Abstract
Background
Given the association between obesity and kidney disease, transplant professionals have debated the appropriateness of accepting obese live kidney donors. We hypothesized that compared to normal weight donors, donors with elevated body mass index (BMI) would have 1) more peri-operative re-admissions and re-operations, and 2) a greater rise in blood pressure, greater percent rise in serum creatinine, and a greater loss of estimated glomerular filtration rate (eGFR) following nephrectomy.
Methods
Retrospective cohort study using Organ Procurement and Transplantation Network data on live donors who donated kidneys from 7/1/2004 –12/31/2005.
Results
9319 live donor kidney transplants were performed. After eliminating donors with missing BMI data, 5304 donors were analyzed, among whom 2108 (40.0%) were overweight (25 ≤ BMI < 30), 944 (17.8%) were obese (30 ≤ BMI < 35), and 250 (4.7%) were very obese (BMI>=35). Re-admission and re-operation rates did not differ across donor BMI categories. At baseline and at 6 months after nephrectomy, higher BMI was associated with higher blood pressure (p<0.01), but changes in systolic blood pressure from baseline were similar across BMI categories (p=0.40). At six months, decline in eGFR from baseline (p=0.63) and percent change in creatinine (p=0.11) did not differ significantly across groups. Delayed graft function was more common among recipients of kidneys from very obese donors (OR 2.16, CI 1.20 – 3.89, p=0.01), but the rates of recipient allograft failure and mortality across donor BMI groups were similar.
Conclusion
Short-term follow-up data show good outcomes for donors with elevated BMI and their recipients.
Keywords: live donor, obesity, kidney transplantation
Background
Increasing wait-time for deceased donor kidney transplantation and superior outcomes with live donor transplantation have led to a rise in the volume of live donor transplants in the United States (US) over the past decade(1). Recognition of increasing body mass index (BMI) as a risk factor for chronic kidney disease (CKD), however, has raised concerns about the appropriateness of accepting obese live kidney donors (2–4). Reflecting this uncertainty about obese live kidney donors, there is wide variation in transplant center policy regarding exclusion of donors on the basis of BMI (5).
Obesity has long been recognized as a cause of proteinuria and glomerular disease and could also cause renal disease indirectly through hypertension or diabetes (6, 7). Studies of the general population have also demonstrated increased risk of CKD with obesity even after adjustment for blood pressure and diabetes (8–11). Biopsies of obese patients commonly show glomerular changes such as glomerulomegaly and increased mesangial matrix (7, 12). Additionally, physiologic studies have revealed that obese patients have glomerular hyperfiltration and elevation of measured glomerular filtration rate (mGFR) (13, 14). Notably, following nephrectomy, live kidney donors are known to have compensatory hyperfiltration in the remaining kidney (15). Therefore, a live donor with pre-existing obesity-related hyperfiltration may have a diminished capacity to undergo further adaptive hyperfiltration after nephrectomy compared to a normal weight donor.
Although long-term studies suggest that donor nephrectomy does not increase risk of mortality or end-stage renal disease (ESRD), little is known about outcomes for overweight and obese live kidney donors (16–21). Single center reports have suggested that obese live donors have a higher rate of peri-operative complications, such as wound infections (2, 3). A study of predominantly Caucasian live donors at the Mayo Clinic showed that elevations in BMI were associated with higher blood pressure before and after nephrectomy compared to normal weight donors (2). A meta-analysis that examined outcomes for obese live donors found that change in GFR after nephrectomy was not greater than among non-obese donors (22–24).
The primary aim of this study was to compare short-term outcomes for live donors according to BMI category at renal transplant centers across the US. We hypothesized that compared to normal weight donors, donors with elevated BMI would have 1) higher rates of peri-operative re-admission and re-operation, and 2) a greater rise in blood pressure, greater rise in serum creatinine, and a greater loss of estimated glomerular filtration rate (eGFR) following nephrectomy.
A secondary aim of the study was to compare short-term outcomes for recipients of live donor kidney transplants across donor BMI categories. We hypothesized that there is no association between recipient outcome and donor BMI category.
Methods
OPTN registry data were used to conduct a retrospective cohort study of US live kidney donors during the 18 months from July 2004 through December 2005. Renal transplant centers first began reporting donor weight and height to the OPTN in July 2004.
Generation of cohort
We restricted our analysis to donors with complete data related to BMI at the time of nephrectomy (the primary exposure). We categorized body mass index into 4 groups: normal weight (BMI<25), overweight (BMI >=25 & <30), obese (BMI>=30 & <35) and very obese (BMI>=35), as defined by the World Health Organization.(25) We also examined outcomes for recipients of kidneys donated by this group of living donors with complete BMI data.
Primary and secondary donor outcomes
Primary outcomes were changes in blood pressure, estimated glomerular filtration rate (eGFR), and percent change in serum creatinine (calculated as: [6month creatinine – baseline creatinine]/baseline creatinine) at 6 months compared to pre-nephrectomy values. The reference group was donors with normal weight.
eGFR was calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation, which uses age, race, gender and serum creatinine (26). The MDRD equation is known to underestimate mGFR in individuals without CKD (27). A prior study of renal function in obese and non-obese patients without CKD, however, showed that the underestimation of renal function when using the MDRD equation is consistent across categories of BMI (28). For our analysis, we used intra-individual change in eGFR for comparing renal function across donor BMI categories.
The primary peri-operative complications of interest among donors were re-operation and re-admission within 6 weeks. We also assessed, secondarily, the rates of conversion from laparoscopic to open surgery, length of stay, and vascular and non-vascular complications. Vascular and non-vascular complications were not treated as primary outcomes because the OPTN live donor form does not define in detail the range of complications that should be reported.
Additionally, we compared donors at 6 months after nephrectomy with regard to the following secondary endpoints: serum creatinine; eGFR; percent of remaining eGFR (calculated as: 6 month eGFR/baseline eGFR), systolic and diastolic blood pressure; and presence of hypertension. We categorized donors as hypertensive if they had systolic blood pressure >=140 mm Hg, diastolic blood pressure >=90 mm Hg, or if a clinical history of hypertension was reported (29). Donor blood pressure, creatinine, and 1/Cr were also compared across BMI categories using linear regression adjusted for donor age, race and gender.
Additionally, we reported analyses of donor eGFR and blood pressure at one year following nephrectomy. These one year outcomes were treated as secondary because a much greater number of donors had missing data at that time point compared to six months after nephrectomy.
