Summary of recent advances
Axon guidance and synapse formation are important developmental events for establishing a functional neuronal circuitry. These two related cellular processes occur in a coordinated fashion but previous studies from multiple model organisms seemed to suggest that axon guidance and synapse formation are mediated by distinct molecular cues. Thus, axon guidance molecules are responsible for guiding the navigating axon toward its target area, while other adhesion or ligand-receptor molecules specify the synapse formation within the target area. However, accumulative evidence has shown that axon guidance molecules can regulate the localization and formation of pre- and post-synaptic components during synapse formation. These results demonstrate a role for axon guidance molecules in synapse formation and provide insight into how axon guidance and synapse formation are coordinated at the molecular level.
Introduction
The hallmark of our nervous system is its ability to propagate information. This unique feature relies on proper establishment of the entire network of neuronal connections. Axon guidance and synapse formation are two distinct, but closely related, developmental processes that are essential for establishing functional neuronal connections in our nervous system. In the past decades, much progress has been made toward understanding the molecular and cellular mechanisms underlying these processes[1–6].
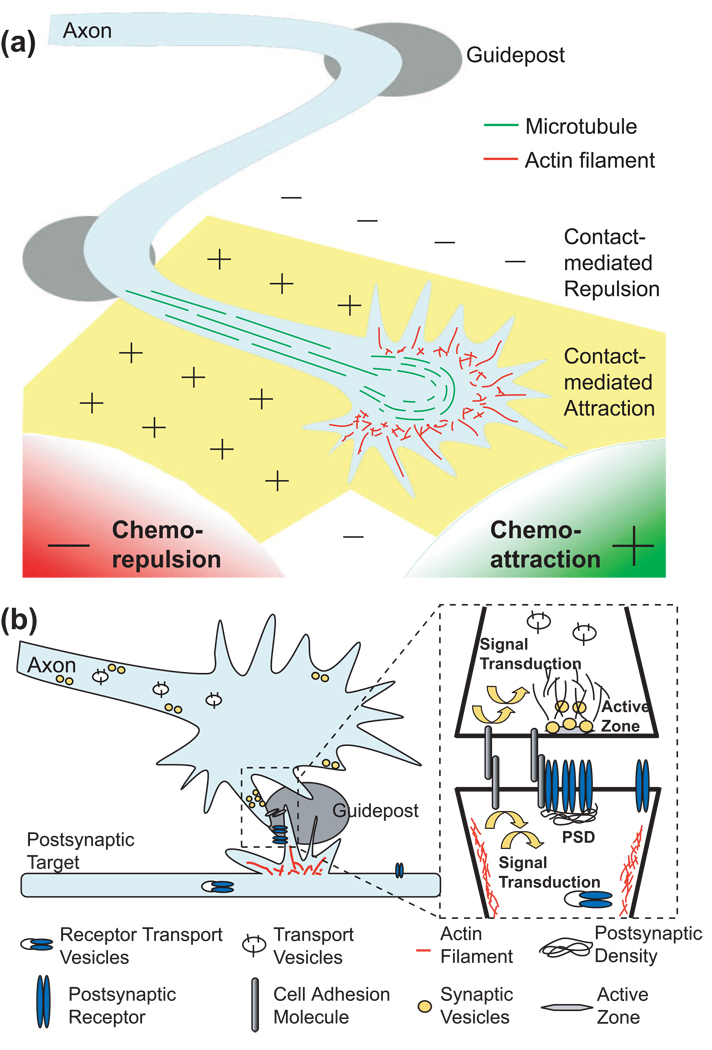
In the process of axon guidance, it is recognized that the entire axon trajectory can be divided into short steps with each controlled by intermediate targets or “guideposts”[1,2,7]. During development, the growth cone at the tip of the axon navigates the microenvironment between the guideposts until it reaches its final target area (Figure 1a). The guidance of the growth cone between each guidepost depends on the actions of four groups of environmental cues: long-range chemoattractants and chemorepellents, and short-range contact-mediated attractants and repellents. The cues that are involved are generally regarded as axon guidance molecules, and their functions are well conserved among species[1,2].
Figure 1. Schematic illustrations of the processes of axon guidance and synapse formation.
(a) The axon trajectory can be divided into multiple segments between guideposts. The guidance process between each guidepost is mediated by four types of forces, long-range chemoattraction or chemorepulsion, and short-range contact-mediated attraction or contact-mediated repulsion. (b) Synapse formation is a process of forming specialized pre- and post-synaptic structures for neurotransmission. It includes cell-cell contact, assembly of the presynaptic active zone for neurotransmitter release, and clustering of postsynaptic receptors in the post-synaptic density (PSD). Guideposts within the target area can provide spatial cues to instruct synapse formation.
