE. In a separate study, culture of purified peripheral blood basophils in the presence of 500 ng/ml IgE resulted in a 4.0 ± 0.6-fold (n=4; P=0.02; |Ho=1.0) increase in FcεRI expression but did not alter syk or lyn kinase as determined by Western blotting (1.30±0.24- and 1.42±0.30-fold, respectively). These results were consistent with the CD34+ cultures and show that an increase in FcεRI induced by the presence of IgE was not accompanied by changes in two early signaling elements.
However, when IgE and anti-IgE were included in the culture medium, a bimodal distribution of syk was observed, and one population expressed syk at levels observed in the control cells, and the other population expressed approximately tenfold less syk (Fig. 4B). At the 10:1 IgE:anti-IgE ratio, 17 ± 6% of the cells gated had syk levels that were in the range of peripheral blood basophils (MFI=82±4; Fig. 4C). This effect was more pronounced at the 2:1 ratio of IgE:anti-IgE in the number of cells expressing less syk (49±9%) and the absolute amount of syk expressed in the down-regulated population (MFI=37±4). The amount of syk in the remaining cells in the 10:1 and 2:1 cultures was similar to the control (Fig. 4D). Approximately 25% of the cells have low syk by Day 14 (with a MFI=25), and 51% of the cells had down-regulated syk by Day 21 (MFI=37±4). Receptor expression in cells that had not yet down-regulated syk was lower than in cells where syk was down-regulated (Fig. 5), suggesting that less-mature basophils did not yet down-regulate syk. Basophils in the cultures containing the IgE:anti-IgE mixtures continued to stain with Alcian blue. The fraction of Alcian blue+ cells in the cultures was 68 ± 10%, 75 ± 7%, 79 ± 2%, and 81 ± 7% in the control, IgE-alone, 10:1, and 2:1 cultures, respectively (n=4). Histamine content was measured in two of these cultures, and relative to the control and +IgE-alone cultures (1.0±0.29 pg/cell), the 10:1 and 2:1 content levels were not different (0.8±0.2- and 1.9±0.9-fold, respectively). These values were also within the wide distribution found for the untreated cells (see above). Thus, syk can be down-regulated in differentiating basophils via stimulation of the IgER, and the cells retain their phenotype with respect to the presence of granules, histamine content, and receptor expression.
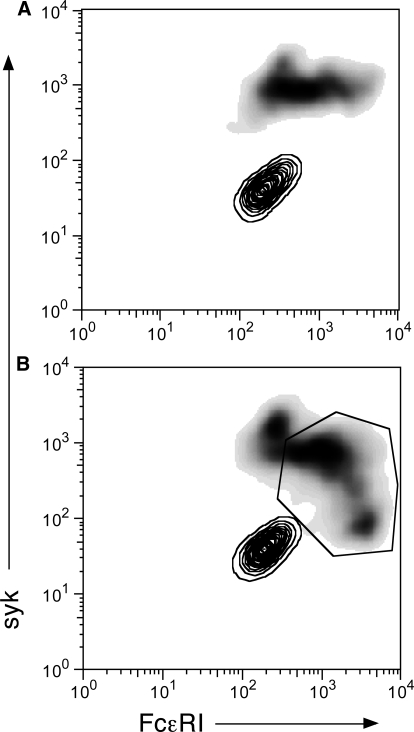
Figure 5.
2-D flow cytograms for control and IgE:anti-IgE-stimulated cultures of CD34 progenitors. After14 days of culture, cells were harvested and labeled with a mixture of anti-syk antibodies and anti-FcεRIα antibodies (see Materials and Methods). (A) Nonstimulated cultures (no IgE and no anti-IgE antibody); two analyses are superimposed: The contour plot shows the 2-D distributions for the labeling isotype control antibodies (IgG2a and IgG2b), and the gray-scale density plot shows the distribution for cells labeled with 4D10 (anti-syk) and CRA-1 (anti-FcεRIα). (B) Cultures containing a 2:1 mixture of IgE:anti-IgE; two analyses superimposed as described for A. Within the polygon gate, which is considered positive with respect to syk and FcεRI expression, relative to the isotype control distribution, the Pearson R correlation for the relationship between FcεRIα and syk expression is R = 0.44 (n=435 cells; P<0.0001; |Ho=0.0).
Down-regulation of syk in an 18-h culture
To determine if syk could be down-regulated in more mature, cultured basophils in a manner similar to peripheral blood basophils, CD34-derived basophils (grown in the absence of any IgE or anti-IgE) were sensitized on Day 21 and stimulated with an optimal concentration of anti-IgE antibody for 18 h. There was only a modest loss of syk after 18 h in the culture-derived basophils (0.78±0.08 of control cells; n=2).
