Abstract
GVHD is a major barrier to broader use of allogenic HSCT for nonmalignancy clinical applications such as the treatment of primary immunodeficiencies and hemoglobinopathies. We show in a murine model of C57BL/6J (H2-kb) → B6D2F1/J (H2-kb/d) acute GVHD that when initiated 2 days before transplant, the activation of the adenosine A2AR with the selective agonist ATL146e inhibits the weight loss and mortality associated with disease progression. Furthermore, circulating levels of proinflammatory cytokines and chemokines, including IFN-γ, IL-6, CCL2, KC, and G-CSF, are reduced significantly by 14-day ATL146e treatment. The up-regulation of CD25, CD69, and CD40L expression by donor CD4+ and CD8+ T cells is inhibited by A2AR activation; fewer CD3+ T cells are found in the liver, skin, and colon of ATL146e-treated mice as compared with vehicle-treated controls; and associated tissue injury is lessened. The delayed administration of ATL146e, beginning 9 days after HSCT, reverses GVHD-associated body weight loss successfully, and improvement is sustained for the duration of treatment. We conclude that the selective activation of the A2AR has therapeutic potential in the prevention and treatment of acute GVHD.
Keywords: T lymphocytes, inflammation, immunodeficiency
Introduction
A major complication of allogenic HSCT is GVHD, a donor-derived immune response against recipient antigens. The overall efficacy of HSCT is impaired by the significant morbidity and mortality associated with GVHD. Concern for the development of GVHD is a major barrier to the broader use of HSCT for treatment of nonmalignancy monogenic hematopoietic disorders such as primary immunodeficiencies and hemoglobinopathies, where graft-versus-tumor effect is not a consideration. Although significant progress has been made in understanding the pathophysiology of GVHD [1], clinical progress in the treatment of the disorder has been slow, with overall success rates remaining unchanged in recent years. Moreover, significant morbidity is associated with standard frontline therapies [2,3,4,5], and although many pharmacological approaches have been investigated with varying success, no standard second line therapy has been developed [6,7,8,9].
Agents that inhibit alloreactive T cell activation and/or limit T cell migration to tissue have shown promise in the treatment of GVHD [10, 11], and cAMP-elevating agents are known to induce alloantigen-specific T cell hyporesponsiveness and prevent GVHD lethality in murine models [12]. As the activation of the Gs-coupled adenosine A2AR plays a role in terminating inflammation via the regulation of cells involved in innate and adaptive immunity, it is an interesting pharmacological target for the treatment of GVHD [13]. The selective activation of the A2AR limits inflammation and injury effectively in many different inflammatory pathologies: A2AR agonists have significant protective effects in multiple models of ischemia-reperfusion injury, reduce joint destruction as a result of septic arthrosis, and limit the progression of inflammatory bowel disease [14,15,16,17,18]. Specifically, the potent and selective A2AR agonist ATL146e has been shown to limit tissue damage in murine models of hepatic, renal, and cardiac ischemia-reperfusion injury, attenuate inflammation and injury in diabetic nephropathy, reduce stress-induced gastric lesions in rats, and improve survival in mouse models of endotoxemia and sepsis [14, 15, 19,20,21,22]. Additionally, it has been demonstrated that the anti-inflammatory effects of methotrexate are mediated by the induction of adenine nucleotide release from injured tissue and the subsequent activation of A2ARs on local immune cells [23, 24]. Importantly, T cell anergy can be induced by A2AR agonist treatment [25].
In this study, we sought to determine if A2AR activation limits disease progression in a murine model of acute GVHD. In murine models, GVHD can be induced by the transfer of parental cells into F1 recipients, and immune system dysregulation results from the reactivity of lymphocytes in the donor inoculum to H2 alloantigens expressed by F1 cells. Models involving MHC class I disparities result in acute GVHD, which is characterized by extensive donor cell engraftment, repopulation of F1 cells with lymphoid and myeloid cells of donor origin, early proinflammatory cytokine release, and development of cytotoxic T lymphocytes with specificity for host alloantigens [26,27,28]. We show that the administration of the potent and selective A2AR agonist ATL146e, beginning 2 days prior to HSCT, results in a sustained protection from GVHD, and the delayed treatment with ATL146e successfully improves physiological symptoms of established GVHD.
MATERIALS AND METHODS
Mice
C57BL/6J (H2-kb) and B6D2F1/J (H2-kb/d) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The National Institute of Allergy and Infectious Disease Animal Care and Use Committee (Bethesda, MD, USA) approved all animal studies.
Reagents
ATL146e was a gift from Adenosine Therapeutics, LLC (Charlottesville, VA, USA).
Induction of GVHD
Female B6D2F1/J recipients (8–12 weeks old) were exposed to 850 rads total body irradiation. Twenty-four hours later, 10 × 106 bone marrow cells and 10 × 106 purified CD3+ T lymphocytes from female C57BL/6 donors (8–12 weeks old) were injected into the lateral tail veins of recipients. Bone marrow cells were collected from the femurs and tibia of donors, and CD3+ T cells were purified from the spleens of donors using mouse CD3+ T cell enrichment columns (R&D Systems, Minneapolis, MN, USA), resulting in 93–97% pure CD3+ T cells. For select experiments, primed Alzet® osmotic minipumps (Durect Corp., Cupertino, CA, USA) were implanted s.c. 24 h before irradiation, and 10 ng/kg/min ATL146e or vehicle was delivered for 7, 14, or 28 days. Alternately, animals received daily i.p. injections of 1 μg/kg ATL146e or vehicle for varying lengths of time. Weights were monitored and animals killed when total weight loss exceeded 25% of starting body weight. Control HSCTs were performed in which B6D2F1/J recipients received 10 × 106 bone marrow cells from syngenic B6D2F1/J donors.
