Abstract
Brain tumor cells respond poorly to radiotherapy and chemotherapy due to inherently efficient anti-apoptotic and DNA repair mechanisms. This necessitates the development of new strategies for brain cancer therapy. Here, we report that the DNA-demethylating agent Zebularine preferentially sensitizes the killing of human glioblastomas deficient in DNA-dependent protein kinase (DNA-PK). In contrast to DNA-PK-proficient human glioblastoma cells (MO59K), cytotoxicity assay with increasing Zebularine concentrations up to 300 μM resulted in a specific elevation of cell killing in DNA-PK-deficient MO59J cells. Further, an elevated frequency of polyploid cells observed in MO59J cells after Zebularine treatment pointed out a deficiency in mitotic checkpoint control. Existence of mitotic checkpoint deficiency in MO59J cells was confirmed by the abnormal centrosome number observed in Zebularine-treated MO59J cells. Although depletion of DNA methyltransferase 1 by Zebularine occurred at similar levels in both cell lines, MO59J cells displayed increased extent of DNA demethylation detected both at the gene promoter-specific level and at the genome overall level. Consistent with increased sensitivity, deoxy-Zebularine adduct level in the genomic DNA was 3- to 6-fold higher in MO59J than in MO59K cells. Elevated micronuclei frequency observed after Zebularine treatment in MO59J cells indicates the impairment of DNA repair response in MO59J cells. Collectively, our study suggests that DNA-PK is the major determining factor for cellular response to Zebularine.
Introduction
Glioblastoma multiforme is one of the most lethal forms of brain cancer with a median survival rate of <12 months and a high rate of recurrence. Treatment strategies for glioblastoma multiforme are extremely difficult due to efficient DNA repair and anti-apoptotic mechanisms that render gliomas resistant to chemotherapy and radiotherapy (1,2). Many tumors have characteristic global hypomethylation and local gene promoter hypermethylation at CpG dinucleotide sequences which result in the aberrant silencing of genes necessary for DNA damage response, apoptosis and other genome maintenance processes (3,4). DNA methylation occurs through addition of a covalently bound methyl group to DNA, commonly occurring at the fifth position of cytosine, and is carried out by three enzymes, DNA methyltransferases (DNMTs) 1, 3a and 3b (5). Therefore, cancer therapies aimed at the restoration of gene expression by DNA-demethylating agents may prove successful.
Recent studies have illustrated the usefulness of epigenome targeting by histone deacetylase or DNMT inhibitors in cancer treatment (3,6–8). The reversible nature of epigenome makes it as an effective target for pharmacological research in cancer cells. DNA methyltransferase inhibitors have been extensively studied and tested in clinical trials. Two common nucleoside DNMT inhibitors are 5-azacytidine (5-azaCR; i.e. Vidaza) and its deoxyribose analogue 5-aza-2′-deoxycytidine [5-azadCdR, i.e. decitabine; (9)]. These inhibitors get incorporated into DNA and trap the DNMT leading to a covalent protein-DNA adduct, depletion of DNMTs and subsequent demethylation of genomic DNA during replication (9). Although proven to be potentially effective as antitumor agents in laboratory experiments and in clinical trials (10–17), both inhibitors are unstable in solution and can be toxic (14).
Zebularine is demonstrated to be a potent inhibitor of DNMTs and is superior to 5-AzaCR in terms of lower cytotoxicity and increased stability in aqueous solutions (18). Zebularine is a proven nucleoside DNMT inhibitor effective at reactivating the silenced genes in vitro and in vivo (19) with a high specificity for cancer cells relative to normal cells (20). As brain tumor cells are often refractory to radiotherapy and chemotherapy, we sought to determine the effects of Zebularine on brain tumor cells. Our findings demonstrate that Zebularine selectively sensitizes the brain tumor cells that are deficient in DNA-dependent protein kinase (DNA-PK). The sensitization of cell killing by Zebularine is mediated by a combination of DNA repair and cell cycle checkpoint defects in DNA-PK-deficient glioblastoma cells.
Materials and methods
Cell lines and treatments
MO59J and MO59K cell lines were purchased from the American Type Culture Collection (Rockville, MD). MO59J and MO59K cells were cultured in OPTI-MEM I + GlutaMAX-I (Gibco BRL, NY) supplemented with 10% fetal bovine serum, vitamins and antibiotics. Zebularine [1-(beta-D-ribofuranosyl)-1, 2-dihydropyrimidin-2-one] was purchased from EMD Biosciences (San Diego, CA). Zebularine was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 100 mM and stored at 4°C.