DISCUSSION
This study demonstrated that with the exception of peripheral blood T cells, syk expression in basophils is uniquely low. To put the flow cytometric values in context, previous studies have calibrated the syk expression in basophils so that it is possible to state that with the flow cytometric settings used, a MFI value of 100 is roughly equivalent to 25,000 molecules of syk/cell [6]. CD34+ progenitors expressed 11-fold more syk than circulating basophils. This level was quite similar to other types of leukocytes, most notably, eosinophils, neutrophils, and B cells. Monocytes and pDCs express considerably more syk than progenitors. As the majority of cells in the CD34+ pool includes lineage-committed progenitors [26], this indicates that each type of leukocyte starts out with the same relatively large amount of syk, and for most paths of maturation, syk levels must be maintained or up-regulated to the levels that were measured in the terminally differentiated cells (Fig. 1). Population variation in syk expression was minimal in other types of leukocytes, and it was marked, as previously found, in basophils. The population variation was small in other types of leukocytes, and although there were some apparent correlations in syk expression in other leukocytes, none reached statistical significance. There were also no correlations found between basophils and other types of leukocytes. This result suggests that the variation in syk expression in basophils did not result from a shared mechanism of regulation with other leukocytes.
In a separate study (manuscript in preparation), we found that lung tissue mast cells, on average, express syk at a level that is similar to the high end of peripheral blood basophils, ∼250 MFI. Thus, like basophils, mast cells express lower levels of syk compared with other leukocytes, which suggests that the down-regulation of syk that occurs in basophils may be shared with mast cells. Although the mechanism for syk down-regulation is not yet understood for developing basophils, it is useful to note that if the normal maturation of basophils simply included a “stop synthesizing syk signal,” two to four divisions would be sufficient to reduce syk to levels found in peripheral blood basophils without invoking an explicit mechanism of down-regulation. The CFSE proliferation studies indicate that these cultures experience at least eight to 10 divisions, sufficient for a putative stop synthesizing syk signal to operate within the time-frame of the cultures. However, it is useful to note that the levels of syk mRNA in peripheral blood basophils are not 9–10% of CD34+ progenitors but closer to one-third, suggesting that the synthesis of the mRNA continues (in Day 21 cells, mRNA levels approached that of peripheral blood basophils; it is possible that the asynchronous nature of these cultures and the fact that the technique sampled all cells prevented this number from being more similar). It was also found that the decay rate of the mRNA in mature peripheral blood basophils is as fast as the decay rate of the mRNA for IL-4 [29], a transiently transcribed cytokine mRNA. Previous studies have shown that syk protein is stably expressed in peripheral blood basophils and that there is little synthesis [13]. Therefore, these observations (the mRNA steady-state is dynamic, but protein is not synthesized), taken together, suggest that there is translational control being exerted in the mature basophil.
These studies then focused attention on a portion of the basophil’s life cycle, the transition from progenitor to mature leukocyte, which has not been studied in the context of signal element regulation. Culture models of CD34+ progenitor differentiation do not generally produce cells indistinguishable from their mature counterparts found in peripheral blood. Nevertheless, the characteristics may be similar enough to explore some general principles of development. The expression of several basophil markers on CD34-derived “basophils” was similar to levels expressed on circulating basophils. Kepley et al. [22] noted the presence of basophil granule protein in basophils derived from cord blood CD34+ progenitors and found levels of β1 and β2 integrins similar to peripheral blood basophils. Our culture methods followed methods published previously used to produce basophil-like cells. Day 21 cells generated by this method release IL-4 upon IgE-mediated stimulation, and cultured mast cells do not release this cytokine [23]. In addition, the concentration dependence for stimulation with goat polyclonal anti-IgE antibody (which distinguishes tissue and cultured mast cells from peripheral blood basophils) is similar to peripheral blood basophils, not mast cells [23]. The cells generated in the current study were positive for markers known to be highly expressed on basophils but not mast cells (IL-3Rα and fMLP-R). Nevertheless, with respect to syk expression, they were not similar to mature peripheral blood basophils. It is apparent that this model of basophil maturation distinguishes among maturation signals that produce expression of markers such as FcεRI, fMLP-R, histamine, and granules or IL-3R and signal(s) that induce changes in syk expression. Whether this difference reflects an inadequate culture environment or a missing extrinsic factor is not clear. However, it also possible that this behavior may accurately reflect maturation in vivo and that the lower syk levels observed in mature peripheral blood basophils are regulated, not by additional factors but by the presence, in vivo, of chronic stimulation of some form.