Flow cytometry of cell-surface markers
Spleens were harvested, passed through a 40-mm nylon cell strainer (BD Biosciences, San Jose, CA, USA), and collected in PBS. Alternately, bone marrow was collected from femurs and tibia. RBCs were removed with lysing buffer (Sigma-Aldrich, St. Louis, MO, USA). Cells were washed and resuspended at 5 × 106 cells/ml in PBS supplemented with 5% FBS and 0.1% NaN3. Aliquots (0.1 ml) were placed on ice and labeled for 30 min in the dark with fluorochrome-labeled anti-mouse CD3, anti-mouse CD4, anti-mouse CD8, anti-mouse CD25, anti-mouse CD69, anti-mouse CD40L (eBioscience, San Diego, CA, USA), anti-mouse H-2Kb, and/or anti-mouse H-2Kd (BD Biosciences). Control samples were labeled with isotype-matched control antibodies. Stained cells were washed with 1 ml iced PBS and resuspended in PBS containing 1% paraformaldehyde. The fluorescence intensity was measured with a dual laser benchtop flow cytometer (FACSCalibur, Becton Dickinson, San Diego, CA, USA) with a minimum of 10,000 events collected. An excitation wavelength of 488 nm and an emission wavelength of 530 nm were used for FITC-stained cells; an excitation wavelength of 488 nm and an emission wavelength of 585 nm were used for PE-stained cells; and an excitation wavelength of 635 nm and an emission wavelength of 661 nm were used for allophycocyanin-stained cells. Analysis was performed with FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Detection of intracellular cytokines
Recipient mice were killed 4 weeks after HSCT, spleens were collected, and CD3+ T cells were column-purified. Purified cells were restimulated for 24 h at 37°C in 5% CO2 by incubation with 5 μg/mL anti-CD28 in 96-well plates coated with 2–10 μg/mL-immobilized anti-CD3 mAb (BD Biosciences). Brefeldin A (3 μg/mL) was added after 1 h of incubation. Intracellular IFN-γ and IL-2 were detected in T lymphocytes by FACS analysis using FIX and PERM cell permeabilization reagents (Caltag Laboratories, S. San Francisco, CA, USA), according to the manufacturer’s protocol. Cell-surface CD3 was detected by standard protocol.
Measurement of serum cytokines
Cytokine concentrations in serum samples were measured using mouse cytokine/chemokine multiplex immunoassay kits (Linco, St. Charles, MO, USA) with the BioPlex system (BioRad, Hercules, CA, USA) using Luminex technology.
Histology and imunohistochemistry
Mice were killed, and skin, liver, and colon were harvested and fixed in 4% paraformaldehyde in PBS, pH 7.4, and embedded in paraffin. Sections (4 μm) were subjected to standard H&E staining. Immunostaining for CD3+ T cells was performed using an anti-CD3 primary antibody (Dako, Carpinteria, CA, USA). Tissue sections were analyzed in a blinded manner.
Transmigration assays
The transmigration of purified C57BL/6 CD3+ T lymphocytes across microporous membranes toward 100 ng/mL CCL20, CXCL-12, or CXCL-10 was measured using QCM™ chemotaxis 5 μm 96-well cell migration assay kits (Chemicon International, El Segundo, CA, USA). Assays were allowed to proceed for 4 h at 37°C before migratory cells were labeled with the fluorescent nucleic acid- binding dye CyQuant® GR (Molecular Probes, Eugene, OR, USA), and fluorescence was measured with a 480/520-nm filter set. The amount of migration was expressed as RFU emitted from the migratory cells.
Statistics
Prism software (GraphPad, San Diego, CA, USA) was used for all statistical analyses. Unpaired t-tests, K-M curves, or one-way ANOVA with post-hoc Dunnett’s multiple comparison were used to compare experimental groups with a control group.
RESULTS
ATL146e limits weight loss and mortality as a result of GVHD
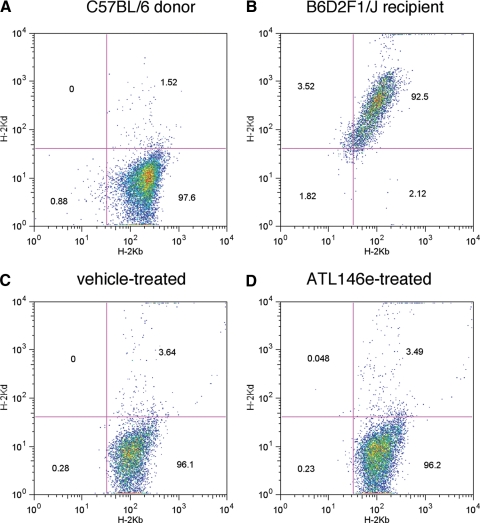
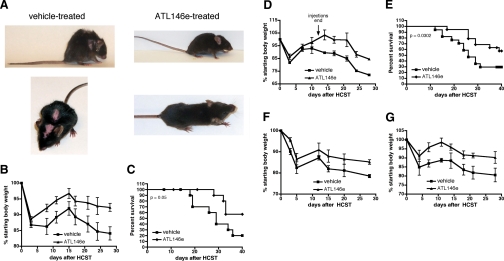
The i.v. inoculation of lethally irradiated B6D2F1/J mice with 10 × 106 bone marrow cells and 10 × 106-purified CD3+ T cells from C57BL/6 parental donors results in acute GVHD, which is characterized by progressive weight loss, tissue inflammation, and ultimately, death. Extensive parental cell engraftment is observed and is unaffected by ATL146e treatment (Fig. 1). The lethargy, hunched posture, diarrhea, and skin pathologies characteristic of acute GVHD are abrogated by the continuous, 14-day s.c. administration of 10 ng/kg/min ATL146e (Fig. 2A). Furthermore, ATL146e treatment reduces the weight loss and mortality observed in vehicle-treated mice, and more than 50% of the ATL146e-treated animals survived 40 days after transplant as compared with 20% of vehicle-treated controls (Fig. 2, B and C). The administration of 50 ng/kg/min ATL146e inhibited weight loss and enhanced survival similarly to 10 ng/kg/min; the administration of 1 ng/kg/min ATL146e did not improve weight loss or survival significantly, as compared with vehicle-treated controls (data not shown). When ATL146e is administered for 14 days via daily i.p. injections rather than continuously via osmotic minipumps, the inhibition of weight loss is somewhat minimized, but decreased mortality, as compared with vehicle-treated mice, is preserved (60% vs. 30%, respectively; Fig. 2, D and E). Alternate schedules of ATL146e treatment, including continuous s.c. delivery for 7 or 28 days, limit GVHD-associated weight loss to a similar but somewhat lesser degree than 14-day treatment (Fig. 2, F and G), with overall survival not significantly different from 14-day treatment (data not shown). GVHD is not induced in control B6D2F1/J recipients receiving matched bone marrow cells (data not shown).