Clonogenic survival
Cell survival was assessed using a standard colony-forming assay. 1 × 103 cells were seeded in triplicate dishes 1 day prior to treating with Zebularine at concentrations of 10–300 μM for 72 h. Following treatment, the cells were washed several times with phosphate-buffered saline and allowed to grow in fresh media without Zebularine for 10 days. The cells were then fixed in 70% ethanol for 15 min and stained with crystal violet overnight, and colonies with >50 cells were counted manually. The surviving fraction was determined by the plating efficiency normalized to sham-treated control cells. To verify the role of DNA-PK, MO59K cells were pretreated for 2–6 h with DNA-PK inhibitor NU7026 (10 μM) followed by exposure to Zebularine. As both Zebularine and DNA-PK inhibitor NU7026 were dissolved in DMSO, sham control cells were treated with the same amount of DMSO as that of cells treated with NU7026 and Zebularine.
Cell proliferation assay
For proliferation assay, 5 × 103 cells in exponential growth phase were seeded onto 96-well dishes 18 h prior to treatment with Zebularine. Proliferation was assessed using the CyQuant NF Cell Proliferation Assay (Molecular Probes, Carlsbad, CA) after 65 h of continuous treatment with different concentrations of Zebularine as described before (21). Cells treated with DMSO served as sham control. To verify the role of DNA-PK, MO59K cells were pretreated for 2–6 h with DNA-PK inhibitor NU7026 (10 μM) followed by exposure to Zebularine.
Terminal deoxy nucleotidyl transferase-mediated deoxyuridine triphosphate fluorescein nick end labeling assay for apoptosis
For detection of apoptotic sub-G1 population, Apo-BrdU terminal deoxy nucleotidyl transferase-mediated deoxyuridine triphosphate fluorescein nick end labeling assay was performed essentially as described before (21). Cells were subjected to the assay after a continuous treatment of Zebularine for 72 h.
Nocodazole treatment and cell cycle
Cells were seeded at a density of 5 × 105 cells in 10 cm dishes 24 h prior to nocodazole treatment. Fresh media containing 4 μg/μl−1 nocodazole was added and cells were processed for flow cytometry after 12 h.
Gene promoter methylation analysis
Gene-specific promoter methylation was analyzed in MO59K and MO59J cells using the TransSignal Methylation Array (Panomics, Fremont, CA). Cells were treated for 72 h with 200 μM of Zebularine and the total genomic DNA was isolated. Procedures for the precipitation of methylated DNA, labeling and hybridization were essentially performed according to the manufacturer's instructions.
High-performance liquid chromatography measurement of 5-methylcytosine and deoxy-Zebularine adducts
High-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS) was used to detect both 5′-methyl deoxycytidine and deoxycytidine in hydrolyzed DNA samples as described previously for the detection of oxidative DNA lesions (22,23). Conditions for quantitative enzymatic hydrolysis of DNA were essentially the same as described before (24). Detailed procedure for deoxy-Zebularine adduct detection by HPLC-MS/MS is given in the supplementary data (available at Carcinogenesis Online).
Results
DNA-PK-deficient MO59J cells are preferentially sensitive to the cytotoxic effects of Zebularine
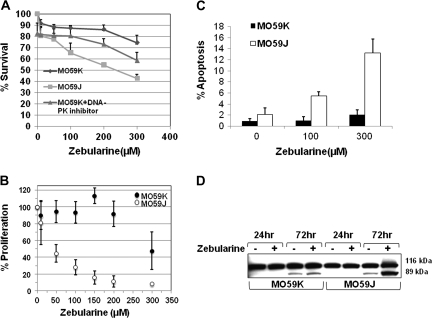
To determine whether Zebularine sensitizes human gliomas, DNA-PK-proficient (MO59K) cells and DNA-PK-deficient (MO59J) cells were exposed to different Zebularine concentrations (10–300 μM) for 72 h and assayed for clonogenic survival. The lethal concentration that kills 50% of the cell population (LC50) value of Zebularine was found to be 245 μM for MO59J cells. In contrast, MO59K cells showed only 20% reduction in survival at the same Zebularine concentration (Figure 1A). Preferential sensitization of DNA-PK-defective MO59J cells seems to suggest that DNA-PK is a critical factor for protecting the brain tumor cells from the cytotoxic effects of Zebularine. To directly assess the role of DNA-PK in Zebularine-mediated cytotoxic effects, we utilized a highly specific DNA-PK inhibitor (NU7026) to