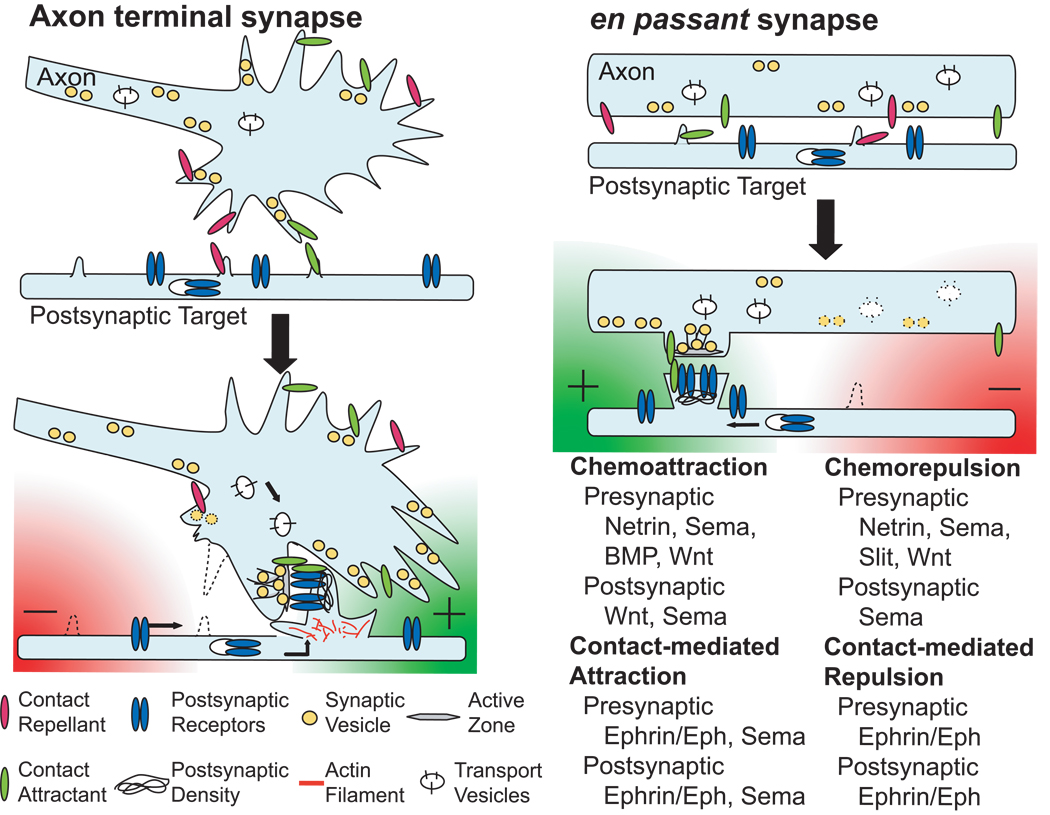
After a growing axon reaches its target area, its terminal starts to branch into multiple tiny arbors to initiate the next developmental process – the formation of synapses (Figure 1b). Synapses are often formed at the end of the axon, but there are also en passant synapses that occur along interior axon segments (Figure 2). Synapse formation involves at least three major stages: (1) localized cellular interactions or adhesions between presynaptic axons and postsynaptic cell body or dendrites, (2) presynaptic differentiation to build up synaptic transmission machinery, and (3) specialized postsynaptic differentiation to receive neurotransmitter signals[3–6]. Although it has been well recognized that specific pre- and post-synaptic components exist in neurites before actual contacts occur, many studies have shown that stable cell-cell contact promotes the accumulation of these synaptic components to the nascent synapse[3–6]. Because of the requirement of synaptic diversities in the peripheral and central nervous systems, multiple signaling cues are often required to specify the formation of synapses. Several specific adhesion molecules and ligand-receptor pairs have been identified as synaptogenic molecules[8].
Figure 2. Mechanisms of axon guidance molecules in synapse formation.
Four general molecular mechanisms are utilized by axon guidance molecules to regulate synapse formation. Molecular attractions have pro-synaptogenic effects, while molecular repulsions result in anti-synaptogenic effects. Chemoattractants (green radial gradient) regulate subcellular cytoskeletal dynamics to facilitate synapse formation and recruit synaptic components to build up neurotransmission machinery in pre- and post-synaptic cells. Contact-mediated attractants (green bar) use similar mechanisms to stabilize the initial synaptic contact and promote the maturation of pre- and post-synaptic structures. In contrast, chemorepellents (red radial gradient) and contact-mediated repellents (red bar) have opposite effects to inhibit synapse formation.
Accumulative evidence has shown that axon guidance molecules also play an important role in regulating synapse formation. These previously known molecules are able to determine synaptic sites and regulate the pre- or post-synaptic differentiations. Here, we review recent findings about how major groups of axon guidance molecules regulate synapse formation and summarize their major functions. Two morphogens, BMPs (bone morphogenic proteins) and Wnts, are also included in the discussion because of their newly recognized roles in axon guidance[9,10]. Due to space limitations, cell surface adhesion molecules like cadherins and members of the immunoglobulin (Ig) superfamily are not discussed. The roles of these molecules in synapse formation can be found elsewhere[3,11,12].