Recipient outcomes
We compared delayed graft function (DGF, defined as need for dialysis in the first week after transplantation), primary non-function (PNF, defined as allograft survival <3 days), acute rejection within the first post-transplant year, one-year allograft survival, and one-year mortality.
Elimination of clinically suspect data related to complexity
To eliminate clinically suspicious values, we coded as “missing” those values that seemed implausible in a donor. We assumed that these data were incorrectly entered into the registry database. For instance, we re-coded as missing pre-nephrectomy eGFR <30 ml/min/m2, and post-nephrectomy rise in eGFR>10 ml/min/m2 from baseline. Less than 1.5% of data points for any variable were re-coded as missing.
Statistical analysis
Analyses were performed using Stata software (Stata 10.0, Stata Corporation, College Station, Texas). Two-sided tests of hypotheses were conducted, with a p-value < 0.05 as the criterion for statistical significance. Means were reported with standard errors.
Unadjusted analyses
For univariate comparisons of means between subjects with versus without missing data related to BMI, we used the two-sample t-test with unequal variances. For comparisons of means across BMI groups, we used the ANOVA test. For categorical variables, we used chi-square tests.
Primary non-function, delayed allograft function and acute rejection
We compared the risk of PNF using logistic regression. No independent variables were added to the model due to a very small number of events; the addition of independent variables (other than donor BMI category) would run the risk of overfitting the model. The multivariable logistic regression models for DGF and acute rejection included terms for the following variables: recipient age, race (black/not black), gender, prior transplant, hepatitis C seropositivity, prior dialysis, diabetes, body mass index, elevated panel reactive antibody titer (defined as >=20%), use of antibody induction therapy, baseline immunosuppression regimen, human leukocyte antigen mismatch (an ordinal variable defined as 0=zero mismatch, 1=one mismatch, and 2=greater than one mismatch); donor age, race, gender, and BMI category. The hypothesis of good fit for these models was not rejected by the Hosmer-Lemeshow test.
Allograft failure and patient mortality
We assessed the assumption of proportional hazards and model fit by assessing log-log plots for one-year allograft failure and one-year patient mortality. The multivariable Cox regression model for allograft survival included the same independent variables listed above for the logistic regression analysis. The model for patient mortality included donor and recipient age, gender and race, but not other variables due to a limited number of deaths and lack of power.
Results
During the 18-month study period, 9319 live kidney donor transplants were performed in the US. A substantial number of donors – 4015 (43%) – had missing data related to BMI. After eliminating those with missing BMI data, 5304 donors from 207 transplant centers were analyzed. Among donors analyzed, two thousand and two (37.8%) were normal weight (BMI <25), 2108 donors (39.7%) were overweight (BMI >=25 and <30), 944 donors (17.8%) were obese (BMI >=30 and <35), and 250 donors (4.7%) were very obese (BMI > 35).
We compared donors who were excluded due to missing data related to BMI versus those included in the study. This comparison showed no difference between excluded and included donors in gender (p=0.83), relationship to recipient (p=0.10), and black race. Excluded donors were slightly older (age 41.0 versus 39.9 years for included donors, p<0.001), and less likely to be Hispanic (10.6% versus 14.0% of included donors, p<0.001). Excluded donors had clinically unimportant differences in baseline systolic blood pressure (121.3 mm Hg for excluded donors versus 120.4 mm Hg for included donors, p=0.004) and in baseline eGFR (89.8 ml/min/m2 for excluded donors versus 91.8 ml/min/m2 for included donors, p<0.001). No significant difference existed between the mean diastolic blood pressure of excluded and included donors (p=0.10).
Pre-nephrectomy characteristics of donors by BMI category (Table 1)
Table 1.
Demographic and clinical characteristics of live donors and their recipients
| Donor characteristic | BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | p |
|---|---|---|---|---|---|
| N | 2002 | 2108 | 944 | 250 | |
| Age, years (s.e.) | 39.4 ± 0.3 | 40.6 ± 0.2 | 40.0 ± 0.4 | 37.4 ± 0.6 | <0.01 |
| Female (%) | 1345 (67.2) | 1112 (52.8) | 537 (56.9) | 170 (68.0) | <0.01 |
| Race (%) | <0.01 | ||||
| White | 1393 (69.6) | 1384 (65.7) | 600 (63.6) | 147 (58.8) | |
| Black | 237 (11.8) | 314 (14.9) | 159 (16.8) | 63 (25.2) | |
| Hispanic | 229 (11.4) | 320 (15.2) | 158 (16.7) | 33 (13.2) | |
| Asian | 118 (5.9) | 52 (2.5) | 13 (1.4) | 2 (0.8) | |
| Other | 25 (1.3) | 38 (1.8) | 14 (1.5) | 5 (2.0) | |