There are some underlying issues that can be framed about an immature cell just beginning to put FcεRI onto the cell surface. For example, for immature cells, it isn’t clear whether the normal down-regulatory machinery for managing aggregation is in place, whether a low number of receptors just emerging in a “pre-basophil” can engage signaling effectively, and whether persistent low-level signaling (and down-regulation) alters the phenotype of basophils. More specifically, we asked whether chronic stimulation of a maturing basophil, using CD34+ hematopoietic progenitors cultured in the presence of IL-3 [21,22,23] to differentiate into basophil-like cells as a model, would down-regulate syk expression but not alter other phenotypic characteristics. This latter point is important, as in general, all subjects—atopic and nonatopic—circulate basophils that express high levels of the receptor (concordant with circulating IgE levels), contain histamine, and stain with Alcian blue. If chronic aggregation were occurring in vivo, one would conclude that it does not alter the rough phenotype of basophils that are typically observed in circulation. In this respect, these studies demonstrated that it was possible to down-regulate the syk levels and yet maintain cell-surface FcεRI densities that were similar to those found in the presence of IgE alone. Unlike the expectation for mature basophils stimulated with an optimal aggregating stimulus [13], the cells did not appear to be depleted of granules or granule contents such as histamine (although the assessment of Alican blue staining is crude, and this influenced the calculation of histamine content as well). Whether they are altered in less-obvious ways will need further study.
It was also noted that the Day-21 basophils, cultured without IgE and without the IgE:anti-IgE mixture, express levels of syk, which if expressed in peripheral blood basophils, would be equated with strong histamine release (>70%). However, the average histamine release was only 40% (Fig. 2F). This result may reflect the asynchronous nature of these cultures; i.e., some cells contain histamine but do not have a fully mature signaling apparatus. With this explanation, 40% histamine release would result from some cells releasing >70%, averaged with those that could not yet respond through FcεRI. A more subtle variation on this possibility is that the asynchronous nature of the cultures produces some cells that respond better to FcεRI-mediated stimulation and others responding better to non-FcεRI-dependent stimuli (such as fMLP). There are alternative explanations. For example, syk is not the only determinant of maximum histamine release in these cells. Even if this explanation were true, it doesn’t change the relationship in naturally mature basophils obtained from peripheral blood. In addition, such an explanation could highlight another technical artifact of these cultures or could indicate that the final maturation step includes a transition from multiple determinants of release to the single determinant found for peripheral blood basophils.
As the manipulation of syk expression via stimulation of FcεRI was possible in this culture system without altering other basophil phenotypic markers, these results raise the possibility that exposure of maturing basophils in the bone marrow to IgE and antigen could potentially influence syk levels in mature circulating basophils. In a recent study of omalizumab treatment (submitted for publication), it was observed that the down-regulation of FcεRI that occurs during treatment with this therapeutic [30,31,32] was also accompanied by an increase in the expression of syk in peripheral blood basophils. An increase in syk expression would not be expected if the cytokine environment that might lead to increased IL-3 were suppressed. Several studies of omalizumab treatment have noted suppression of the presence of CD4-positive T cells, suggesting that there might also be suppressed Th2 cytokines such as IL-3 [33,34,35]. However, in light of the current study and with knowledge that free IgE levels and FcεRI expression are suppressed markedly following omalizumab, an increase in syk expression is a possible outcome if there were a natural chronic stimulation that was blunted during treatment. There are ways that chronic stimulation could occur in vivo. For example, many normal and atopic individuals have circulating anti-IgE or anti-FcεRI antibodies [36, 37]. The function of these antibodies is unknown, but they offer the possibility that maturing basophils experience chronic stimulation through FcεRI.
The kinetics of syk down-regulation in these cultures indicates that significant down-regulation lags behind the appearance of FcεRI. This would suggest that the machinery of syk down-regulation is not expressed simultaneously with FcεRI. However, as histamine content and FcεRI expression also appear not to be down-regulated, the full machinery for secretion may require longer than the mechanism of syk down-regulation to appear. These studies suggest that the various mechanisms involved in IgE-mediated secretion asynchronously appear during maturation of basophils, leading to the suppression of syk expression and unchanged histamine content, granule, and FcεRI expression.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI20253 and AI070345 to D. W. M. The authors thank Valerie Alexander for technical assistance, Dr. Peter Peachell (University of Sheffield) for providing mast cells to assess c-kit levels in tissue mast cells, and Jackie Langdon for providing knowledge about culturing CD34+ progenitors.