Figures 1.
Donor cell engraftment is unaffected by ATL146e treatment. Bone marrow was collected from the femurs and tibia of naïve mice or mice in which GVHD was induced by allogenic HSCT. Cell-surface expression of H-2Kb and/or H-2Kd was assessed by FACS. (A) Bone marrow was collected from naïve C57BL/6 donors. (B) Bone marrow was collected from naïve B6D2F1/J recipients. (C) Bone marrow was collected 4 weeks after HSCT from mice, in which primed Alzet® osmotic minipumps were implanted s.c. 48 h before transplant, and vehicle was delivered for 14 days. (D) Bone marrow was collected 4 weeks after HSCT from mice, in which primed Alzet® osmotic minipumps were implanted s.c. 48 h before transplant, and 10 ng/kg/min ATL146e was delivered for 14 days. Data shown are from one experiment representative of three independent experiments (in all experiments, n=10 mice each for vehicle-treated and ATL146e-treated groups).
Figure 2.
ATL146e inhibits GVHD after allogenic HSCT. B6D2F1/J recipients were exposed to 850 rads total body irradiation, and 24 h later, 10 × 106 bone marrow cells and 10 × 106-purified CD3+ T lymphocytes from C57BL/6 donors were transplanted via lateral tail vein injection. (A) Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. Photographs were taken 4 weeks after transplant. (B and C) Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. (D and E) Recipients received daily i.p. injections of 1 μg/kg ATL146e or vehicle. Injections were initiated 48 h before HSCT and continued for 14 days. (F) Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 7 days. (G) Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 28 days. Weights were monitored for 4 weeks, and animals were killed when total weight loss exceeded 25% of starting body weight. Mortality was defined as mice that were found dead or had reached a moribund state as a result of excessive body weight loss, necessitating euthanasia. In all panels, data shown are from one experiment representative of three independent experiments (in all experiments, n=10 mice each for vehicle-treated and ATL146e-treated groups); error bars indicate sem.
T lymphocyte tissue infiltration and tissue inflammation/necrosis/damage are inhibited by A2AR activation
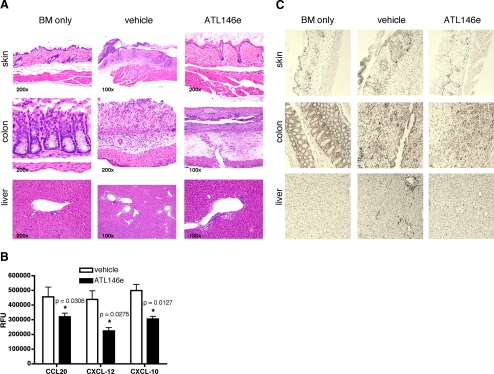
A hallmark of acute GVHD is inflammation and tissue injury in target organs including the skin, liver, and gastrointestinal tract. When observed 4 weeks after HSCT, vehicle-treated mice exhibit marked inflammation with ulceration and pustule formation in the skin, severe inflammation, edema, necrosis, and abundant amyloid-like material in the lamina propria of the colon and moderate lymphoplasmacytic vasculiltis in the liver. In contrast, mice receiving ATL146e had mostly normal-appearing skin with minimal focal ulceration, a less-pronounced submucosal edema in the colon, and mild lymphoplasmacytic vasculiltis in the liver. Tissue injury and inflammation are not merely a byproduct of irradiation and/or HSCT, as injury is absent in the tissue of control mice receiving bone marrow cells from syngenic donors (Fig. 3A). The inflammatory processes observed in target organs during acute GVHD are driven by the infiltration of alloantigen-specific T lymphocytes [29]. It has been shown that CXCL-12, CXCL-10, and CCL20 are involved in inflammatory cell migration during GVHD [30,31,32,33,34]. The in vitro transmigration of purified C57BL/6 CD3+ T cells toward CXCL-12, CXCL-10, and CCL20 is inhibited by the addition of 100 nM ATL146e (Fig. 3B). Correspondingly, when measured 4 weeks post-HSCT, fewer CD3+ cells are found in the skin, colon, and liver of mice treated with ATL146e than in vehicle-treated controls (Fig. 3C).