| US Citizen (%) | 1879 (94.3) | 1989 (94.9) | 892 (94.9) | 242 (97.2) | 0.02 |
| Education (%) | <0.01 | ||||
| Grade school or less | 34 (1.7) | 59 (2.8) | 18 (1.9) | 2 (0.8) | |
| High school | 501 (25.0) | 554 (26.3) | 283 (30.0) | 83 (33.2) | |
| Attended college/technical school | 418 (20.9) | 455 (21.6) | 223 (23.6) | 51 (20.4) | |
| Associate/Bachelor degree | 403 (20.1) | 394 (18.7) | 136 (14.4) | 35 (14.0) | |
| Graduate degree | 151 (7.5) | 136 (6.5) | 45 (4.8) | 6 (2.4) | |
| Unknown | 495 (24.7) | 510 (25.3) | 239 (25.3) | 73 (29.2) | |
| Relation to recipient (%) | <0.01 | ||||
| Full sibling | 554 (27.7) | 553 (26.2) | 245 (25.6) | 65 (26.0) | |
| Child | 345 (17.2) | 406 (19.3) | 209 (22.1) | 65 (26.0) | |
| Spouse | 234 (11.7) | 254 (12.1) | 128 (13.6) | 36 (14.4) | |
| Other biological | 170 (8.5) | 149 (7.1) | 67 (7.1) | 14 (5.6) | |
| Parent | 127 (6.3) | 182 (8.6) | 79 (8.4) | 23 (9.2) | |
| Non-biological, unrelated: Anonymous donation | 34 (1.7) | 24 (1.1) | 5 (0.5) | 2 (0.8) | |
| Half-sibling | 28 (1.4) | 18 (0.9) | 12 (1.3) | 1 (0.4) | |
| Life partner | 20 (1.0) | 11 (0.5) | 7 (0.7) | 2 (0.8) | |
| Non-biological, unrelated: Paired exchange | 9 (0.5) | 10 (0.5) | 1 (0.1) | 0 (0.0) | |
| Twin | 4 (0.2) | 4 (0.2) | 2 (0.2) | 2 (0.8) | |
| Non-biological, live/Deceased Donor Exchange | 4 (0.2) | 4 (0.2) | 2 (0.2) | 0 (0.0) | |
| Pre-nephrectomy BMI (kg/m2) | 22.4 ± 0.0 | 27.3 ± 0.0 | 31.9 ± 0.0 | 38.5 ± 0.3 | <0.01 |
| Pre-nephrectomy weight (kg) | 64.3 ± 0.2 | 79.4 ± 0.2 | 91.9 ± 0.4 | 108.0 ± 1.1 | <0.01 |
| Pre-nephrectomy creatinine (mg/dL) | 0.87 ± 0.00 | 0.92 ± 0.00 | 0.90 ± 0.01 | 0.88 ± 0.01 | <0.01 |
| Pre-nephrectomy eGFR (ml/min/1.73m2)* | 92.4 ± 1.0 | 90.7 ± 0.9 | 92.4 ± 1.4 | 95.1 ± 3.0 | <0.01 |
| Pre-nephrectomy systolic blood pressure (mm Hg) | 117.2 ± 0.6 | 121.4 ± 0.6 | 124.0 ± 0.9 | 124.1 ± 1.7 | <0.01 |
| Pre-nephrectomy diastolic blood pressure (mm Hg) | 72.3 ± 0.3 | 74.2 ± 0.3 | 75.2 ± 0.4 | 76.4 ± 0.8 | < 0.01 |
| Pre-nephrectomy hypertension ** (%) | 129 (6.4) | 233 (11.1) | 150 (15.9) | 39 (15.6) | <0.01 |
| Recipient Characteristic |
Donor BMI < 25 |
Donor 25 ≤ BMI < 30 |
Donor 30 ≤ BMI < 35 |
Donor BMI ≥ 35 |
p |
| N | 2002 | 2108 | 944 | 250 | |
| Age, years | 46.2 ± 0.3 | 46.7 ± 0.3 | 47.0 ± 0.4 | 45.9 ± 0.8 | 0.39 |
| Female (%) | 801 (40.3) | 809 (38.4) | 402 (42.6) | 119 (47.6) | 0.01 |
| Race (%) | <0.01 | ||||
| White | 1349 (67.4) | 1346 (63.9) | 575 (60.9) | 146 (58.4) | |
| Black | 263 (13.1) | 355 (16.8) | 176 (18.6) | 65 (26.0) | |
| Hispanic | 236 (11.8) | 309 (14.7) | 160 (17.0) | 32 (12.8) | |
| Asian | 121 (6.0) | 63 (3.0) | 14 (1.5) | 4 (1.6) | |
| Other | 33 (1.7) | 35 (1.7) | 19 (2.0) | 3 (1.2) | |
| Education (%) | <0.01 | ||||
| Grade school or less | 81 (4.1) | 81 (3.7) | 49 (5.2) | 11 (4.4) | |
| High school | 678 (33.9) | 798 (37.9) | 357 (37.8) | 107 (42.8) | |
| Attended college/technical school | 449 (22.5) | 460 (21.8) | 214 (22.7) | 51 (20.4) | |
| Associate/Bachelor degree | 349 (14.5) | 336 (16.0) | 137 (14.5) | 33 (13.2) | |
| Graduate degree | 174 (8.7) | 126 (6.0) | 53 (5.6) | 10 (4.0) | |
| Unknown | 269 (13.5) | 306 (14.5) | 134 (14.2) | 38 (15.2) | |
| Cause of ESRD (%) | 0.25 | ||||
| Diabetes | 420 (21.0) | 453 (21.5) | 235 (24.9) | 58 (23.2) | |
| Glomerular disease | 585 (29.2) | 583 (27.7) | 247 (26.2) | 57 (22.8) | |
| Hypertension | 308 (15.4) | 357 (16.9) | 172 (18.2) | 50 (20.0) | |
| Obstruction / hypoplasia / reflux | 65 (3.3) | 72 (3.4) | 26 (2.8) | 8 (3.2) | |
| Other | 396 (19.8) | 394 (18.7) | 168 (17.8) | 50 (20.0) | |
| Polycystic kidneys | 213 (10.6) | 229 (10.9) | 87 (9.2) | 27 (10.8) | |
| Unknown | 16 (0.8) | 20 (1.0) | 9 (1.0) | 0 | |
| Peak PRA | 9.7 ± 0.6 | 9.0 ± 0.6 | 9.5 ± 0.9 | 11.3 ± 2.0 | 0.61 |
| High PRA (%)*** | 181 (9.0) | 183 (8.7) | 86 (9.1) | 22 (8.8) | 0.97 |
| Hepatitis C (%) | 48 (2.4) | 69 (3.3) | 29 (3.1) | 1 (0.4) | 0.04 |
| Dialysis (%) | 1233 (61.6) | 1325 (62.9) | 607 (64.3) | 167 (66.8) | 0.27 |
| Prior transplant (%) | 194 (9.7) | 200 (9.5) | 96 (10.2) | 20 (8.0) | 0.77 |
BMI: Body mass index; eGFR: Estimated glomerular filtration rate; ESRD: End-stage renal disease; PRA: Panel reactive antibody
Calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation
Defined as clinical hypertension reported on donor form, systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg
Defined as >=20% PRA
Very obese and obese live donors were more likely to be black (25.2% of very obese donors and 16.8% of obese live donors were black, compared to 14.9% of overweight donors and 11.8% of normal weight donors, p<0.001) Very obese live donors were younger (37.4 years for very obese, versus 40.0 years for obese, 40.6 years for overweight, and 39.4 years for normal weight donors, p<0.01), although the differences in age was not clinically important. Other differences in demographic characteristics did not follow a clear pattern across BMI categories.
