Abstract
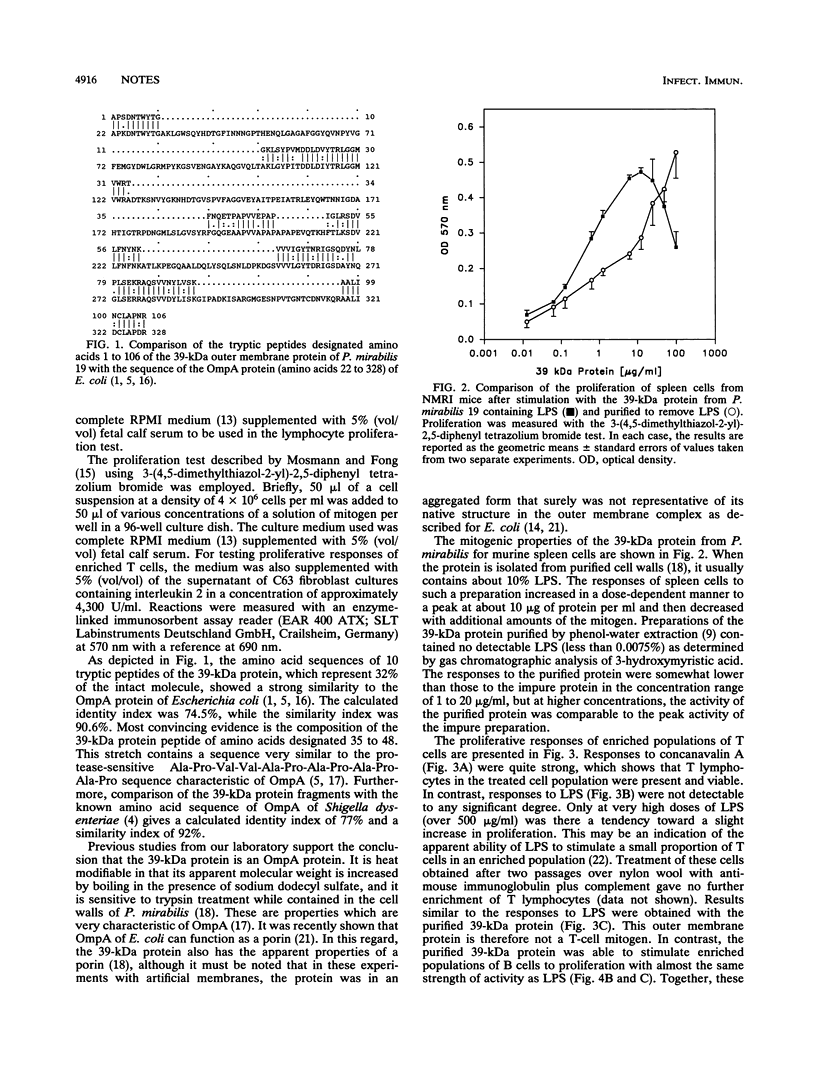
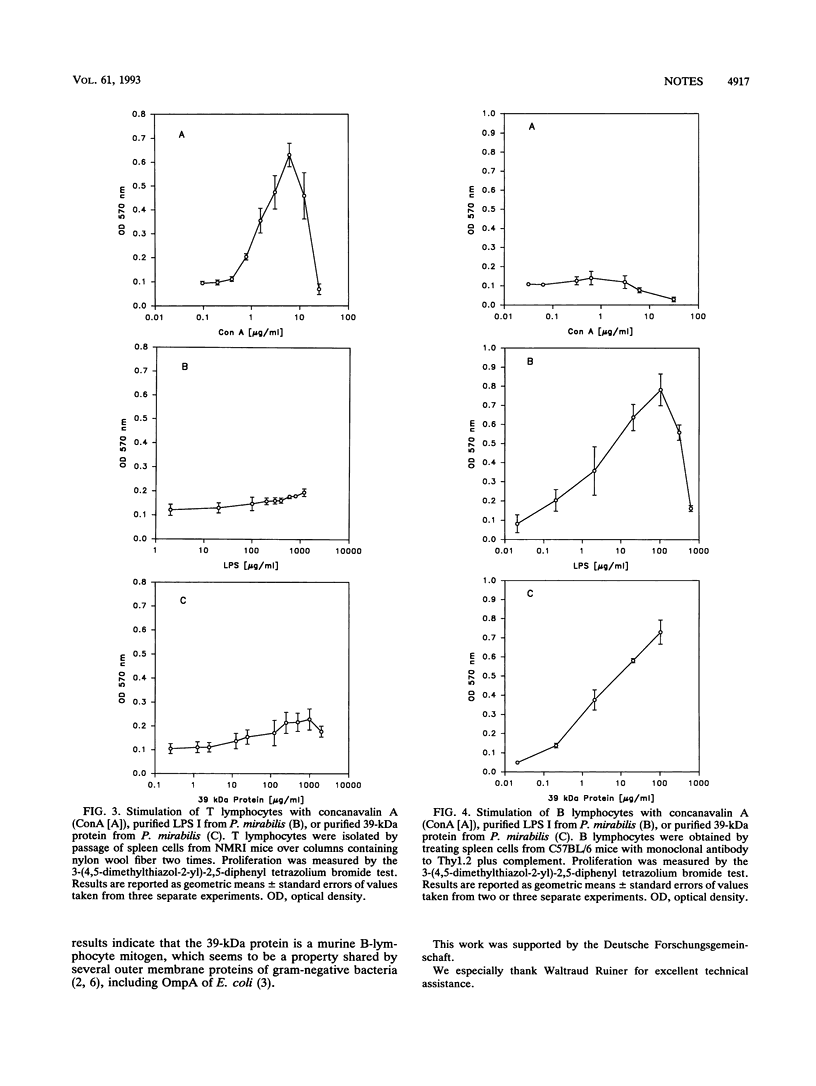
Partial amino acid sequence analysis of a major outer membrane protein of Proteus mirabilis (39-kDa protein) indicates that it is an OmpA protein. The mitogenic activities of the 39-kDa protein for murine lymphocytes were also investigated with T lymphocytes isolated by passing spleen cells over columns of nylon wool fiber and B lymphocytes obtained by treating spleen cells with monoclonal antibodies to Thy1 plus complement. The 39-kDa protein showed little activity in stimulating T cells to proliferate but was strongly mitogenic for B cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler W. G., Henning U. Protein I and protein II from the outer membrane of Escherichia coli are mouse B-lymphocyte mitogens. Z Immunitatsforsch Immunobiol. 1979 Jun;155(5):387–398. [PubMed] [Google Scholar]
- Braun G., Cole S. T. The nucleotide sequence coding for major outer membrane protein OmpA of Shigella dysenteriae. Nucleic Acids Res. 1982 Apr 10;10(7):2367–2378. doi: 10.1093/nar/10.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Hancock R. E., Mishell R. I. Mitogenic effects of purified outer membrane proteins from Pseudomonas aeruginosa. Infect Immun. 1980 Apr;28(1):178–184. doi: 10.1128/iai.28.1.178-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Karch H., Gmeiner J., Nixdorff K. Alteration of the immunoglobulin G subclass responses in mice to lipopolysaccharide: effects of nonbacterial proteins and bacterial membrane phospholipids or outer membrane proteins of Proteus mirabilis. Infect Immun. 