suppress DNA-PK in MO59K cells (25). The study by Nutley et al. (25) demonstrated that treatment of human ovarian cancer cells with NU7026 at a concentration of 10 μM, which is largely non-toxic to the cells, resulted in the complete sensitization of cells after exposure to 3 Gy of ionizing radiation. MO59K cells were pretreated for 2–6 h with 10 μM of DNA-PK-specific inhibitor NU7026 (EMD Chemicals, Gibbstown, NJ) followed by subsequent exposure to different concentrations of Zebularine. Mock-treated cells received the same amount of DMSO as that of cells treated with both Zebularine and DNA-PK inhibitor (NU7026). MO59K cells treated with DNA-PK inhibitor clearly showed a reduced survival after Zebularine treatment illustrating that the sensitizing effects of Zebularine in human gliomas is potentiated by the loss of DNA-PK (Figure 1A). In contrast to MO59K cells, MO59J cells showed a 20% reduction in survival even at the lowest Zebularine concentration (10 μM) used in this study. In corroboration with cellular sensitivity, a dose-dependent effect of Zebularine on cellular proliferation was also observed in MO59J. While 50 μM Zebularine reduced the proliferation by 50% in MO59J cells, a relatively high Zebularine concentration (300 μM) was required to achieve a similar level of proliferation inhibition in MO59K cells (Figure 1B). To verify whether inhibition of DNA-PK in MO59K cells also reduces the cellular proliferation after Zebularine treatment, MO59K cells were pretreated for 2–6 h with NU7026 followed by exposure to different concentrations of Zebularine (10–300 μM). As compared to MO59K cells treated with Zebularine alone, cells treated with DNA-PK inhibitor (10 μM) and Zebularine reduced the cellular proliferation by 14–28% after 96 h of treatment and the inhibitory effect was more pronounced at Zebularine concentrations ranging from 50–150 μM (supplementary Figure 1 is available at Carcinogenesis Online).
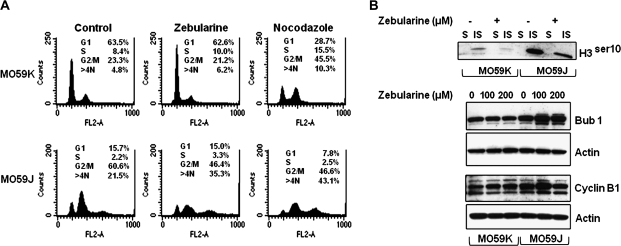
Fig. 1.
Zebularine selectively sensitizes DNA-PK-deficient MO59J cells to cell killing. (A) MO59J cells had reduced clonogenic survival with increasing concentrations of Zebularine. The clonogenic survival was reduced in MO59K cells that were pretreated with 10 μM of DNA-PK inhibitor (NU7026) followed by treatment with indicated concentrations of Zebularine. (B) MO59J cells showed reduced cellular proliferation with increasing concentrations of Zebularine. (C) Zebularine exposure induced a dose-dependent apoptosis in MO59J. Apoptosis was determined by flow cytometry of cells labeled by Apo-BrdU terminal deoxy nucleotidyl transferase-mediated deoxyuridine triphosphate fluorescein nick end labeling assay (Invitrogen, Carlsbad, CA). (D) Zebularine treatment specifically increased the level of cleaved poly(ADP) ribose polymerase-1 fragment (89 kDa) in MO59J cells following exposure to 200 μM Zebularine for 72 h.
Reduced clonogenic survival and cellular proliferation observed in MO59J cells prompted us to determine whether MO59J cells undergo apoptosis after Zebularine treatment. Although cellular proliferation was significantly affected by the highest concentration of Zebularine (300 μM) in both cell lines, apoptosis measured by bromodeoxyuridine terminal deoxy nucleotidyl transferase-mediated deoxyuridine triphosphate fluorescein nick end labeling assay was much higher in MO59J (14%) than in MO59K (2%) cells after 72 h of Zebularine exposure (Figure 1C). To confirm this observation, poly(ADP) ribose polymerase-1 cleavage indicative of apoptotic death was analyzed. The intensity of poly(ADP) ribose polymerase-1 cleaved fragment (85 kDa) was ∼3.5-fold higher in MO59J than in MO59K cells demonstrating the enhanced sensitivity of DNA-PK-deficient gliomas to Zebularine (Figure 1D). Consistent with reduced cell survival and proliferation, inhibition of DNA-PK in MO59K cells resulted in a 1.25- to 1.5-fold increase in apoptotic death over cells treated with Zebularine alone (200 and 300 μM). This finding demonstrates that the loss of DNA-PK potentiates the cytotoxic effects of Zebularine in human glioblastoma cells.