Footnotes
Abbreviations: 2-D=two dimensional, BDCA-2=blood DC antigen-2, CV=coefficient of variation, DC=dendritic cell, h=human, HSA=human serum albumin, m=murine, MFI=mean fluorescence intensity, PAG=PIPES-albumin-glucose, pDC=plasmacytoid DC, RIN=RNA Integrity Number
References
- Conroy M C, Adkinson N F J, Lichtenstein L M. Measurement of IgE on human basophils: relation to serum IgE and anti-IgE-induced histamine release. J Immunol. 1977;118:1317–1321. [PubMed] [Google Scholar]
- Marone G, Poto S, Celestino D, Bonini S. Human basophil releasability. III. Genetic control of human basophil releasability. J Immunol. 1986;137:3588–3592. [PubMed] [Google Scholar]
- Lichtenstein L M, MacGlashan D W., Jr The concept of basophil releasability. J Allergy Clin Immunol. 1986;77:291–294. doi: 10.1016/s0091-6749(86)80106-3. [DOI] [PubMed] [Google Scholar]
- Kepley C L, Youssef L, Andrews R P, Wilson B S, Oliver J M. Syk deficiency in nonreleaser basophils. J Allergy Clin Immunol. 1999;104:279–284. doi: 10.1016/s0091-6749(99)70367-2. [DOI] [PubMed] [Google Scholar]
- Lavens-Phillips S E, MacGlashan D W., Jr The tyrosine kinases, p53/56lyn and p72syk, are differentially expressed at the protein level but not at the mRNA level in non-releasing human basophils. Am J Respir Cell Mol Biol. 2000;23:566–571. doi: 10.1165/ajrcmb.23.4.4123. [DOI] [PubMed] [Google Scholar]
- MacGlashan D W., Jr Relationship between Syk and SHIP expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–633. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hirai K, Ohta K, Suzuki K, Kitani S, Takaishi T, Ito K, Ra C, Morita Y. Nonreleasing basophils convert to releasing basophils by culturing with IL-3. J Allergy Clin Immunol. 1996;97:1279–1287. doi: 10.1016/s0091-6749(96)70196-3. [DOI] [PubMed] [Google Scholar]
- Kepley C L, Youssef L, Andrews R P, Wilson B S, Oliver J M. Multiple defects in Fc ε RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol. 2000;165:5913–5920. doi: 10.4049/jimmunol.165.10.5913. [DOI] [PubMed] [Google Scholar]
- MacGlashan D W, Jr, Ishmael S, Macdonald S M, Langdon J M, Arm J P, Sloane D E. Induced loss of Syk in human basophils by non-IgE-dependent stimuli. J Immunol. 2008;180:4208–4217. doi: 10.4049/jimmunol.180.6.4208. [DOI] [PubMed] [Google Scholar]
- Youssef L A, Wilson B S, Oliver J M. Proteasome-dependent regulation of Syk tyrosine kinase levels in human basophils. J Allergy Clin Immunol. 2002;110:366–373. doi: 10.1067/mai.2002.127562. [DOI] [PubMed] [Google Scholar]
- Kepley C L. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005;138:29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- MacGlashan D. Loss of receptors and IgE in vivo during treatment with anti-IgE antibody. J Allergy Clin Immunol. 2004;114:1472–1474. doi: 10.1016/j.jaci.2004.07.064. [DOI] [PubMed] [Google Scholar]
- MacGlashan D, Miura K. Loss of syk kinase during IgE-mediated stimulation of human basophils. J Allergy Clin Immunol. 2004;114:1317–1324. doi: 10.1016/j.jaci.2004.08.037. [DOI] [PubMed] [Google Scholar]
- MacGlashan D W, Jr, Peters S P, Warner J, Lichtenstein L M. Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol. 1986;136:2231–2239. [PubMed] [Google Scholar]
- Gilbert H S, Ornstein L. Basophil counting with a new staining method using Alcian blue. Blood. 1975;46:279–286. [PubMed] [Google Scholar]
- Siraganian R P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- MacGlashan D W, Jr, White J M, Huang S K, Ono S J, Schroeder J, Lichtenstein L M. Secretion of interleukin-4 from human basophils: the relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–3016. [PubMed] [Google Scholar]
- Ishmael S, MacGlashan D W., Jr Early signal protein expression profiles in basophils: a population study. J Leukoc Biol. 2009;86:313–325. doi: 10.1189/jlb.1208724. [DOI] [PubMed] [Google Scholar]
- Law C L, Sidorenko S P, Chandran K A, Draves K E, Chan A C, Weiss A, Edelhoff S, Disteche C M, Clark A. Molecular cloning of human Syk. A B cell protein-tyrosine kinase associated with the surface immunoglobulin M-B cell receptor complex. J Biol Chem. 1994;269:12310–12320. [PubMed] [Google Scholar]