Prior to nephrectomy, increases in BMI were associated with higher systolic blood pressures (117.2 mm Hg for normal weight, 121.4 mm Hg for overweight, 124.0 mm Hg for obese and 124.1 mm Hg for very obese donors, p<0.01) and higher diastolic blood pressures (72.3 mm Hg for normal weight, 74.2 mm Hg for overweight, 75.2 mm Hg for obese and 76.4 mm Hg for very obese, p<0.01).
eGFR did not consistently increase across BMI categories, although very obese donors did have the highest eGFR (95.1 ml/min/1.73m2 for very obese donors) compared to other groups (92.4 ml/min/1.73m2 for normal weight, 90.7 ml/min/1.73m2 for overweight, and 92.4 ml/min/1.73m2 for obese, p<0.004).
Peri-operative events for donors (Table 2)
Table 2.
Peri-operative outcomes for live donors, by BMI category
| Outcome | Total | BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | p | |
|---|---|---|---|---|---|---|---|
| Length of stay in days (s.e.) | 3.1 ± 0.0 | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.3 ± 0.2 | 3.4 ± 0.3 | 0.15 | |
| Laparoscopic nephrectomy (%) | 4418 (83.3) | 1685 (84.2) | 1736 (82.4) | 785 (83.2) | 212 (84.8) | 0.41 | |
| Complications | Total | BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | p | |
| Re-operation (%) | 17 (0.3) | 9 (0.5) | 5 (0.2) | 2 (0.2) | 1 (0.4) | 0.59 | |
| Re-admission (%) | 79 (1.5) | 36 (1.8) | 22 (1.0) | 17 (1.8) | 4 (1.6) | 0.19 | |
| Conversion to open surgery (%) | 70 (1.3) | 21 (1.1) | 29 (1.4) | 16 (1.7) | 4 (1.6) | 0.50 | |
| Vascular and other complications (%) | 116 (2.2) | 42 (2.1) | 47 (2.3) | 20 (2.2) | 7 (2.8) | 0.82 |
There were no peri-operative deaths. Re-operation and re-admission within 6 weeks of surgery were rare and occurred in similar proportions across donor BMI categories. Mean length of stay was 3.0 days for normal weight donors, 3.1 days for overweight donors, 3.3 days for obese donors, and 3.4 days for very obese donors (p=0.15).
Six-month outcomes for donors
Many donors did not have six-month outcome data reported to UNOS by their centers. In the primary cohort of 5304 donors, 3193 (60.2%) had 6-month creatinine data, 3011 (56.8%) had weights, and 2659 (50.1%) had blood pressures. The proportion of donors with missing data for creatinine (p=0.30), blood pressure (p=0.25), and weight (p=0.14) did not differ significantly across BMI category (Table 3).
Table 3.
Six month outcomes for live donors, by BMI category *
| Outcome | BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | P |
|---|---|---|---|---|---|
| Primary outcomes (continuous) | |||||
| Change in eGFR (ml/min/1.73m2) | −29.9 ± 0.5 | −30.5 ± 0.5 | −30.9 ± 0.7 | −30.9 ± 1.5 | 0.62 |
| Percent rise in creatinine (%) ** | 41 ± 1 | 43 ± 1 | 44 ± 1 | 43 ± 2 | 0.11 |
| Change in systolic blood pressure (mm Hg) | 0.15 ± 0.45 | −0.62 ± 0.46 | −0.95 ± 0.67 | 0.66 ± 1.24 | 0.40 |
| Change in diastolic blood pressure (mm Hg) | 0.60 ± 0.33 | −0.03 ± 0.34 | −0.40 ± 0.50 | 0.33 ± 0.95 | 0.34 |
| Secondary outcomes (continuous) | |||||
| eGFR (ml/min/1.73m2) | 62.0 ± 0.4 | 59.9 ± 0.4 | 60.6 ± 0.6 | 62.7 ± 1.3 | <0.01 |
| Percent remaining eGFR (%) *** | 70 ± 0.0 | 69 ± 0.0 | 68 ± 0.0 | 68 ± 0.0 | 0.17 |
| Mean serum creatinine (mg/dL) | 1.20 ± 0.01 | 1.28 ± 0.01 | 1.28 ± 0.01 | 1.22 ± 0.02 | <0.01 |
| Systolic blood pressure (mm Hg) | 117.7 ± 0.4 | 121.0 ± 0.4 | 122.6 ± 0.5 | 124.3 ± 1.1 | <0.01 |
| Diastolic blood pressure (mm Hg) | 72.3 ± 0.3 | 74.2 ± 0.3 | 75.2 ± 0.4 | 76.4 ± 0.8 | <0.01 |
| Weight (kg) | 65.2 ± 0.3 | 79.4 ± 0.3 | 90.8 ± 0.5 | 103.6 ± 1.3 | <0.01 |
| Change in weight (kg) | 1.3 ± 0.2 | − 0.0 ± 0.1 | −0.7 ± 0.2 | −3.8 ± 1.4 | <0.01 |
| Secondary outcomes (categorical) | |||||
| Hypertension | 84 (8.2) | 97 (9.4) | 46 (9.3) | 22 (17.2) | 0.01**** |
In the cohort of 5304 donors, 3193 (60.2%) had 6-month creatinine values reported, 3011 (56.8%) had weights reported and 2659 (50.1%) had blood pressure values reported. There were no significant differences in missing creatinine, weight or blood pressure across BMI categories.
Defined as (rise in creatinine from pre-nephrectomy to 6 months)/(pre-nephrectomy creatinine)
Defined as (6 month eGFR)/(Pre-nephrectomy eGFR)
When adjusted for pre-nephrectomy hypertension, the increased prevalence of hypertension among very obese donors remained significant (p=0.03).