Main text of review
Diffusible netrins determine the distribution of presynaptic components during synapse formation
In axon guidance, netrin/UNC-6 acts as a chemoattractant through the DCC/UNC-40 receptor or as a chemorepellent through the UNC-5 receptor. Two recent studies in C. elegans show that netrin/UNC-6 can function in synapse formation by regulating the assembly of presynaptic component proteins[13,14]. Similar to its functions in axon guidance, netrin/UNC-6 either facilitates or excludes the presynaptic protein assembly.
The pro-synaptogenic activities of netrin/UNC-6 are demonstrated in the synapse formation between the pre-synaptic AIY neuron and the postsynaptic RIA neuron in C. elegans [13]. A mutation in the DCC/unc-40 gene was isolated from a visual genetic screen for mutants with perturbed pre-synaptic marker distribution in AIY. Mosaic analysis indicates that the DCC/UNC-40 functions in the pre-synaptic AIY neuron. The netrin/UNC-6 cue is secreted from the glia-like sheath cell that contacts both AIY and RIA in the synaptogenic region. Mutants with distended sheath cell morphology exhibit ectopic synapse formation at previous asynaptic areas. These results indicate that netrin/UNC-6 from the sheath cell, which in a sense is similar to the guidepost cell in axon guidance, dictates where the synapse can be formed in the AIY axon by recruiting the presynaptic components.
Netrin/UNC-6 can also exclude presynaptic assembly through the UNC-5 receptor[14]. In C. elegans, the DA9 motor neuron is located on the ventral side with its dendrite extending along the ventral nerve cord. The DA9 axon, however, is repelled by a high ventral low dorsal netrin/UNC-6 gradient in early development and guided dorsally to reach the dorsal cord, where it forms en passant synapses with dorsal muscle cells. In wild-type animals, the presynaptic components of DA9 only accumulate in the dorsal segment of the axon and are totally excluded from the dendrite, while in unc-5 mutant animals, ectopic presynaptic components are assembled in the dendrite. A silencing intron cassette to specifically remove UNC-5 after the guidance of the DA9 axon was used to confirm that the synaptic exclusion effect of UNC-5 is specific, and ectopic netrin/UNC-6 results in elimination of synapses in nearby axon segments.
The studies in the C. elegans DA9 neuron demonstrate the combinatorial effects of different axon guidance molecules on synapse formation. As will be discussed later, the high posterior low anterior wnt/LIN-44 gradient also regulates the distribution of synapses in the DA9 axon. Together with the ventral-dorsal netrin/UNC-6 gradient, these two sets of diffusible cues cooperate to determine the location of presynaptic assembly in the axon by exclusion mechanisms. Earlier mutant analysis in Drosophila also suggests that coordination between netrin and semaphorin signals regulates target selection in the peripheral neuromuscular junction (NMJ)[15,16]. Thus, as in axon guidance, synapse formation can be regulated by the coordinated actions of the pro- and anti-synaptogenic cues in the target area (Figure 2).
Bidirectional signaling of ephrin and Eph at the synaptic site regulates synapse formation
Eph receptors are a subfamily of receptor tyrosine kinases that bind to membrane-associated ligands, ephrins. They mediate short-range contact interactions. One unique feature of ephrin/Eph interactions is the bidirectional signaling toward either the Eph-expressing cell (forward signaling) or the ephrin-presenting cell (reverse signaling)[17]. The roles of ephrin/Eph during synapse formation are both presynaptic and postsynaptic.
The first evidence suggesting the involvement of ephrin/Eph in synapse formation is from the finding that EphB receptors form protein complexes with glutamatergic NMDA receptors and the binding of ephrinB1 to EphBs induces clustering of EphBs and NMDA receptors[18]. In the triple knockout mice of EphB1, EphB2, and EphB3, the formation of glutamatergic synapses and dendritic spines are impaired[19,20] and the motility of postsynaptic dendritic filopodia is reduced[21]. These findings establish the role of EphB forward signaling in postsynaptic receptor clustering and cytoskeletal remodeling.