1983 Apr;40(1):157–165. doi: 10.1128/iai.40.1.157-165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Modulation of the IgG subclass responses to lipopolysaccharide by bacterial membrane components: differential adjuvant effects produced by primary and secondary stimulation. J Immunol. 1983 Jul;131(1):6–8. [PubMed] [Google Scholar]
- Morona R., Klose M., Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984 Aug;159(2):570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980 Nov 5;143(3):317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Nixdorff K., Weber G., Kaniecki K., Ruiner W., Schell S. Bacterial protein-LPS complexes and immunomodulation. Adv Exp Med Biol. 1992;319:49–61. doi: 10.1007/978-1-4615-3434-1_6. [DOI] [PubMed] [Google Scholar]
- Siekmann J., Wenzel H. R., Schröder W., Tschesche H. Characterization and sequence determination of six aprotinin homologues from bovine lungs. Biol Chem Hoppe Seyler. 1988 Mar;369(3):157–163. [PubMed] [Google Scholar]
- Sugawara E., Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992 Feb 5;267(4):2507–2511. [PubMed] [Google Scholar]
- Weber G., Heck D., Bartlett R. R., Nixdorff K. Modulation of effects of lipopolysaccharide on macrophages by a major outer membrane protein of Proteus mirabilis as measured in a chemiluminescence assay. Infect Immun. 1992 Mar;60(3):1069–1075. doi: 10.1128/iai.60.3.1069-1075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Link F., Ferber E., Munder P. G., Zeitter D., Bartlett R. R., Nixdorff K. Differential modulation of the effects of lipopolysaccharide on macrophages by a major outer membrane protein of Proteus mirabilis. J Immunol. 1993 Jul 1;151(1):415–424. [PubMed] [Google Scholar]