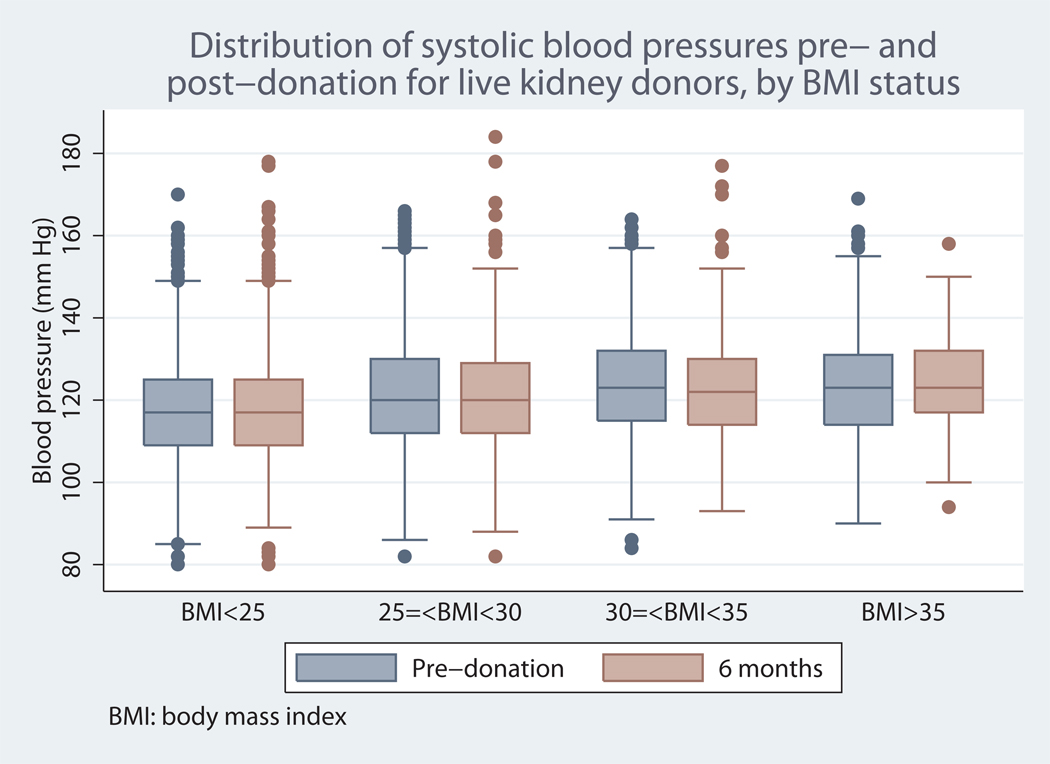
At six months, mean systolic and diastolic blood pressure rose consistently across donor BMI categories, but there were no significant or clinically meaningful differences in the magnitude of change from baseline, suggesting that differences in follow-up blood pressures reflected differences in baseline blood pressures. A box plot of the distribution of pre- and post-nephrectomy systolic blood pressures is displayed in Figure 1.
Figure 1.
* p<0.01 for comparisons of systolic blood pressure across donor BMI categories prior to nephrectomy, and at 6 months
Hypertension was significantly more common among very obese donors (17.2% of very obese, versus 8.2% of normal weight, 9.4% of overweight and 9.3% of obese had hypertension at six months; p=0.01 for the contrast of very obese to normal weight donors). Notably, many of these donors met our criteria for hypertension pre-nephrectomy. When adjusted for pre-nephrectomy hypertension, the increased prevalence of hypertension among very obese donors remained significant (p=0.03) (Table 3).
At six months after nephrectomy, decline in eGFR from baseline was similar across donor BMI categories (p=0.62). At this time point, differences in mean absolute eGFR across BMI groups, although statistically significant, were not clinically important (62.0 ml/min/1.73 m2 for normal weight donors, versus 59.9 for overweight donors, 60.6 for obese donors and 62.7 for very obese donors, p<0.01) and did not rise consistently across BMI category (p=0.62) (Table 3).
Percent of remaining eGFR (p=0.17) and percent rise in creatinine (p=0.11) also did not differ across groups. A multivariable linear regression analysis of percent rise in serum creatinine (adjusted for donor age, race and gender) showed that obese donors had a significantly higher rise (coefficient=3%) compared to normal weight donors (p=0.04), but no significant differences were found among overweight (p=0.07) or very obese donors (p=0.45) (Table 4).
Table 4.
Multivariable adjusted outcomes for live donors, by BMI category *
| Outcome | BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | |
|---|---|---|---|---|---|
| Pre-nephrectomy creatinine (mg/dL) | Coefficient | Reference | 0.01 | 0.00 | 0.00 |
| p | 0.04 | 0.54 | 0.94 | ||
| Intercept | 0.73 | ||||
| Post-nephrectomy creatinine (mg/dL) | Coefficient | Reference | 0.03 | 0.03 | 0.00 |
| p | <0.01 | <0.01 | 0.83 | ||
| Intercept | 0.95 | ||||
| Percent rise creatinine (%) | Coefficient | Reference | 2 | 3 | 2 |
| p | 0.07 | 0.04 | 0.45 | ||
| Intercept | 35 | ||||
| 1/Pre-nephrectomy creatinine | Coefficient | Reference | −0.01 | 0.00 | 0.00 |
| p | 0.09 | 0.68 | 0.91 | ||
| Intercept | 1.39 | ||||
| 1/Post-nephrectomy creatinine | Coefficient | Reference | −.02 | −0.01 | 0.00 |
| p | <0.01 | 0.04 | 0.91 | ||
| Intercept | 1.03 | ||||
| 1/Change in creatinine | Coefficient | Reference | −0.28 | −0.40 | −0.24 |
| p | 0.01 | <0.01 | 0.33 | ||
| Intercept | 4.04 | ||||
| Pre-nephrectomy systolic blood pressure (mm Hg) | Coefficient | Reference | 3.3 | 6.1 | 7.1 |
| p | <0.01 | <0.01 | <0.01 | ||
| Intercept | 109.0 | ||||
| Post-nephrectomy systolic blood pressure (mm Hg) | Coefficient | Reference | 2.0 | 4.2 | 6.4 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | |
| Intercept | 108.0 | ||||
| Change in systolic blood pressure (mm Hg) | Coefficient | Reference | −1.1 | −1.3 | −0.4 |
| p | 0.09 | 0.11 | 0.79 | ||
| Intercept | −1.1 | ||||
| Pre-nephrectomy diastolic blood pressure (mm Hg) | Coefficient | Reference | 2.1 | 4.0 | 4.5 |
| p | <0.01 | <0.01 | <0.01 | ||
| Intercept | 66.1 | ||||
| Post-nephrectomy diastolic blood pressure (mm Hg) | Coefficient | Reference | 1.1 | 2.5 | 3.8 |
| p | <0.01 | <0.01 | <0.01 | ||
| Intercept | 67.2 | ||||
| Change in diastolic blood pressure (mm Hg) | Coefficient | Reference | −0.8 | −1.1 | −0.4 |
| p | 0.10 | 0.06 | 0.67 | ||
| Intercept | 0.6 |
Results from linear regression, adjusted for donor age, race and gender. Post-nephrectomy values are at 6 months after donor surgery.
An inverse relationship existed between pre-nephrectomy weight and 6-month change in weight, such that normal weight donors gained a mean of 1.3 kg by 6 months, whereas overweight donors had no mean change in weight, obese donors lost a mean of 0.7 kg and very obese donors lost a mean of 3.8 kg (Table 3).