Figure 3.
T lymphcoyte infiltration into target organs and accompanying tissue injury are inhibited by A2AR activation. (A) Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. Alternately, control HSCTs were performed, in which B6D2F1/J recipients received 10 × 106 bone marrow (BM) cells from congenic B6D2F1/J donors. Mice were killed 4 weeks after transplant, and skin, liver, and colon were harvested and fixed in 4% paraformaldehyde in PBS, pH 7.4, and embedded in paraffin. Sections (4 μm) were subjected to standard H&E staining, which shown, is representative of three ×100 or ×200 fields of view photographed for each of three animals/treatment group in three independent experiments (for a total of nine animals/treatment group). (B) CD3+ T lymphocytes were column-purified from the spleens of C57BL/6 mice. Migration across 5 μm microporous membranes toward 100 ng/mL CCL20, CXCL-12, or CXCL-10 was measured after 4 h using QCM chemotaxis 5 μm 96-well cell migration assay kits. Data are shown as the mean ± sem from three independent experiments performed in triplicate. *, P < 0.05, versus vehicle control, as assessed by unpaired t-test. (C) Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. Alternately, control HSCTs were performed, in which B6D2F1/J recipients received 10 × 106 bone marrow cells from congenic B6D2F1/J donors. Mice were killed 4 weeks after transplant, and skin, liver, and colon were harvested and fixed in 4% paraformaldehyde in PBS, pH 7.4, and embedded in paraffin. Immunostaining for CD3+ T cells was performed on 4 μm sections using an anti-CD3 primary antibody. Immunostaining shown is representative of three ×100 fields of view photographed for each of three animals/treatment group in three independent experiments (for a total of nine animals/treatment group).
Alloreactive donor T cell activation is inhibited by ATL146e treatment
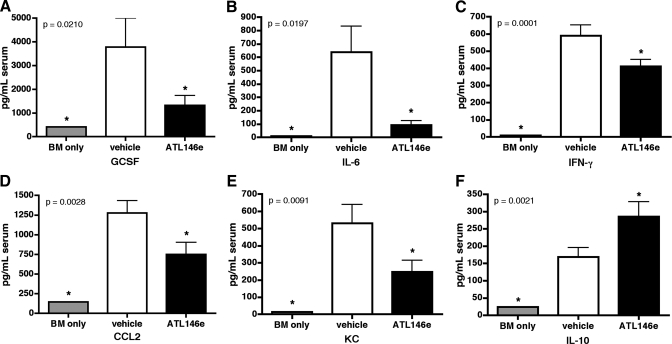
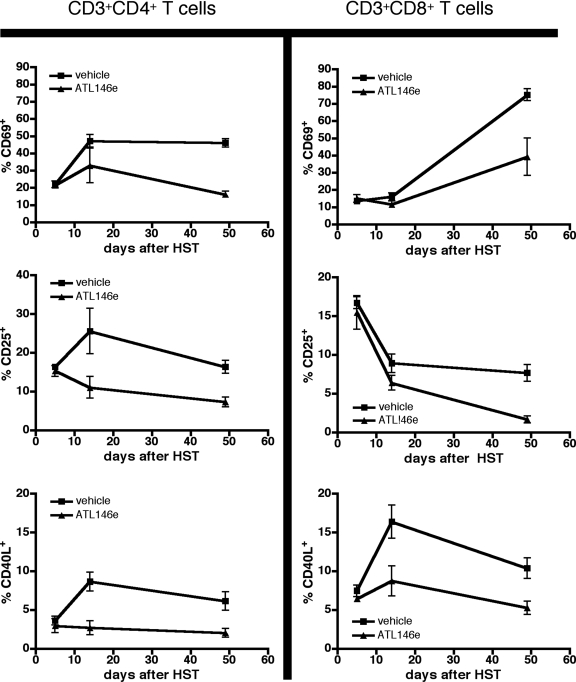
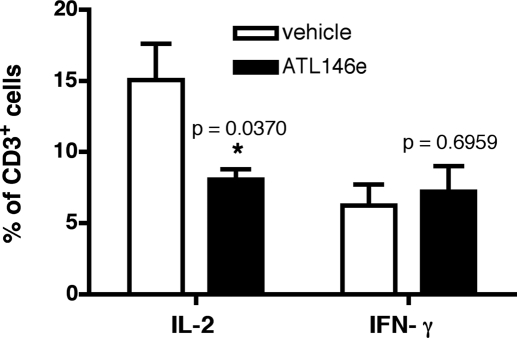
In models of acute GVHD, disease progression is accompanied by the production and release of proinflammatory cytokines and chemokines [35, 36]. We show that 4 weeks after HSCT, serum levels of IFN-γ, IL-6, CCL2, KC, and G-CSF are elevated above baseline in vehicle-treated mice, but circulating levels of these cytokines are reduced by A2AR activation (Fig. 4, A–E). Additionally, serum levels of the anti-inflammatory, tolerance-inducing cytokine IL-10 are enhanced by ATL146e treatment (Fig. 4F). Moreover, up-regulation of the cell-surface activation markers CD25, CD69, and CD40L by CD4+ and CD8+ T cells is minimized by ATL146e treatment, as compared with expression levels in vehicle controls (Fig. 5). Although the inhibition of alloreactive T lymphocyte activity is essential to limit the progression of acute GVHD, the induction of a permanent hyporesponsiveness in these cells would likely have untoward consequences, including increased susceptibility to infection. When CD3+ T cells are collected from the spleens of F1 recipients 4 weeks post-HSCT and restimulated ex vivo, cells collected from ATL146e and vehicle-treated mice produce IFN-γ comparably, but IL-2 production by cells exposed to ATL146e is impaired (Fig. 6).