- MacGlashan D W, Jr, Schroeder J T. Functional consequences of FcεRIa up-regulation by IgE in human basophils. J Leukoc Biol. 2000;68:479–486. [PubMed] [Google Scholar]
- Arock M, Schneider E, Boissan M, Tricottet V, Dy M. Differentiation of human basophils: an overview of recent advances and pending questions. J Leukoc Biol. 2002;71:557–564. [PubMed] [Google Scholar]
- Kepley C L, Pfeiffer J R, Schwartz L B, Wilson B S, Oliver J M. The identification and characterization of umbilical cord blood-derived human basophils. J Leukoc Biol. 1998;64:474–483. doi: 10.1002/jlb.64.4.474. [DOI] [PubMed] [Google Scholar]
- Langdon J M, Schroeder J T, Vonakis B M, Bieneman A P, Chichester K, Macdonald S M. Histamine-releasing factor/translationally controlled tumor protein (HRF/TCTP)-induced histamine release is enhanced with SHIP-1 knockdown in cultured human mast cell and basophil models. J Leukoc Biol. 2008;84:1151–1158. doi: 10.1189/jlb.0308172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGlashan D W., Jr Endocytosis, re-cycling and degradation of unoccupied FcεRI in human basophils. J Leukoc Biol. 2007;82:1003–1010. doi: 10.1189/jlb.0207103. [DOI] [PubMed] [Google Scholar]
- Thompson H L, Metcalfe D D, Kinet J P. Early expression of high-affinity receptor for immunoglobulin E (Fc ε RI) during differentiation of mouse mast cells and human basophils. J Clin Invest. 1990;85:1227–1233. doi: 10.1172/JCI114557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Wagers A J, Manz M G, Prohaska S S, Scherer D C, Beilhack G F, Shizuru J A, Weissman I L. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Columbo M, Horowitz E M, Botana L M, MacGlashan D W, Jr, Bochner B S, Gillis S, Zsebo K M, Galli S J, Lichtenstein L M. The human recombinant c-kit receptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- MacGlashan D W., Jr Two regions of down-regulation in the IgE-mediated signaling pathway in human basophils. J Immunol. 2003;170:4914–4925. doi: 10.4049/jimmunol.170.10.4914. [DOI] [PubMed] [Google Scholar]
- MacGlashan D, Jr, Xia H Z, Schwartz L B, Gong J. IgE-regulated loss, not IgE-regulated synthesis, controls expression of FcεRI in human basophils. J Leukoc Biol. 2001;70:207–218. [PubMed] [Google Scholar]
- MacGlashan D W, Jr, Bochner B S, Adelman D C, Jardieu P M, Togias A, Mckenzie-White J, Sterbinsky S A, Hamilton R G, Lichtenstein L M. Down-regulation of Fc(ε)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- Beck L A, Marcotte G V, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fcε RI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Lin H, Boesel K M, Griffith D T, Prussin C, Foster B, Romero F A, Townley R, Casale T B. Omalizumab rapidly decreases nasal allergic response and FcεRI on basophils. J Allergy Clin Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- Ong Y E, Menzies-Gow A, Barkans J, Benyahia F, Ou T T, Ying S, Kay A B. Anti-IgE (omalizumab) inhibits late-phase reactions and inflammatory cells after repeat skin allergen challenge. J Allergy Clin Immunol. 2005;116:558–564. doi: 10.1016/j.jaci.2005.05.035. [DOI] [PubMed] [Google Scholar]
- van Rensen E L, Evertse C E, van Schadewijk W A, van Wijngaarden S, Ayre G, Mauad T, Hiemstra P S, Sterk P J, Rabe K F. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy. 2009;64:72–80. doi: 10.1111/j.1398-9995.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- Djukanovic R, Wilson S J, Kraft M, Jarjour N N, Steel M, Chung K F, Bao W, Fowler-Taylor A, Matthews J, Busse W W, Holgate S T, Fahy J V. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- Soundararajan S, Kikuchi Y, Joseph K, Kaplan A P. Functional assessment of pathogenic IgG subclasses in chronic autoimmune urticaria. J Allergy Clin Immunol. 2005;115:815–821. doi: 10.1016/j.jaci.2004.12.1120. [DOI] [PubMed] [Google Scholar]
- Eckman J A, Hamilton R G, Gober L M, Sterba P M, Saini S S. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956–1963. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]