Secondary analysis of one-year donor outcomes
Two donors had died by one year; one was normal weight and the other was overweight. The number of donors with missing data related to blood pressure or renal function increased substantially at one year, but there was no difference in the proportion of missing data across BMI categories. Out of 5304 donors, 1816 (34.2%) of donors had 12-month creatinine values reported and 1546 (29.1%) of donors had blood pressure values reported.
Similar to our findings at 6 months, changes from pre-nephrectomy values in eGFR (p=0.79), percent rise in creatinine (0.82), systolic blood pressure (p=0.09) and diastolic blood pressure (p=0.42) were not statistically different across donor BMI categories at one year following nephrectomy. (Data not presented in tables).
Recipient analyses (Table 1)
There were no differences in recipient age across donor BMI groups. Recipients of kidneys from very obese live donors were more likely to be black (26.0% of recipients of kidneys from very obese live donors were black, compared to 13.1% of recipients of kidneys from normal weight donors, 16.8% of recipients from obese donors, and 18.6% of recipients from obese donors, p<0.01).
One-year outcomes for recipients of kidneys from live donors
PNF of kidney allografts was a rare event that was experienced by 1% of recipients overall. Compared to the reference group of recipients of kidneys from normal weight donors, only the category of recipients of kidneys from very obese donors were more likely to suffer PNF (0.8% of normal weight donors, 1.1% of overweight donors, 0.7% of obese donors and 2.8% of very obese donors experienced PNF; the odds ratio for very obese donors compared to normal weight donors was 3.58, CI 1.46 – 8.78, p<0.01). In multivariable logistic regression, recipients of kidneys from very obese donors were also more likely to experience DGF (OR 2.16, CI 1.20 – 3.89, p=0.01).
In multivariable analyses, no significant differences in recipient acute rejection, allograft survival, or patient mortality were evident across donor BMI categories. (Table 5)
Table 5.
Outcomes for recipients of live donor kidneys, by donor BMI category
| Outcome | BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | BMI ≥ 35 | Total | |
|---|---|---|---|---|---|---|
| Delayed graft function* | N (%) | 76 (3.8) | 96 (4.6) | 48 (5.1) | 18 (7.2) | 238 (4.5) |
| OR | Reference | 1.17 | 1.22 | 2.16 | ||
| p-value | 0.36 | 0.33 | 0.01 | |||
| Acute rejection within the first year* | N (%) | 69 (3.5) | 94 (4.5) | 38 (4.0) | 14 (5.6) | 215 (4.0) |
| OR | Reference | 1.19 | 1.07 | 1.71 | ||
| p-value | 0.31 | 0.77 | 0.09 | |||
| 1 year allograft failure* | N (%) | 96 (4.8) | 118 (5.6) | 51 (5.4) | 16 (6.4) | 281 (5.3) |
| HR | Reference | 1.05 | 0.99 | 1.39 | ||
| p-value | 0.75 | 0.97 | 0.25 | |||
| 1 year patient mortality** | N (%) | 47 (2.9) | 43 (2.5) | 22 (2.9) | 8 (3.9) | 120 (2.8) |
| HR | Reference | 0.85 | 0.95 | 1.39 | ||
| p-value | 0.47 | 0.85 | 0.43 | |||
Logistic regression models were fit for the outcomes of delayed graft function and acute rejection. A Cox regression model was fit for the outcome of allograft failure. These multivariable models included terms for the following independent variables: recipient age, race (black/not black), gender, prior transplant, hepatitis C seropositivity, prior dialysis, diabetes, body mass index, elevated panel reactive antibody titer (defined as >=20%), use of antibody induction therapy, baseline immunosuppression regimen, human leukocyte antigen mismatch (an ordinal variable defined as 0=zero mismatch, 1=one mismatch, and 2=greater than one mismatch); donor age, race, gender and BMI category.
This multivariable Cox model for the outcome of recipient mortality was adjusted for donor and recipient age, gender and race
Discussion
Despite concerns about increased health risks for obese live kidney donors, limited data exist about comparative outcomes for donors across BMI categories (22, 30). Using a national dataset with a racially diverse population, this study showed that donors with elevated BMI have higher mean blood pressures at baseline and after nephrectomy, but that changes in blood pressure were not related to BMI. Higher donor BMI did not increase the risk of re-operation or re-admission or increase length of stay for the donor. At six months, the relative changes in donor creatinine and eGFR were similar across BMI categories, and differences in absolute level of creatinine and eGFR were not clinically important. One-year allograft survival and patient mortality were also not different across BMI groups. Taken together, these findings suggest that obesity itself should not limit acceptability for organ donation; however, further studies are necessary to ensure that outcomes for donors with elevated BMI do not worsen in the long term.
The decision to accept or reject an obese donor is a frequent and challenging dilemma for transplant professionals (30). Although trends in average weight among live kidney donors have not been published, our clinical impression is that the population of potential donors has become more obese -- similar to trends in the US population (25). Our group has reported previously that use of obese kidney donors is common among US transplant centers (31). But as evidence has accumulated that obesity is an independent risk factor for subsequent renal disease, limited studies have been available to counsel obese donors and their recipients about outcomes after nephrectomy.
The variation in standards across centers for accepting obese live donors appears to reflect a lack of consensus about appropriate clinical practice for these donors (5). A 2007 questionnaire answered by 53% of US renal transplant programs showed that 12% had no policy for excluding donors on the basis of BMI, 10% excluded donors with BMI>30, 52% excluded donors with BMI>35, 20% excluded donors with BMI>40, and 6% only excluded donors on the basis of BMI only if other cardiovascular risks were also present (5). Additionally, a United Network for Organ Sharing resource document for transplant programs lists “morbid obesity” as a “possible exclusion” criterion for live kidney donation.(32)
Our findings that donor death, re-admission, re-operation and length of stay were not associated with elevated BMI are generally confirmed by prior studies. In a meta-analysis that examined studies of obese donors, Young et al. reported that obese live donors had no increase in surgical complications (22). The Young study found a small increased length of stay associated with obesity, but the increase was small and barely met statistical significance (22). Heimbach et al. reported that only 0.2% of donors at Mayo clinic required re-operation. Donors with higher BMI at Mayo were not more likely to have re-operation and did not have increased length of stay (2). We believe that most major complications would be captured by differences in the rates of donor death, re-admission, re-operation or length of stay; all of these outcomes in our study were similar across BMI categories.