Figure 4.
GVHD-induced elevation of proinflammatory cytokine and chemokine levels is reduced by ATL146e administration. Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. Alternately, control HSCTs were performed in which B6D2F1/J recipients received 10 × 106 bone marrow cells from congenic B6D2F1/J donors. Blood was collected 4 weeks after transplant, and serum concentrations of G-CSF (A), IL-6 (B), IFN-γ (C), CCL2 (D), KC (E), and IL-10 (F) were measured using multiplex immunoassay kits with the BioPlex system. Data shown are from three independent experiments (in all experiments, n=3 mice/treatment group); error bars indicate sem; *, P < 0.05, versus vehicle control, as assessed by one-way ANOVA, followed by Dunnetts multiple comparison test.
Figure 5.
Up-regulation of activation marker expression by donor T cells is reduced by ATL146e. Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. Mice were killed at various time-points after transplant, and spleens were collected. Cell-surface expression of CD69, CD25, and CD40L was assessed by FACS. CD3+CD4+ or CD3+CD8+ T cells were gated on for analysis. Data shown are from three independent experiments (in all experiments, n=3 mice/treatment group); error bars indicate sem.
Figure 6.
Prolonged activation of the A2AR modifies T cell cytokine production profiles in response to restimulation. Osmotic minipumps were implanted s.c. in recipients 48 h before HSCT, and vehicle or 10 ng/kg/min ATL146e was delivered for 14 days. Mice were killed 4 weeks after transplant, spleens were collected, and CD3+ T cells were column-purified. Purified cells were restimulated with immobilized anti-CD3 mAb and anti-CD28 mAb for 24 h at 37°C in 5% CO2. FACS analysis was used to measure intracellular IL-2 and IFN-γ; CD3+ cells were gated on for analysis. Data shown are from three independent experiments (in all experiments, n=3 mice/treatment group); error bars indicate sem; *, P < 0.05, versus vehicle control, as assessed by unpaired t-test.
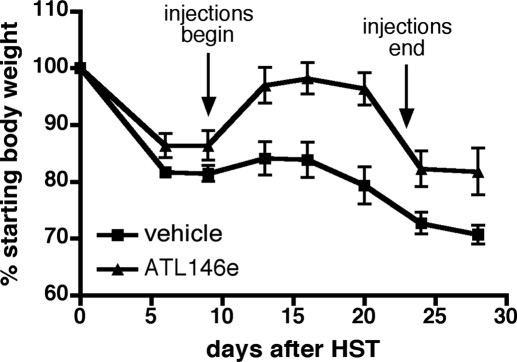
ATL146e administration inhibits the progression of established GVHD
Although prophylactic therapies for GVHD are important, perhaps equally important are therapies for established GVHD and/or GVHD, which is refractory to frontline therapy [37]. As A2AR agonists are known to terminate the proinflammatory activities of activated T cells, we hypothesized that ATL146e might be effective at stabilizing or reversing the symptoms of active GVHD. In support of this hypothesis, we show that when daily i.p. injections of ATL146 are delayed until 9 days after HSCT, weight loss is reversed, and the animals return to nearly pre-transplant weights for the duration of treatment. Although a loss in body weight is observed upon the termination of ATL146e treatment, a sustained improvement over vehicle controls is maintained (Fig. 7).
Figure 7.
Delayed administration of ATL146e reverses GVHD-associated weight loss successfully. Recipient mice received daily i.p. injections of 1 μg/kg ATL146e or vehicle. Injections were initiated 9 days after HSCT and continued for 14 days. Weights were monitored for 4 weeks, and animals were killed when total weight loss exceeded 25% of starting body weight. Data shown are from one experiment representative of three independent experiments (in all experiments, n=10 mice each for vehicle-treated and ATL146e-treated groups); error bars indicate sem.
DISCUSSION
Although the pathophysiology of acute GVHD is complex, accumulating evidence suggests that pharmacologic agents that inhibit the activity of alloreactive T lymphocytes can effectively limit the morbidity and mortality associated with the disorder. Unfortunately, many such agents carry with them significant side-effects, including increased incidence of infection and delayed immune reconstitution that diminish their use [37]. We show that the selective activation of the A2AR with the synthetic agonist ATL146e effectively protects from acute GVHD, as manifested by decreased weight loss and mortality. Moreover, our results suggest that this effect is elicited by the inhibition of donor T cell activation and migration to target organs and also possibly by the induction of an anti-inflammatory, tolerance-inducing environment. It is well established that the activation of the A2AR limits many functions of T effector cells including proinflammatory cytokine production, proliferation, and activation marker expression [38,39,40,41]. It has also been shown recently in vitro that when T cells are activated in the presence of an A2AR agonist, they are rendered anergic, as evidenced by their failure to proliferate or produce IL-2 or IFN-γ upon restimulation. Furthermore, the activation of the A2AR inhibits the generation of adaptive T effector cells and promotes the generation of adaptive Tregs by reducing the production of IL-6 and enhancing the production of TGF-β after antigen stimulation [25]. In accord with these data, we show in the current study that the production of IL-6 during acute GVHD is limited by ATL146e treatment, the production of the anti-inflammatory cytokine IL-10 is elevated, and the in vivo exposure of donor T cells to ATL146e results in their reduced capacity to produce IL-2 upon restimulation ex vivo. These data are consistent with the demonstrated activity of A2AR agonists to induce anergy and skew T cell differentiation from proinflammtory Th1 cells to tolerance-inducing Tregs. IL-2 has long been recognized to play a critical role in the pathogenesis of acute GVHD, a role that is underscored by the beneficial effects often elicited by cyclosporine prophylaxis [42]. The effect of ATL146e treatment to evoke a sustained decrease in IL-2 production by donor T cells is therefore quite important.