Zebularine causes a rapid global and gene-specific demethylation in DNA-PK-defective MO59J cells
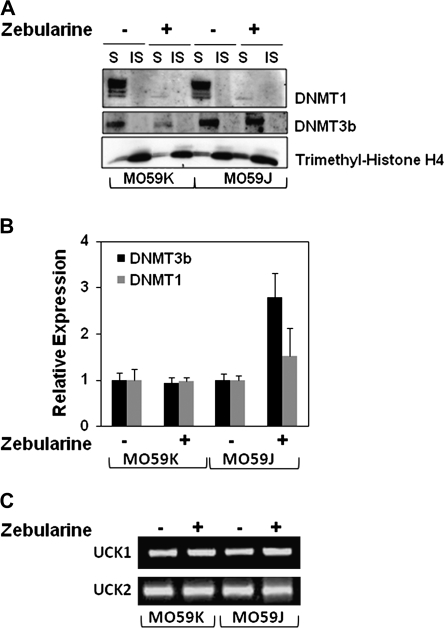
Zebularine is a known nucleoside analog which, when incorporated into newly synthesized DNA, covalently traps DNMT1 with chromatin leading to DNA demethylation. To verify whether or not the enhanced Zebularine sensitivity of MO59J cells is due to variations in DNMT expression, expression levels of DNMT1 and DNMT3b proteins were analyzed after Zebularine treatment in both cell lines. As DNMT1 binds to chromatin, DNMT1 level after Zebularine treatment was analyzed in both soluble and chromatin-bound insoluble protein fractions (26,27). In mock-treated cells, DNMT1 was enriched only in the soluble protein fraction in both cell lines. In corroboration with an earlier report (28), complete DNMT1 protein depletion from both soluble and insoluble fractions was observed after Zebularine treatment in both cell lines (Figure 2A). To verify the soluble and insoluble protein fractionation, the blot was probed with trimethyl-Histone H4, which is known to be enriched in the chromatin-bound fractions (Figure 2A). As DNMT1 messenger RNA expression level measured by quantitative real-time polymerase chain reaction did not drastically change either with or without Zebularine treatment in both cell lines, DNMT1 depletion at the protein level was presumably due to trapping of the enzyme to the DNA (Figure 2B). Interestingly, Zebularine significantly depleted DNMT3b only in MO59K cells but not in MO59J cells. The relative expression level of DNMT3b messenger RNA was increased in MO59J cells after Zebularine treatment suggesting that DNMT3b may be upregulated through a DNA-damage response pathway. Ben-Kasus et al. (29) have demonstrated that Zebularine is phosphorylated and incorporated into DNA and the phosphorylation is mediated by uridine cytidine kinases (UCKs; UCK1 and UCK2). To test whether there are differences in UCK levels between MO59J and MO59K cells, expression levels of UCK1 and UCK2 were analyzed by reverse transcription–polymerase chain reaction. The expression levels of UCK1 and UCK2 were found to be similar in both cell lines (Figure 2C).
Fig. 2.
Zebularine depletes DNMT protein levels in MO59J and MO59K cells. (A) MO59J and MO59K cell lines were incubated with 200 μM Zebularine for 72 h. DNMT1 and DNMT3b protein levels were measured in total soluble (S) and insoluble (IS; chromatin bound) fractions. Trimethyl-Histone H4 was used to verify the fractionation of S and IS proteins. (B) Quantitative real-time polymerase chain reaction analysis of DNMT1 and DNMT3b messenger RNA in MO59K and MO59J following 200 μM Zebularine for 72 h. Actin was used as an internal control for normalization. (C) Reverse transcription–polymerase chain reaction analysis of UCK1 and UCK2 messenger RNA expression. Total RNA from MO59K and MO59J was isolated in untreated and 200 μM Zebularine-treated cells for 72 h.