On the other hand, the OPTN dataset reported a much lower rate of overall complications than prior studies (33, 34). For instance, the rate of overall complications was 12.8% in the Heimbach study and 16.8% in the Pesavento study, whereas <5% of donors had any reported complication in our study (2, 3). In the single center Heimbach and Pesavento studies, these complications were pre-dominantly wound-related (e.g. cellulitis), and did not affect duration of hospitalization. The much lower rate of overall complications in our dataset, however, suggests that some peri-operative problems are under-reported to OPTN.
Donors with elevated BMI had higher blood pressure, but did not have greater changes in blood pressure from pre-nephrectomy levels compared to normal weight donors. The slightly higher blood pressure associated with obesity (before and after nephrectomy) in our study has previously been described in the general public and also in kidney donors (23). At one year after nephrectomy, Heimbach et al. reported a mean systolic blood pressure of 137 mm Hg among very obese live donors versus 126 mm Hg in normal weight donors (2). Notably, these absolute blood pressure values are higher than in our study (124.3 mm Hg for very obese versus 117.7 mmHg for normal weight donors at 6 months), but the association between blood pressure and obesity is marked in both populations. In the study by Heimbach et al., the blood pressures of obese donors at one year after nephrectomy were either unchanged or lower than at baseline (2). Using a national dataset, our study provides confirmatory evidence that donor nephrectomy does not lead to greater increases in blood pressure among donors with elevated BMI. Nonetheless, the higher absolute blood pressures associated with higher BMI could expose these donors to cardiovascular or other risks over the long term. For instance, the 6.6 mm Hg difference in mean post-nephrectomy blood pressure between very obese and normal weight donors in our study is comparable in magnitude to differences in blood pressure observed between treatment groups in important clinical trials of hypertensive agents.(35, 36) All prior kidney donors should have their blood pressure assessed regularly, but obese donors should also be counseled about the association between BMI and hypertension.
We also compared changes in renal function between donors at six months using decline in eGFR from baseline. Prior studies have shown that underestimation of mGFR using the MDRD equation is consistent across BMI categories, and that obesity causes elevation in mGFR (13, 28). Therefore, comparison of the intra-individual decline in eGFR after nephrectomy should be less susceptible to bias than comparisons of absolute levels of eGFR across donor BMI categories. We hypothesized that donors with elevated BMI, whose kidneys were likely to hyperfilter at baseline, would be less able to adapt to nephrectomy with additional compensatory glomerular hyperfiltration in the remaining kidney.
Contrary to our hypothesis, however, we did not find a greater decline in eGFR among donors with elevated BMI compared to normal weight donors. The percent rise in serum creatinine (adjusted for donor demographics) was slightly higher among obese donors compared to normal weight donors, but there was no significant elevation in percent rise in creatinine among very obese donors. These findings bear comparison to the study by Heimbach et al., in which mGFR was measured using iothalamate and compared by donor BMI category. These authors did not report the mean intra-individual decline in renal function, but mGFR was not significantly different between donors in different BMI categories (2). In sum, our analysis of OPTN data also suggests that higher BMI does not lead to greater loss of renal function for live kidney donors in the short term.
Weight control before and after donor nephrectomy has also been a focus of attention by renal transplant clinicians (30). Obese potential live donors are often counseled to lose weight, and some have been referred for gastric bypass surgery (37). Among the general public, outcomes from weight control programs typically show that substantial weight loss is difficult to maintain (38). Our study provides evidence that live donors with elevated BMI tend to maintain their weight or lose weight by six months after nephrectomy.
We also found that short-term allograft survival for recipients of kidneys from donors with elevated BMI did not differ from outcomes for recipients of kidneys from normal weight live donors. The similar rate of allograft survival suggests that recipients of kidneys from obese live donors have the potential to enjoy the same high level of renal function in the short term as live donor renal transplant recipients in general. Interestingly, recipients of kidneys from very obese donors did have an increased risk of PNF and DGF, which may stem from technical challenges in performing nephrectomy on large patients. These novel findings should be examined in subsequent studies.
Compared to prior studies of obese donors, our analysis has a number of strengths, including a diverse population of donors from over two hundred transplant centers. Unlike some prior single center reports of Caucasian donors (2, 39), the OPTN population included in our analysis was 18.6% black and 16.0% Hispanic. Additionally, we found that black and Hispanic donors were more likely to be obese or very obese. Therefore, our findings related to blood pressure and obesity may be more reasonably generalized to broader populations and may be particularly relevant to centers that evaluate a substantial number of minority donors.
Similar to other analyses of registry data, however, our analysis is limited by information collected by OPTN. For instance, we classified donors as hypertensive on the basis of the blood pressures reported by the renal transplant program, but did not have data on blood pressure medication use or other blood pressure evaluation that some centers perform, such as 24-hour ambulatory monitoring (1). Additionally, given that obese patients may require a larger cuff size to obtain an accurate blood pressure reading, we acknowledge that obese donors might be more likely to be misclassified as hypertensive. Most renal transplant programs quantify renal function with 24-hour urine creatinine clearance, or assess mGFR with methods such as iothalamate clearance, but these data were not available from the OPTN (5). Owing to the known physiologic increase in glomerular filtration rate associated with obesity, we chose intra-individual percent rise in serum creatinine and decline in eGFR as our primary outcomes to compare renal function across donor BMI categories (13).
Missing data are also a limitation of this study. A substantial proportion of donors had missing data about BMI at the time of nephrectomy. We addressed this limitation by restricting our analysis to donors with complete BMI information. Many donors also did not have follow-up data, although the proportions of missing data did not differ between obese and non-obese donors, suggesting that our analyses of outcomes was not biased with regard to the outcomes of interest (40).
Conclusions
Our findings suggest that carefully evaluated live donors with elevated BMI do not experience worse short-term changes in blood pressure or renal function compared to non-obese donors. Recipients of kidneys from obese live donors have an increased risk of PNF and DGF, but have excellent one-year allograft survival. Additional follow-up of donors with elevated BMI will be necessary to ascertain the long-term impact of obesity of residual renal function after donor nephrectomy.