The involvement of ephrinB1 reverse signaling in the presynaptic differentiation has been demonstrated in the Xenopus retinotectal system[22]. Infusion of fusion of extracellular EphB2 with immunoglobulin Fc (EphB2-Fc) in retina ganglion cells (RGCs) activates ephrinB1 and promotes presynaptic specialization, while expression of dominant negative ephrinB1 to block the ephrinB1/EphBs interactions reduces synaptic density and presynaptic neurotransmitter release. Furthermore, EphB2 expressed in fibroblasts is sufficient to promote the accumulation of presynaptic synaptic vesicles in the axons from cortical neurons[20]. However, ephrinB reverse signaling is not restricted to the presynaptic neurons. Application of EphB2-Fc in hippocampal neuronal culture increases the number of postsynaptic dendritic spines[23]. EphB2-Fc application also inhibits AMPA receptor internalization postsynaptically[24,25]. Importantly, these in vitro results are supported by in vivo analysis of mutant mice: the number of excitatory synapses in the hippocampal CA1 region is reduced in ephrinB3 knockout mice[26], and the AMPA receptor stabilizing effect of ephrinB reverse signaling is disrupted in ephrinB2 mutant mice. Taken together, ephrinB reverse signaling can regulate both pre- and post-synaptic differentiations during synapse formation. It is important to note that trans-synaptic binding between ephrin and Eph might also be essential for synapse formation. Recent in vitro studies show that rescuing postsynaptic dendritic filopodia motility without trans-synaptic ephrin/Eph binding is not sufficient to restore synaptic formation defects in EphB1-3 triple mutant mice[21].
EphrinA and EphA signaling has also been implicated in synapse formation. The activation of EphA4 in hippocampal slice culture by ephrinA3 induces spine retraction in postsynaptic neurons[27]. In EphA4 knockout mice, distorted spines are observed, suggesting that EphA4 regulates postsynaptic spine morphology. EphA4 forward signaling is associated with cdk5 activation and results in ephexin1 phosphorylation[28]. This then regulates the activation of the small GTPase RhoA, which is involved in cytoskeletal dynamics. These data suggest a role for EphA4 forward signaling in postsynaptic cytoskeletal regulation, but whether such regulation is sufficient to affect synapse formation is still unclear.
Both transmembrane and diffusible semaphorins contribute to synapse formation
Earlier studies in Drosophila have indicated that semaphorins play a role in synapse formation[29,30]. Two transmembrane class 4 semaphorins have recently been shown to regulate postsynaptic differentiation in an RNAi based genetic screen[31]. Knockdown of Sema4B in cultured hippocampal neurons reduces the density of postsynaptic PSD-95 but not presynaptic synapsin I puncta, suggesting a role for Sema4B in post-synaptic specialization of glutamatergic synapses. This finding is consistent with a previous report that Sema4B interacts with PSD-95 and localizes to PSD-95 containing synapses[32]. In contrast, knockdown of Sema4D down-regulates GABAergic, but not glutamatergic, synapse density through a specific reduction in the number of postsynaptic GABA receptors. Analysis of knockout mice also suggests the involvement of Sema4D in GABAergic synapse formation. Although it is still unclear whether or not Sema4 reverse signaling is involved, these results reveal the specific requirements of different members of the class 4 semaphorins in the development of excitatory and inhibitory synapse formation.
Diffusible semaphorins are also involved in synapse formation, but the mechanisms are far from clear. Sema3A has been shown to promote both pre- and post-synaptic differentiations when applied to cultured cortical neurons[33,34]. These effects depend on cdk5 and crmp1. Consistent with this, analysis of Sema3A or crmp1 knockout mice shows lower dendritic spine density in layer V cerebral cortex. In contrast, Sema3F mutant mice exhibit an increased number of dendritic spines in both hippocampal and layer V cortical neurons[36], and Sema3F signaling is required for synapse elimination before axon pruning in hippocampal and cortical neurons[37,38]. How the two diffusible semaphorins exhibit such different effects is not clear. Diffusible semaphorins were initially identified as chemorepellents, but some of them are known to be chemoattractants as well [39]. In addition, Sema3A has also been shown to regulate axoplasmic transport, which is independent of its repellent activities[40]. These findings highlight the complexity of these semaphorins’ functions and might explain why various effects have been observed. Further studies are required to pinpoint the functions of diffusible semaphorins in synapse formation.
Diffusible slit may inhibit synaptogenesis
There had been very few reports on the role of slits and robos in synapse formation [41], but one recent study in the zebrafish retinotectal system revealed a possible role for slit-robo signaling in inhibiting synapse formation[42]. Loss of either slit1a or robo2 causes increases in the complexity of RGC arbors and the number of synaptic puncta and area. The effects of robo2 in RGCs are cell-autonomous. Time-lapse imaging of RGC further demonstrates that arborization and synaptogenesis mature faster in robo2 mutants or slit1a morphants. Together, these findings indicate that slit1a in the tectum prevents RGC axons arborization and synaptogenesis probably by regulating cytoskeleton dynamics. The mechanism may be presynaptic, since the role of robo2 is cell-autonomous in presynaptic RGC axons.