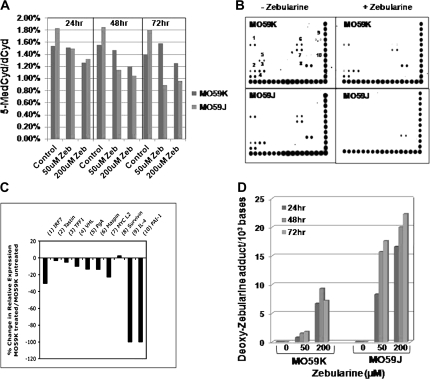
The impact of Zebularine on DNA demethylation was next analyzed both at the overall genome and gene-specific levels. The genome wide demethylation measured by HPLC-MS/MS showed a dependency both on dose and treatment time of Zebularine (Figure 3A). In MO59J cells, Zebularine exposure at a low concentration (50 μM) for 24 h was sufficient to reduce the 5-methylcytosine content by 40% of that observed in sham-treated control cells. In contrast, a 200 μM dose of Zebularine was required to yield about a 30% reduction in global DNA methylation in MO59K cells. Interestingly, the basal level of DNA methylation observed in sham-treated MO59J cells was higher than that of sham-treated MO59K cells. This finding clearly indicates that DNA-PK is critical for the maintenance of epigenome integrity in human cancer cells. Although our western blot analysis demonstrated the depletion of DNMT1 protein (Figure 2A), it was important to investigate whether DNMT1 depletion results in demethylation of gene promoter regions. For this purpose, a gene promoter methylation array comprising of a total of 82 genes (Panomics) was utilized. Procedures for the precipitation of methylated DNA, labeling and hybridization were essentially performed according to the manufacturer's instructions. Using this array, we identified 10 genes (IRF7, Tastin, TFF1, VHL, PgA, Maspin, MYCL2, Survivin, IL-4 and PAI-1) in sham-treated MO59K cells and 7 genes (IRF7, Tastin, TFF1, VHL, Maspin, MYCL2 and Survivin) in sham-treated MO59J cells that were methylated at the promoter regions (Figure 3B). After Zebularine treatment, the intensity of signal was markedly reduced (loss of methylation) in the promoter regions of all the genes in MO59J. Although demethylation of all the genes was apparent in MO59K (Figure 3C) after Zebularine treatment, the extent of demethylation (judged by the loss of signal intensity) was much less in MO59K as compared with MO59J cells. Interestingly, methylation status of Survivin did not get drastically altered by Zebularine in MO59K cells, whereas the loss of methylation was readily noticeable in MO59J cells (Figure 3B). Thus, the rapid loss of DNA methylation observed at the gene promoter regions after Zebularine treatment in MO59J cells correlated well with the loss of methylation measured at the genome overall by HPLC-MS/MS detection of 5-methylcytosine level.
Fig. 3.
Zebularine-induced DNA demethylation is rapid in DNA-PK-defective MO59J cells. (A) Zebularine (Zeb) treatment caused a decrease in 5-methylcytosine at the genome overall level in both MO59K and MO59J in a time and dose-dependent manner. (B) Gene-specific promoter DNA methylation was analyzed in MO59K and MO59J cells using the TransSignal Methylation Array. Both cell lines were mock treated or treated with 200 μM Zebularine for 72 h and DNA was isolated, digested with MseI, fragments were incubated with methylation-binding protein and amplified by polymerase chain reaction. The denatured PCR product was hybridized to the methylation array. (C) Histogram showing the quantification of hybridization signal observed at various genes (numbered) using Image J software (National Institutes of Health) and normalized to the intensity of reference dots provided at the bottom and right sides of the arrays. The average density for the Zebularine treated and untreated blots were compared and represented as percentage change in expression in MO59K cells. (D) HPLC-MS/MS detection of deoxy-Zebularine adduct levels in the genomic DNA isolated from MO59J and MO59K cells after treatment with Zebularine at the indicated concentrations and treatment times.
Although UCK1 and UCK2 levels were essentially similar, differences observed in the extent of DNA demethylation both at the gene-specific level and at the genome overall level prompted us to analyze whether the differential uptake of Zebularine by MO59J and MO59K cells was responsible for the observed effects on DNA demethylation. To verify this important aspect, Zebularine incorporation was measured for the first time in the genomic DNA as deoxy-Zebularine adduct by HPLC-MS/MS. The results showed that the level of deoxy-Zebularine adduct was 3- to 6-fold higher in MO59J cells than in MO59K cells (Figure 3D). The deoxy-Zebularine adduct level increased in both cell lines in a dose-dependent manner. Interestingly, the deoxy-Zebularine adduct level measured at 72 h after Zebularine treatment (200 μM) showed a slight reduction in MO59K cells but not in MO59J cells. The time dependent increase observed in deoxy-Zebularine adduct level in MO59J cells may be due to impaired DNA repair efficiency.