Interestingly, the capacity of T cells from ATL146e-treated mice to produce IFN-γ upon restimulation is preserved, perhaps explaining why these mice show no increased susceptibility to infection despite the immunosuppressive activities of A2AR activation. The mechanism by which prolonged ATL146e treatment elicits this unique T cell phenotype warrants further investigation, as do the physiological effects of such a phenotype, which may be beneficial in limiting T cell proliferation in response to alloantigen stimulation, while preserving effector activity in response to infectious stimuli. It will be interesting to see if this change in T cell cytokine profile post-ATL146e treatment is transient or sustained upon the termination of agonist administration. The activation of the Gs-coupled A2AR on CD4+ T cells is known to result in a sustained elevation of intracellular cAMP [38], and a previous study has demonstrated that cAMP-elevating agents induce long-term, alloantigen-specific tolerance in CD4+ T cells without impairing responses to nonspecific mitogens [12]. Taken together with our observation of preserved IFN-γ production in ATL146e-treated cells, these data suggest that A2AR activation during GVHD likely, selectively impairs alloantigen-specific T cell responses without altering proinflammatory activity triggered by global simuli. These observations are important, as A2ARs are known to play a critical and nonredundant role in the regulation of antipathogen immunity, with receptor activation by endogenous adenosine functioning to limit tissue damage, resulting from immune responses to bacterial endotoxin and exotoxin [43]. Thus, although A2AR activation is a vital part of the physiological negative-feedback mechanism for limiting potentially harmful inflammation, the prolonged administration of A2AR agonists has the potential to increase susceptibility to infection by causing the premature or inappropriate termination of immune responses, an effect that we did not observe in our study. Moreover, the administration of ATL146e does not affect donor cell engraftment, thereby making it unlikely that A2AR activation affects transplant success or immune system reconstitution after HSCT.
Although it has been shown that alloreactive T cells enter peripheral lymphoid organs within hours after HSCT, the initial proliferation of CD4+ and CD8+ T cells in secondary lymphoid organs occurs rapidly, and alloreactive lymphocytes migrate to target organs within days of HSCT [29, 44], we show that ATL146e acts to reverse symptoms of acute GVHD, even when administration is delayed until 9 days after HSCT. This observation suggests that the initial events in alloreactive T cell activation need not be prevented to limit the progression of GVHD and also that ATL146e may be efficacious, not only as a prophylactic agent but also as a second-line therapy for steroid-refractory GVHD. It is notable, however, that the most significant and persistent effects of ATL146e treatment were garnered when the drug was administered continuously via an osmotic pump, beginning 2 days before transplant, and similar, if slightly less dramatic, protection was imparted by daily i.p. injections beginning 2 days before HSCT. The reduced efficacy of daily injections as compared with continuous delivery can possibly be explained by the fact that 1 μg/kg ATL146e daily likely only occupies the A2AR with compound for a short period of time, as the half-life of ATL146e is less than 10 min. Once-a-day injections may therefore be insufficient to establish the steady-state level of drug needed to elicit maximal effect. Interestingly, increasing the dose of ATL146e administered (to 50 ng/kg/min) did not improve the outcome significantly: reduction in weight loss and mortality was similar to that elicited by the lower dose of drug. We therefore used the lower dose of ATL146e in our experiments to minimize the risk of possible cardiovascular side-effects and/or dampening of antipathogen immunity, while still maintaining optimal drug efficacy. These findings are consistent with previous studies that have found the administration of 10 ng/kg/min ATL146e (the lower dose) to be effective in limiting inflammation in murine models of reperfusion injury [15]. Importantly, when treatment was initiated before HSCT, whether via pump or daily injections, the inhibition of weight loss was preserved to a greater extent after termination of treatment than when treatment was delayed to 9 days. Similarly, one study has found that improved clinical outcomes are achieved when corticosteroid treatment is initiated early after transplant (before Day 5) [45]. It is possible that an early step in the inflammatory cascade must be blocked to induce lasting tolerance as opposed to temporary improvement; this warrants further investigation. These observations correlate well with the results of a previous study demonstrating that the pharmacological elevation of cAMP induces long-term hyporesponsiveness in T cells only when antigen stimulation occurs at the time of cAMP elevation [12]. Consequently, we hypothesize that the early administration of ATL146e may induce tolerance, and delayed treatment may act to limit the inflammatory assault of activated, alloreactive T cells. The favorable effects achieved by delayed ATL146e treatment must not be minimized, however, as most currently used salvage therapies elicit low patient-response rates [6, 7, 9], and a dramatic improvement is elicited while ATL146e is actively administered. It bears investigation whether a more chronic activation of the A2AR would result in a persistent protection from GVHD, but in any case, it is promising that a response is observed even if therapy is delayed until GVHD is actively progressing.