BMPs have a role in presynaptic growth
BMPs are members of the TGF-β superfamily. They bind to a receptor complex of type I and type II receptors and activate the signaling molecule receptor Smad (R-Smad). Activated R-Smad then associates with common mediator Smad (co-Smad) and translocates into the nucleus to regulate transcriptional activities[43].
Previous studies at the Drosophila NMJ indicate BMPs regulate presynaptic growth. Mutations in BMP, their receptors, or signaling molecules cause impaired neurotransmitter release and reduced synaptic size and number[44–47]. A Smad-independent LIMK pathway has also been reported to mediate BMP receptor’s presynaptic function[48]. Recent reports further indicate that presynaptic BMP receptors at the Drosophila NMJ are regulated by endocytic trafficking. Such regulation can be controlled by NIPA1/spichthyin, vps35 and nerve wreck. Mutations in these genes affect the presynaptic BMP signaling and synapse formation[49–51]. Recent studies in mutant mice also suggest that BMP signaling regulates synapse formation. Chordin is a secreted extracellular antagonist of BMP signaling. Chordin mutant mice exhibit increased frequency of mESPC and enhanced paired-pulse facilitation, suggesting its role in controlling presynaptic transmitter release[52].
Wnt signaling has diverse pre- and post-synaptic effects
Wnt signaling has long been recognized to promote presynaptic differentiation. Studies in mouse cerebellum and spinal cord sensory-motor connection demonstrate that Wnts are secreted from the postsynaptic neurons and function as retrograde signals to induce the accumulation of presynaptic components during synapse formation[53]. In vivo analysis of mutants of wnt-7a or its receptor dishevelled-1 reveals defect in presynaptic protein assembly[53–55]. At the Drosophila NMJ, Wnt/Wingless regulates presynaptic microtubule dynamics and assembly of presynaptic proteins, although Wnt/Wingless is secreted from presynaptic fibers and might act in an autocrine fashion [56,57].
Wnt signaling also participates in post-synaptic differentiation. At the vertebrate NMJ, the Wnt receptor, Dishevelled, interacts with MuSK (muscle-specific kinase) to activate PAK1 kinase and regulate the clustering of postsynaptic acetylcholine receptors (AChRs)[58]. Recent studies further demonstrate that Wnt11r is the ligand that binds to MuSK/unplugged and induces AChR clustering in the NMJ in zebrafish[59] and that Wnt works with agrin, the ligand of MuSK, to promote the AChR aggregation at the NMJ[60]. The effects of Wnt signaling on postsynaptic development are also observed at the Drosophila NMJ[56], and an unexpected nuclear translocation and transcription regulation mechanism has been proposed[61].
In addition to pro-synaptogenic activity, Wnt signaling can inhibit synapse formation. In C. elegans, Wnt/Lin-44 is expressed in a high posterior-low anterior gradient to prevent the presynaptic protein assembly of DA9 axon at the tail region[62]. In wnt/lin-44, frizzled/lin-17 or dishevelled/dsh-1 mutant animals, ectopic presynaptic proteins accumulate in the otherwise asynaptic region. At the Drosophila NMJ, Wnt-4 has been identified as a local repulsive cue for target discrimination[63].
Finally, it is interesting to note that the effects of BMPs and Wnts on synapse formation seem to be complimentary. For example, at the Drosophila NMJ, the wnt mutants have ghost boutons - boutons that contain synaptic vesicles but without active zone and post-synaptic specialization[56]. In contrast, mutants of BMP signaling often exhibit defects in transmitter release though they have intact active zones[44,45]. In addition, Wnt signaling seems to involve presynaptic microtubule remodeling[56,57], while BMP signaling is likely to control presynaptic actin dynamics[48]. Together, these results imply that morphogens such as BMPs and Wnts may function complimentarily in many aspects of development to, for example, pattern the wing formation during development, guide the projection of commissural axons in the spinal cord, and control synapse formation.
Conclusions
An emerging picture of the functions of axon guidance molecules in synapse formation
A general picture of how axon guidance molecules contribute to synapse formation is beginning to emerge from the studies summarized above. Guidepost cells within the target area – resembling the guideposts in axon guidance – help define microenvironments for the axon terminal arbors to determine their final synaptic contact points (Figure 1b and 2). Axon guidance molecules in the target area establish a local field composed of pro- and anti-synaptogenic cues to control synapse formation. The pre- and post-synaptic cells sense these combinatorial signals to determine the subcellular distributions of synaptic components and the specific synaptic contact. Thus, axon guidance molecules use similar molecular mechanisms in axon guidance - chemoattraction, chemorepulsion, contact-mediated attraction or contact-mediated repulsion - to specify synapse formation.