Zebularine treatment results in DNA damage induction
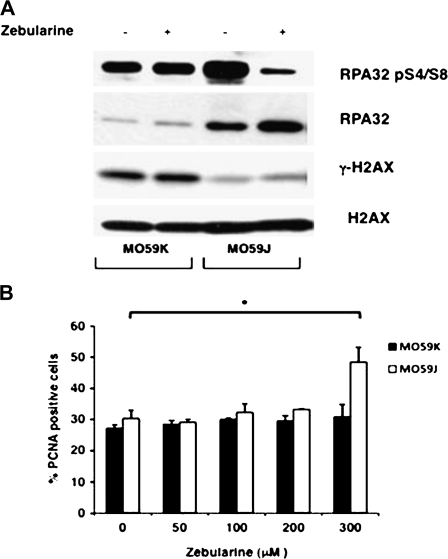
Given the deficiency of MO59J cells in DNA double-strand break (DSB) repair pathway, we hypothesized that the increased Zebularine-mediated killing of MO59J cells is due to the generation of DNA strand breaks in general and DSB in particular. To test this hypothesis, single-strand breaks and DSB induced by Zebularine were indirectly monitored by determining the relative levels of replication protein A (RPA) (a single-strand break marker) and γ-H2AX (a DSB marker) after Zebularine treatment in chromatin-bound protein fractions of both cell lines. Although basal γ-H2AX level was higher in MO59K cells than MO59J cells, Zebularine treatment did not increase the levels of γ-H2AX in both cell lines (Figure 4A). The reduced level of γ-H2AX in MO59J cells is probably due to the lack of DNA-PK that redundantly phosphorylates H2AX in an overlapping manner with ataxia telangiectasia mutated (ATM) and ATM and Rad3 related (ATR) kinases (30,31). Further, the demonstrated reduction in ATM kinase level can also account for the reduced γ-H2AX level in MO59J cells (32,33). In striking contrast to γ-H2AX, the basal levels of both phosphorylated (serine 4 and 8) and non-phosphorylated RPA were higher in MO59J than in MO59K cells. Although RPA phosphorylation is shown to be mediated through DNA-PK (34), DNA-damage induced chromatin association of RPA is DNA-PK independent (35). Interestingly, Zebularine treatment considerably reduced the accumulation of chromatin-bound phosphorylated RPA in MO59J cells, whereas no significant difference was observed between sham treated and Zebularine-treated MO59K cells. Non-phosphorylated RPA level was also higher in Zebularine-treated MO59J cells in comparison to sham-treated control. The increased RPA level may be due to the elevated accumulation of Zebularine-induced single strand breaks in MO59J cells. Proliferating cell nuclear antigen (PCNA) is recruited to chromatin rapidly after DNA damage and the kinetics of disassembly from the chromatin is considered to reflect the DNA repair efficiency (36,37). We observed a statistically significant (P = 0.01) increase in PCNA-positive cells in MO59J after 300 μM Zebularine treatment. The retention of PCNA in the chromatin-bound form over extended periods of time indicates the impairment of DNA repair response in MO59J cells (Figure 4B). Based on this observation, we suggest that the sensitivity of MO59J cells to Zebularine is probably due to an impaired DNA repair response.
Fig. 4.
Zebularine treatment increases DNA strand breaks in MO59J cells. (A) Asynchronous MO59K and MO59J cells were treated with 200 μM Zebularine for 72 h and chromatin-bound proteins were isolated. DNA damage response proteins phospho-RPA32, RPA32 and γ-H2AX were analyzed by western blotting. H2AX was used as a loading control. (B) Percentage of PCNA-positive cells after exposure to increasing concentrations of Zebularine for 24 h. The histogram shows mean ± SD for three independent experiments of 500 cells scored in triplicate (n = 6); *P < 0.002.