To date, studies using A2AR agonists as anti-inflammatory agents have focused primarily on the effects of short-term treatment on acute inflammatory events, such as reperfusion injury. The efficacy and effects of A2AR agonists in the treatment of more chronic conditions have yet to be fully determined. It is possible that the extended activation of the A2AR will result in receptor desensitization, hypotension, or hemorrhage. Furthermore, immunosuppressive signaling through the A2AR protects cancerous tissues from anti-tumor T lymphocytes by inhibiting the ability of the T cells to limit tumor growth, destroy metastases, and prevent neovascularization. This suggests that in addition to limiting antipathogen immunity, prolonged treatment with A2AR agonists has the potential to compromise anti-tumor immunity [46]. However, there is no evidence of severe toxicity evoked by the chronic A2AR activation resulting from persistent use of methotrexate [23, 24], and it is encouraging that the oral administration of an A2AR agonist for 20 days limits colitis successfully with no observed side-effects [17]. Similarly, we show that the prolonged s.c. or i.p. administration of ATL146e (up to 14 days) results in a continued protection from GVHD, with no identified adverse effects. It will be interesting to see if A2AAR agonists prove to be more efficacious than or can be used synergistically with current therapies in the treatment of acute GVHD, much as short-course methotrexate is used in harmony with corticosteroids as a first-line prophylactic. As the anti-inflammatory activities of methotrexate are mediated by A2AR activation, it seems likely that the use of A2AR-selective agonists might similarly augment the protective effects of steroid treatment.
AUTHORSHIP
C. M. L. and P-C. L. performed experiments; C. M. L. collected and analyzed data; C. M. L., J. L., E. M. K., and H. L. M. designed the research; and C. M. L. wrote the manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Lily Cheng for her invaluable assistance with histopathology and Gabriel Mannis for preliminary work with the GVHD model.
DISCLOSURE
J. L. owns equity in Adenosine Therapeutics, LLC (Charlottesville, VA, USA.), a biopharmaceutical company developing ATL146e for clinical applications. The authors declare no other competing financial interests.
Footnotes
Abbreviations: ATL146e=4-{3-[6-Amino- 9-(5-ethylcarbamoyl-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-2-yl]prop-2-ynyl}cyclohexanecarboxylic acid methyl ester, CD40L=CD40 ligand, GVHD=graft-versus-host disease, HSCT=hematopoietic stem cell transplantation, KC=keratinocyte-derived chemokine, RFU=relative fluorescence unit, Treg=T regulatory cell
References
- Morris E S, Hill G R. Advances in the understanding of acute graft-versus-host disease. Br J Haematol. 2007;137:3–19. doi: 10.1111/j.1365-2141.2007.06510.x. [DOI] [PubMed] [Google Scholar]
- Han S B, Lee C W, Yoon Y D, Kim Y K, Lee K, Park S K, Kim H M. Effective prevention of lethal acute graft-versus-host disease by combined immunosuppressive therapy with prodigiosin and cyclosporine A. Biochem Pharmacol. 2005;70:1518–1526. doi: 10.1016/j.bcp.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Furlong T, Kiem H P, Appelbaum F R, Carpenter P A, Deeg H J, Doney K, Flowers M E, Mielcarek M, Nash R A, Storb R, Martin P J. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14:531–537. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash R A, Antin J H, Karanes C, Fay J W, Avalos B R, Yeager A M, Przepiorka D, Davies S, Petersen F B, Bartels P, Buell D, Fitzsimmons W, Anasetti C, Storb R, Ratanatharathorn V. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- MacMillan M L, Weisdorf D J, Wagner J E, DeFor T E, Burns L J, Ramsay N K, Davies S M, Blazar B R. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A. Management of acute graft-versus-host disease. Br J Haematol. 2007;137:87–98. doi: 10.1111/j.1365-2141.2007.06533.x. [DOI] [PubMed] [Google Scholar]
- Jacobsohn D A. Novel therapeutics for the treatment of graft-versus-host disease. Expert Opin Investig Drugs. 2002;11:1271–1280. doi: 10.1517/13543784.11.9.1271. [DOI] [PubMed] [Google Scholar]
- Jacobsohn D A. Emerging therapies for graft-versus-host disease. Expert Opin Emerg Drugs. 2003;8:323–338. doi: 10.1517/14728214.8.2.323. [DOI] [PubMed] [Google Scholar]
- Holler E. Progress in acute graft versus host disease. Curr Opin Hematol. 2007;14:625–631. doi: 10.1097/MOH.0b013e3282f08dd9. [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Asakura S, Matsuoka K, Sakoda Y, Koyama M, Aoyama K, Tanimoto M, Teshima T. FTY720 enhances the activation-induced apoptosis of donor T cells and modulates graft-versus-host disease. Eur J Immunol. 2007;37:271–281. doi: 10.1002/eji.200636123. [DOI] [PubMed] [Google Scholar]
- Trenado A, Sudres M, Tang Q, Maury S, Charlotte F, Grégoire S, Bonyhadi M, Klatzmann D, Salomon B L, Cohen J L. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol. 2006;176:1266–1273. doi: 10.4049/jimmunol.176.2.1266. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy M J, Chen Z M, Gramaglia I, Taylor P A, Panoskaltsis-Mortari A, Vogtenhuber C, Palmer E, Grader-Beck T, Boussiotis V A, Blazar B R. Elevation of intracellular cyclic AMP in alloreactive CD4(+) T cells induces alloantigen-specific tolerance that can prevent GVHD lethality in vivo. Biol Blood Marrow Transplant. 2007;13:530–542. doi: 10.1016/j.bbmt.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Lappas C M, Sullivan G W, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- Awad A S, Huang L, Ye H, Duong E T, Bolton W K, Linden J, Okusa M D. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- Lappas C M, Day Y J, Marshall M A, Engelhard V H, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Naganuma M, Wiznerowicz E B, Lappas C M, Linden J, Worthington M T, Ernst P B. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast C C, Moskaluk C A, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Day Y J, Huang L, McDuffie M J, Rosin D L, Ye H, Chen J F, Schwarzschild M A, Fink J S, Linden J, Okusa M D. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Day Y, Toufektsian M C, Xu Y, Ramos S I, Marshall M A, French B A, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- Odashima M, Otaka M, Jin M, Horikawa Y, Matsuhashi T, Ohba R, Linden J, Watanabe S. Selective adenosine A receptor agonist, ATL146e, attenuates stress-induced gastric lesions in rats. J Gastroenterol Hepatol. 2005;20:275–280. doi: 10.1111/j.1440-1746.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Sullivan G W, Fang G, Linden J, Scheld W M. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- Montesinos M C, Desai A, Cronstein B N. Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res Ther. 2006;8:R53. doi: 10.1186/ar1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos M C, Yap J S, Desai A, Posadas I, McCrary C T, Cronstein B N. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43:656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Zarek P E, Huang C T, Lutz E R, Kowalski J, Horton M R, Linden J, Drake C G, Powell J D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim F T, Sharrow S O, Payne S, Shearer G M. Repopulation of host lymphohematopoietic systems by donor cells during graft-versus-host reaction in unirradiated adult F1 mice injected with parental lymphocytes. J Immunol. 1991;146:2108–2115. [PubMed] [Google Scholar]
- Via C S, Finkelman F D. Critical role of interleukin-2 in the development of acute graft-versus-host disease. Int Immunol. 1993;5:565–572. doi: 10.1093/intimm/5.6.565. [DOI] [PubMed] [Google Scholar]
- Via C S, Sharrow S O, Shearer G M. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987;139:1840–1849. [PubMed] [Google Scholar]
- Beilhack A, Schulz S, Baker J, Beilhack G F, Wieland C B, Herman E I, Baker E M, Cao Y A, Contag C H, Negrin R S. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapara M Y, Leng C, Kim Y M, Bronson R, Lokshin A, Luster A, Sykes M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12:623–634. doi: 10.1016/j.bbmt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Piper K P, Horlock C, Curnow S J, Arrazi J, Nicholls S, Mahendra P, Craddock C, Moss P A. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz M G, Lira S A, Charo I, Cook D N, Weissman I L, Strober S, Engleman E G. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beider K, Nagler A, Wald O, Franitza S, Dagan-Berger M, Wald H, Giladi H, Brocke S, Hanna J, Mandelboim O, Darash-Yahana M, Galun E, Peled A. Involvement of CXCR4 and IL-2 in the homing and retention of human NK and NK T cells to the bone marrow and spleen of NOD/SCID mice. Blood. 2003;102:1951–1958. doi: 10.1182/blood-2002-10-3293. [DOI] [PubMed] [Google Scholar]
- Varona R, Cadenas V, Gomez L, Martinez A C, Marquez G. CCR6 regulates CD4+ T-cell-mediated acute graft-versus-host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hiraki A, Aoe K, Murakami T, Ikeda K, Masuda K, Matsuo K, Shinagawa K, Ishimaru F, Sugi K, Darzynkiewicz Z, Tanimoto M. Serum cytokine concentrations and acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation: concurrent measurement of ten cytokines and their respective ratios using cytometric bead array. Int J Mol Med. 2006;17:881–885. [PubMed] [Google Scholar]
- Ju X P, Xu B, Xiao Z P, Li J Y, Chen L, Lu S Q, Huang Z X. Cytokine expression during acute graft-versus-host disease after allogeneic peripheral stem cell transplantation. Bone Marrow Transplant. 2005;35:1179–1186. doi: 10.1038/sj.bmt.1704972. [DOI] [PubMed] [Google Scholar]
- Kim S S. Treatment options in steroid-refractory acute graft-versus-host disease following hematopoietic stem cell transplantation. Ann Pharmacother. 2007;41:1436–1444. doi: 10.1345/aph.1K179. [DOI] [PubMed] [Google Scholar]
- Lappas C M, Rieger J M, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Sullivan G W. Adenosine A2A receptor agonists as anti-inflammatory agents. Curr Opin Investig Drugs. 2003;4:1313–1319. [PubMed] [Google Scholar]
- Butler J J, Mader J S, Watson C L, Zhang H, Blay J, Hoskin D W. Adenosine inhibits activation-induced T cell expression of CD2 and CD28 co-stimulatory molecules: role of interleukin-2 and cyclic AMP signaling pathways. J Cell Biochem. 2003;89:975–991. doi: 10.1002/jcb.10562. [DOI] [PubMed] [Google Scholar]
- Apasov S G, Sitkovsky M V. The extracellular versus intracellular mechanisms of inhibition of TCR-triggered activation in thymocytes by adenosine under conditions of inhibited adenosine deaminase. Int Immunol. 1999;11:179–189. doi: 10.1093/intimm/11.2.179. [DOI] [PubMed] [Google Scholar]
- Jaksch M, Mattsson J. The pathophysiology of acute graft-versus-host disease. Scand J Immunol. 2005;61:398–409. doi: 10.1111/j.1365-3083.2005.01595.x. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Panoskaltsis-Mortari A, Price A, Hermanson J R, Taras E, Lees C, Serody J S, Blazar B R. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- Van Lint M T, Milone G, Leotta S, Uderzo C, Scimè R, Dallorso S, Locasciulli A, Guidi S, Mordini N, Sica S, Cudillo L, Fagioli F, Selleri C, Bruno B, Arcese W, Bacigalupo A. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad S J, Ronchese F, Lukashev D, Wong M K, Huang X, Caldwell S, Liu K, Smith P, Chen J F, Jackson E K, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]