One unique feature in synapse formation is the induction of specific pre- and post-synaptic differentiation. Current evidence indicates that axon guidance molecules play roles in synaptic differentiation. Further studies are required to address how they regulate the cytoskeletal dynamics and the transport of various synaptic components to the synaptic sites. It will be interesting to learn whether similar signaling mechanisms are utilized for both axon guidance and synapse formation, and how axon guidance molecules are regulated temporally and spatially so that ectopic synapses are not formed. Finally, it will also be interesting to know how some axon guidance repellents, such as ephrins and transmembrane semaphorins, switch to promote synapse formation after the axons reach their target area. Future studies on how these axon guidance molecules coordinate with synaptogenic adhesion molecules to facilitate synapse formation might provide answers to some of these questions and allow us to better understand the highly specialized process of synapse formation.
Acknowledgements
We would like to thank RL Faulkner, D O’Halloran and TW Cheng for helpful comments on this manuscript; CM Ho, CY Cheng and YT Chang for preparation of this manuscript. Owing to the space limit, we apologize for not being able to cite additional relevant publications. This work was supported by grants from the National Institute of Health (HD045757) and the March of Dimes Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics in Publishing: General Statement
No conflict of interest (S.-Y. C. and H.-J. C.)
References and annotations
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Tessier-Lavigne M, Goodman CS. The Molecular Biology of Axon Guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 2.Dickson BJ. Molecular Mechanisms of Axon Guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 3.Scheiffele P. CELL-CELL SIGNALING DURING SYNAPSE FORMATION IN THE CNS. Annual Review of Neuroscience. 2003;26:485. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 4.Waites CL, Craig AM, Garner CC. MECHANISMS OF VERTEBRATE SYNAPTOGENESIS. Annual Review of Neuroscience. 2005;28:251. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 5.McAllister AK. Dynamic Aspects of CNS Synapse Formation. Annual Review of Neuroscience. 2007;30:425. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y, Garner CC. Molecular Mechanisms of Presynaptic Differentiation. Annual Review of Cell and Developmental Biology. 2008;24:237. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- 7.Chao DL, Ma L, Shen K. Transient cell-cell interactions in neural circuit formation. Nat Rev Neurosci. 2009;10:262–271. doi: 10.1038/nrn2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biederer T, Stagi M. Signaling by synaptogenic molecules. Current Opinion in Neurobiology. 2008;18:261–269. doi: 10.1016/j.conb.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y, Lyuksyutova AI. Morphogens as conserved axon guidance cues. Current Opinion in Neurobiology. 2007;17:22–28. doi: 10.1016/j.conb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends in Neurosciences. 2008;31:487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colon-Ramos DA, Margeta MA, Shen K. Glia Promote Local Synaptogenesis Through UNC-6 (Netrin) Signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762.The authors demonstrate a novel role of netrin/UNC-6 in promoting presynaptic assembly. They show that netrin/UNC-6 is secreted by supporting glia cells to facilitate the local circuit formation between the presynaptic AIY neuron and the postsynaptic RIA neuron. Through its DCC/UNC-40 receptor, netrin/UNC-6 functions as a guidance molecule to guide the RIA neurite, but it regulates the presynaptic assembly of AIY axons
- 14.Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291.The authors elegantly demonstrate that in addition to functioning as a chemorepellent in axon guidance, Netrin/UNC-6, through its UNC-5 receptor, excludes presynaptic components from the dendrites of C. elegans DA9 motor neuron.
- 15.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled Encodes a Drosophila Member of the DCC Immunoglobulin Subfamily and Is Required for CNS and Motor Axon Guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 16.Winberg ML, Mitchell KJ, Goodman CS. Genetic Analysis of the Mechanisms Controlling Target Selection: Complementary and Combinatorial Functions of Netrins, Semaphorins, and IgCAMs. Cell. 1998;93:581–591. doi: 10.1016/s0092-8674(00)81187-3. [DOI] [PubMed] [Google Scholar]
- 17.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 18.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB Receptors Interact with NMDA Receptors and Regulate Excitatory Synapse Formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 19.Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. The Journal of Cell Biology. 2003;163:1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and Trans-Synaptic Regulation of Glutamatergic Synaptogenesis by EphB Receptors. Journal of Neuroscience. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayser MS, Nolt MJ, Dalva MB. EphB Receptors Couple Dendritic Filopodia Motility to Synapse Formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007.Using time-lapse imaging in dissociated cell culture and in slice, the authors dissect the mechanisms of how EphB regulates synaptic formation. In EphB1–3 triple knockout mice, the motility of dendritic filopodia and the density of synapses are significantly decreased. Interestingly, even if the filopodia motility is rescued by expressing a constitutively active downstream effector, PAK, the normal synaptogenesis is only restored when kinase-inactive EphB2 and PAK are co-expressed in the neurons, which suggests EphB-mediated synapse formation requires both forward signaling in regulating dendritic filopodia motility and transcellular EphB-ephrin interactions.