ABBREVIATIONS
- BMI
Body mass index
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- MDRD
Modification of Diet in Renal Disease
- mGFR
Measured glomerular filtration rate
- PRA
Panel reactive antibody
- US
United States
Footnotes
Funding Sources and participation:
Dr. Reese is supported by NIH Career Development Award, K23 - DK078688-01. He participated in research design, data analysis and writing of the paper
Dr. Feldman is supported by an NIH Midcareer Award in Patient Oriented Research, K24 - DK002651. He participated in research design and writing of the paper
Dr. Asch participated in research design and writing of the paper
Dr. Shults participated in data analysis and writing of the paper
Dr. Thomasson participated in data analysis and writing of the paper
Dr. Bloom participated in research design and writing of the paper
The authors have no conflict of interest to declare.
UNOS Disclaimer: "This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government."
References
- 1.Davis CL, Delmonico FL. Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol. 2005;16(7):2098. doi: 10.1681/ASN.2004100824. [DOI] [PubMed] [Google Scholar]
- 2.Heimbach JK, Taler SJ, Prieto M, et al. Obesity in living kidney donors: clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant. 2005;5(5):1057. doi: 10.1111/j.1600-6143.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 3.Pesavento TE, Henry ML, Falkenhain ME, et al. Obese living kidney donors: short-term results and possible implications. Transplantation. 1999;68(10):1491. doi: 10.1097/00007890-199911270-00011. [DOI] [PubMed] [Google Scholar]
- 4.Rea DJ, Heimbach JK, Grande JP, et al. Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int. 2006;70(9):1636. doi: 10.1038/sj.ki.5001799. [DOI] [PubMed] [Google Scholar]
- 5.Mandelbrot DA, Pavlakis M, Danovitch GM, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant. 2007;7(10):2333. doi: 10.1111/j.1600-6143.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol. 2006;17(6):1501. doi: 10.1681/ASN.2006040327. [DOI] [PubMed] [Google Scholar]
- 7.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 9.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46(5):871. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ryu S, Chang Y, Woo HY, et al. Changes in Body Weight Predict CKD in Healthy Men. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73(8):947. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 13.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 14.Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26(4):610. doi: 10.1161/01.hyp.26.4.610. [DOI] [PubMed] [Google Scholar]
- 15.Oh CK, Jeon KO, Kim HJ, Kim SI, Kim YS, Pelletier SJ. Metabolic demand and renal mass supply affecting the early graft function after living donor kidney transplantation. Kidney Int. 2005;67(2):744. doi: 10.1111/j.1523-1755.2005.67136.x. [DOI] [PubMed] [Google Scholar]
- 16.Fehrman-Ekholm I, Duner F, Brink B, Tyden G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72(3):444. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- 17.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tyden G, Groth CG. Kidney donors live longer. Transplantation. 1997;64(7):976. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Narkun-Burgess DM, Nolan CR, Norman JE, Page WF, Miller PL, Meyer TW. Forty-five year follow-up after uninephrectomy. Kidney Int. 1993;43(5):1110. doi: 10.1038/ki.1993.156. [DOI] [PubMed] [Google Scholar]
- 19.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340(8823):807. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 20.Ommen E, Winston J, Murphy B. Medical risks for living donors: absence of proof is not proof of absence. Clinical Journal of the American Society of Nephrology. 2006;1:885. doi: 10.2215/CJN.00840306. [DOI] [PubMed] [Google Scholar]
- 21.Gossmann J, Wilhelm A, Kachel HG, et al. Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant. 2005;5(10):2417. doi: 10.1111/j.1600-6143.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 22.Young A, Storsley L, Garg AX, et al. Health Outcomes for Living Kidney Donors with Isolated Medical Abnormalities: A Systematic Review. Am J Transplant. 2008 doi: 10.1111/j.1600-6143.2008.02339.x. [DOI] [PubMed] [Google Scholar]
- 23.Gracida C, Espinoza R, Cedillo U, Cancino J. Kidney transplantation with living donors: nine years of follow-up of 628 living donors. Transplant Proc. 2003;35(3):946. doi: 10.1016/s0041-1345(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 24.Espinoza R, Gracida C, Cancino J, Ibarra A. Effect of obese living donors on the outcome and metabolic features in recipients of kidney transplantation. Transplant Proc. 2006;38(3):888. doi: 10.1016/j.transproceed.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Obesity. 2007 http://www.who.int/topics/obesity/en/.
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14(10):2573. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 28.Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46(2):233. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 30.Delmonico F. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53. [PubMed] [Google Scholar]
- 31.Reese PP, Feldman HI, McBride MA, Anderson K, Asch DA, Bloom RD. Substantial variation in the acceptance of medically complex live kidney donors across US renal transplant centers. Am J Transplant. 2008;8(10):2062. doi: 10.1111/j.1600-6143.2008.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UNOS. [Accessed: January 30 2009];Guidance for the development of program-specific living kidney donor medical evaluation protocols. URL: http://www.unos.org/SharedContentDocuments/Program_Specific_Living_Kidney_Donor_Med_Eval_Protocols.pdf.
- 33.Nanidis TG, Antcliffe D, Kokkinos C, et al. Laparoscopic versus open live donor nephrectomy in renal transplantation: a meta-analysis. Ann Surg. 2008;247(1):58. doi: 10.1097/SLA.0b013e318153fd13. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Cassuto J, Orloff M, et al. Minimizing morbidity of organ donation: analysis of factors for perioperative complications after living-donor nephrectomy in the United States. Transplantation. 2008;85(4):561. doi: 10.1097/TP.0b013e3181643ce8. [DOI] [PubMed] [Google Scholar]
- 35.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000;283(15):1967. [PubMed] [Google Scholar]
- 36.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 37.Branco AW, Branco Filho AJ, Kondo W. Laparoscopic live donor nephrectomy in patients surgically treated for morbid obesity. Int Braz J Urol. 2007;33(3):377. doi: 10.1590/s1677-55382007000300010. [DOI] [PubMed] [Google Scholar]
- 38.Hainer V, Toplak H, Mitrakou A. Treatment modalities of obesity: what fits whom? Diabetes Care. 2008;31 Suppl 2:S269. doi: 10.2337/dc08-s265. [DOI] [PubMed] [Google Scholar]
- 39.Rook M, Bosma RJ, van Son WJ, et al. Nephrectomy Elicits Impact of Age and BMI on Renal Hemodynamics: Lower Postdonation Reserve Capacity in Older or Overweight Kidney Donors. Am J Transplant. 2008 doi: 10.1111/j.1600-6143.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- 40.Hennekens CH, Buring JE. Epidemiology in Medicine. 1st ed. Philadelphia: Lippincott Williams & Wilkins; 1987. [Google Scholar]