Zebularine-induced G2/M cell cycle arrest is abrogated in the absence of DNA-PK
DNA damage response involves an efficient co-ordination of DNA repair and cell cycle checkpoint activities. Deficient cell cycle checkpoint regulation has been reported in DNA-PK-defective MO59J cells after exposure to bleomycin and ionizing radiation (38,39). To verify whether the impaired cell cycle checkpoint regulation after Zebularine treatment sensitizes the MO59J cells, cell cycle analysis was performed. DNA demethylating agents such as 5-Aza-CdR and Zebularine induce G2/M arrest in different cancer cell types (40) and it has been demonstrated that the release from Zebularine-induced G2/M arrest as well as DNA repair efficiency correlate well with phosphorylation of ATM, p53 and Chk1 proteins (27,32,33). If cells with persistent DNA damage proceed to mitosis due to G2/M checkpoint deficiency, mitotic catastrophe is expected to occur. Consistent with impaired DNA repair response, increased frequency of polyploid (>4N) cells [21.5% in sham-treated cells and 31.5% in Zebularine-treated cells] was observed in MO59J after Zebularine treatment (Figure 5A) indicating that the increased sensitivity of MO59J cells may be due to mitotic checkpoint deficiency. To further verify whether or not MO59J cells have an intrinsic G2/M checkpoint deficiency, cell cycle analysis was performed after nocodazole, a drug that disrupts mitotic spindle assembly and arrests cells at mitosis. In the presence of a functional mitotic checkpoint, nocodazole treatment should trigger a mitotic arrest similar to that observed for MO59K cells (Figure 5A). In contrast, MO59J cells failed to show any accumulation of cells at G2/M phase. Further, the lack of G2/M arrest was evident by the increased fraction of polyploid cell population. We next analyzed the expression levels of some of the mitotic protein markers to determine whether these were altered in MO59J cells (Figure 5B). Western blot analysis of soluble and insoluble (chromatin bound) proteins revealed that the phospho-histone H3 (Ser10) expression was higher in sham-treated MO59J cells than in MO59K cells and the phospho-H3 expression was reduced in MO59J cells after Zebularine treatment (Figure 5B). Cylin B1 expression showed a Zebularine dose dependency in both cell lines. Bub1 (a spindle checkpoint protein that ensures chromosome segregation fidelity during mitosis) level reaches a peak at mitosis and can be used for monitoring G2 to M phase progression. Interestingly, although cyclin B1 levels decreased at 200 μM Zebularine, Bub1 level remained elevated up to 72 h of analysis (Figure 5B). It is likely that Bub1 expression is modulated by DNA damage response or chromosome anomalies. Taken together, these results indicate that the G2/M checkpoint is abrogated at higher doses of Zebularine (>50 μM) in MO59J cells.
Fig. 5.
Zebularine increases the mitotic abnormalities in MO59J cells. (A) Representative cell cycle flow cytometry profiles of DNA content in MO59K and MO59J cells following sham treated control, Zebularine (200 μM for 72 h) and nocodazole (12 h). (B) Western blot analysis of soluble (S) and insoluble (IS) lysates for phospho-histone H3 detection and for mitotic checkpoint proteins (Cyclin B1 and Bub1) after Zebularine treatment (100 and 200 μM for 72 h). Actin was used as a loading control.
Zebularine preferentially increases genomic instability features in MO59J cells
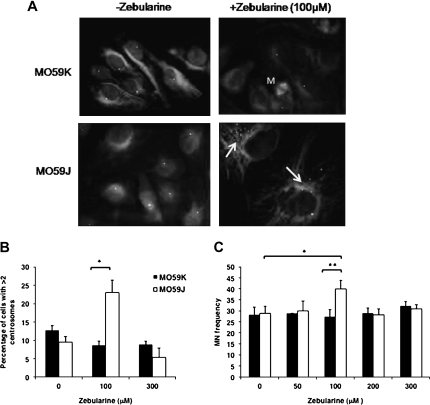
To test whether Zebularine-induced polyploidy is accompanied by spindle checkpoint deficiencies, centrosome number was analyzed by immunoprobing for pericentrin. Increased centrosome number per cell was observed in MO59J after Zebularine treatment up to 100 μM followed by a decline at higher Zebularine concentrations (Figure 6A and B). In addition to centrosome number, micronuclei formation, which is a useful indicator of chromosome breakage and mis-segregation, was also assessed. In MO59J, percentage of micronuclei-positive cells after treatment with 100 μM Zebularine was significantly (P = 0.02) higher than either sham-treated MO59J cells or MO59K (P = 0.009) cells treated with the same Zebularine concentration (Figure 6C). The decline of both centrosome number and micronuclei-positive cells observed in MO59J cell line after exposure to high concentrations of Zebularine is probably due to the selective removal of heavily damaged cells by apoptosis. Collectively, our data indicate that MO59J cells are sensitized to Zebularine due to a combination of deficiencies in DNA repair and mitotic checkpoint activities.
Fig. 6.