- 22.Lim BK, Matsuda N, Poo M-m. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- 23.Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- 24.Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-Synaptic Eph Receptor-Ephrin Signaling in Hippocampal Mossy Fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- 25.Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci. 2008;11:1035–1043. doi: 10.1038/nn.2171. [DOI] [PubMed] [Google Scholar]
- 26.Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, Chen L. Postsynaptic EphrinB3 Promotes Shaft Glutamatergic Synapse Formation. Journal of Neuroscience. 2007;27:7508–7519. doi: 10.1523/JNEUROSCI.0705-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 28.Fu W-Y, Chen Y, Sahin M, Zhao X-S, Shi L, Bikoff JB, Lai K-O, Yung W-H, Fu AKY, Greenberg ME, et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- 29.Matthes DJ, Sink H, Kolodkin AL, Goodman CS. Semaphorin II can function as a selective inhibitor of specific synaptic arborizations. Cell. 1995;81:631–639. doi: 10.1016/0092-8674(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 30.Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- 31.Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-Based Approach Identifies Molecules Required for Glutamatergic and GABAergic Synapse Development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012.Using an RNAi based genetic screen, the authors identify two class 4 semaphorins, Sema4B and Sema4D, as candidates required for synaptic formation by regulating postsynaptic differentiation. However, while Sema4B's effects are in both excitatory glutamatergic and inhibitory GABAergic synapses, Sema4D is specific to inhibitory GABAergic synapses.
- 32.Constanze B, Myriam M, Anja B, Craig CG, Eckart DG, Andreas WP. Semaphorin 4B interacts with the post-synaptic density protein PSD-95/SAP90 and is recruited to synapses through a C-terminal PDZ-binding motif. FEBS letters. 2005;579:3821–3828. doi: 10.1016/j.febslet.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 33.Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R, et al. Regulation of Dendritic Branching and Spine Maturation by Semaphorin3A-Fyn Signaling. Journal of Neuroscience. 2006;26:2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita N, Morita A, Uchida Y, Nakamura F, Usui H, Ohshima T, Taniguchi M, Honnorat J, Thomasset N, Takei K, et al. Regulation of Spine Development by Semaphorin3A through Cyclin-Dependent Kinase 5 Phosphorylation of Collapsin Response Mediator Protein 1. Journal of Neuroscience. 2007;27:12546–12554. doi: 10.1523/JNEUROSCI.3463-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahay A, Kim C-H, Sepkuty JP, Cho E, Huganir RL, Ginty DD, Kolodkin AL. Secreted Semaphorins Modulate Synaptic Transmission in the Adult Hippocampus. Journal of Neuroscience. 2005;25:3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eickholt BJ. Functional diversity and mechanisms of action of the semaphorins. Development. 2008;135:2689–2694. doi: 10.1242/dev.019968. [DOI] [PubMed] [Google Scholar]
- 37.Liu X-B, Low LK, Jones EG, Cheng H-J. Stereotyped Axon Pruning via Plexin Signaling Is Associated with Synaptic Complex Elimination in the Hippocampus. Journal of Neuroscience. 2005;25:9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low LK, Liu X-B, Faulkner RL, Coble J, Cheng H-J. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proceedings of the National Academy of Sciences. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 40.Goshima YKT, Hori H, Sugiyama Y, Takasawa S, Hashimoto Y, Kagoshima-Maezono M, Takenaka T, Misu Y, Strittmatter SM. A novel action of collapsin: Collapsin-1 increases antero- and retrograde axoplasmic transport independently of growth cone collapse. Journal of Neurobiology. 1997;33:316–328. doi: 10.1002/(sici)1097-4695(199709)33:3<316::aid-neu9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Godenschwege TA, Simpson JH, Shan X, Bashaw GJ, Goodman CS, Murphey RK. Ectopic Expression in the Giant Fiber System of Drosophila Reveals Distinct Roles for Roundabout (Robo), Robo2, and Robo3 in Dendritic Guidance and Synaptic Connectivity. Journal of Neuroscience. 2002;22:3117–3129. doi: 10.1523/JNEUROSCI.22-08-03117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell DS, Stringham SA, Timm A, Xiao T, Law M-Y, Baier H, Nonet ML, Chien C-B. Slit1a Inhibits Retinal Ganglion Cell Arborization and Synaptogenesis via Robo2-Dependent and -Independent Pathways. Neuron. 2007;55:231–245. doi: 10.1016/j.neuron.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 43.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- 44.Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. wishful thinking Encodes a BMP Type II Receptor that Regulates Synaptic Growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 45.Marqués G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB. The Drosophila BMP Type II Receptor Wishful Thinking Regulates Neuromuscular Synapse Morphology and Function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- 46.McCabe BD, Marqués G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O'Connor MB. The BMP Homolog Gbb Provides a Retrograde Signal that Regulates Synaptic Growth at the Drosophila Neuromuscular Junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 47.Joel M, Rawson MLELKSBS. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. Journal of Neurobiology. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- 48.Eaton BA, Davis GW. LIM Kinase1 Controls Synaptic Stability Downstream of the Type II BMP Receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Shaw WR, Tsang HTH, Reid E, O'Kane CJ. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat Neurosci. 2007;10:177–185. doi: 10.1038/nn1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connor-Giles KM, Ho LL, Ganetzky B. Nervous Wreck Interacts with Thickveins and the Endocytic Machinery to Attenuate Retrograde BMP Signaling during Synaptic Growth. Neuron. 2008;58:507–518. doi: 10.1016/j.neuron.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korolchuk VI, Schutz MM, Gomez-Llorente C, Rocha J, Lansu NR, Collins SM, Wairkar YP, Robinson IM, O'Kane CJ. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. Journal of Cell Science. 2007;120:4367–4376. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 52.Sun M, Thomas MJ, Herder R, Bofenkamp ML, Selleck SB, O'Connor MB. Presynaptic Contributions of Chordin to Hippocampal Plasticity and Spatial Learning. Journal of Neuroscience. 2007;27:7740–7750. doi: 10.1523/JNEUROSCI.1604-07.2007.The role of retrograde BMP signaling in regulating synaptic function has been demonstrated in Drosophila. In this study, the authors investigate the role of BMP signaling in mammalian synapses by analyzing Chordin mutant mice. Chordin is a secreted extracellular antagonist of BMP signaling. They show that the number of docked vesicles per active zone length and the frequency of mEPSC are increased in mutant mice. These results are consistent with the idea that BMP signaling regulates synaptic function in the pre-synaptic terminal.
- 53.Hall AC, Lucas FR, Salinas PC. Axonal Remodeling and Synaptic Differentiation in the Cerebellum Is Regulated by WNT-7a Signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. The Journal of Cell Biology. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, Expressed by Motoneurons, Regulates Terminal Arborization of Neurotrophin-3-Responsive Spinal Sensory Neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 56.Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, Wingless, Provides an Essential Signal for Pre- and Postsynaptic Differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miech C, Pauer H-U, He X, Schwarz TL. Presynaptic Local Signaling by a Canonical Wingless Pathway Regulates Development of the Drosophila Neuromuscular Junction. Journal of Neuroscience. 2008;28:10875–10884. doi: 10.1523/JNEUROSCI.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, et al. Regulation of AChR Clustering by Dishevelled Interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 59.Jing L, Lefebvre JL, Gordon LR, Granato M. Wnt Signals Organize Synaptic Prepattern and Axon Guidance through the Zebrafish unplugged/MuSK Receptor. Neuron. 2009;61:721–733. doi: 10.1016/j.neuron.2008.12.025.The authors identify a novel MuSK dependent Wnt pathway which regulates the acetylcholine receptor (AChR) clustering at the zebrafish neuromuscular junction. Knocking down or interfering of with either Wnt11r, MuSK isoform SV1 or Dishevelled can cause a reduction of AChR cluster formation prior to the arrival of motor axons. In addition, they provide both genetic and biochemical data to support that Wnt11r signals through MuSK and Dishevelled to regulate the initiation of early nerve-independent neuromuscular junction formation.
- 60.Henriquez JP, Webb A, Bence M, Bildsoe H, Sahores M, Hughes SM, Salinas PC. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proceedings of the National Academy of Sciences. 2008;105:18812–18817. doi: 10.1073/pnas.0806300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless Signaling at Synapses Is Through Cleavage and Nuclear Import of Receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klassen MP, Shen K. Wnt Signaling Positions Neuromuscular Connectivity by Inhibiting Synapse Formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046.The authors use the DA9 neuron in C. elegans to demonstrate that Wnt acts as a negative regulator of synaptogenesis. Mutations in Wnt/lin-44, Wnt receptor lin-17/Frizzled, and its downstream effector dsh-1/Dishevelled cause a redistribution of presynaptic puncta. In addition, they show that ectopically expressed wnt/lin-44 is sufficient to expand the asynaptic region of the DA9 neuron. This suggests an instructive role for the Wnt/Frizzed signaling pathway in synapse exclusion.
- 63.Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 Is a Local Repulsive Cue that Determines Synaptic Target Specificity. Current Biology. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013.Using single cell microarray analysis, the authors show that Wnt4 is differentially enriched in specific muscles M13 but not in its neighboring muscles M12 in Drosophila. Motor neurons (MN12) that normally innervate M12 form smaller synapses on M12 and ectopic synapses on M13 when Wnt4, or its putative receptor Frizzled2 and Derailed-2, or downstream effector Dishevelled, is inhibited. Furthermore, mis-expression of Wnt4 in M12 inhibits the synaptic formation by MN12. Together, these results are consistent with the idea that Wnt4 function as an anti-synaptogenic molecule to regulate target selection.