Zebularine induces genomic instability features in MO59J cells. (A) Centrosome amplification was measured in each cell line following increasing doses of Zebularine at 72 h. Arrows indicate the cells with increased centrosomes in MO59J cells. M indicates the mitotic cell in MO59K cell line. (B) Histogram showing the percentage of cells with >2 centrosomes in MO59K and MO59J cell lines. Values represent the mean of at least two independent biological experiments in which a minimum of 500 randomly chosen cells were analyzed; *P < 0.001. (C) Chromosome breakage was determined by scoring micronuclei (MN) in MO59K and MO59J cells following exposure to Zebularine (0–300 μM) for 72 h. The values represent the mean of three independent experiments where a minimum of 500 cells were scored for each experiment; *P < 0.05 and **P < 0.05. Error bars represent standard deviation.
Discussion
Existence of inherently efficient DNA repair and anti-apoptotic mechanisms in brain tumor cells poses a technical challenge for the development of effective treatment strategies. In this study, we demonstrated that DNA-PK-deficient glioblastoma cells are selectively sensitive to DNA-demethylating agent Zebularine due to the impairment of DNA repair and mitotic checkpoint activities. Further, our study identified DNA-PK as a potential molecular target for Zebularine-mediated sensitization of brain cancer cells. Our study suggests the possibility that development of clinically applicable DNA-PK inhibitor may be effective in sensitizing the brain tumor cells to exogenous DNA damage.
The DNA-demethylating agent Zebularine has gained considerable importance in recent times due to its demonstrated specificity for cancer cells (41). Further, properties of low cytotoxicity and greater stability in solution make Zebularine an attractive agent for targeting the epigenome of cancer cells. However, use of Zebularine has certain limitations and further studies are absolutely necessary for validation of its use under clinical settings. A distinct disadvantage of Zebularine is that a much higher Zebularine concentration as compared with 5-Aza-CdR is required to obtain similar levels of DNA demethylation in cancer cells. Further, Zebularine has been shown to be actively metabolized by aldehyde oxidase thereby reducing the overall oral bioavailability of Zebularine (42). Inhibition of Zebularine metabolism by raloxifene and 5-benzylacyclouridine may be used clinically not only to improve the oral bioavailability of Zebularine but also to reduce its cytotoxicity (42). Although Zebularine is increasingly used in experimental in vitro cancer cell systems, the mode of Zebularine sensitization of cancer cells remains largely unknown. In this study, we have demonstrated that the functional loss of DNA-PK leads to enhanced sensitization of brain cancer cells by Zebularine due to deficiencies in DNA repair and mitotic checkpoint regulation. Zebularine has been shown to form a covalent complex between DNMT1 and the Zebularine-substituted DNA thereby resulting in the depletion of DNMT1 level. An earlier study (29) demonstrated that Zebularine is phosphorylated prior to its incorporation into DNA and the Zebularine is phosphorylated by UCK1 and UCK2. Although we found no difference either in DNMT1 depletion or UCK1 and UCK2 expression levels between DNA-PK-proficient (MO59K) cells and DNA-PK-deficient (MO59J) cells, DNA-PK-deficient cells were found to be much more sensitive than MO59K cells.
DNA-PK belongs to a super family of phosphatidylinositol-3 kinases and is shown to be a critical factor for the removal of a wide spectrum of DNA lesions ranging from simple DSB to complex helix distorting bulky DNA adducts (43–45). Human and mouse cells deficient in DNA-PK are extremely sensitive to ionizing radiation due to impairment of DNA damage response. Although Zebularine has been demonstrated to form a covalent complex with DNMT1 enzyme and Zebularine-substituted DNA, the exact molecular nature of DNA lesions induced either directly or indirectly by Zebularine exposure remains to be identified. Nevertheless, selective enrichment of RPA and PCNA proteins in MO59J cells after Zebularine treatment seems to suggest that failure to remove the DNA lesions may be responsible for the enhanced cytotoxicity. An earlier study has shown that Zebularine exposure followed by ionizing radiation treatment potentiated the killing of cancer cells indicating the possibility that Zebularine has the potential to impair the DNA damage response (40). The present study demonstrates that Zebularine alone is sufficient to sensitize DNA-PK-deficient cancer cells in which the DNA repair efficiency is inherently impaired. However, the precise mechanism by which Zebularine inhibits or impairs the cellular DNA damage response is yet to be defined. As DNMT1 is recruited to ultraviolet-laser-induced DSB sites in a PCNA-dependent manner (26), impairment of DNA damage response by Zebularine may arise due to DNMT1 depletion. It is not clear whether the DNMT1 depletion in the absence of DNA-PK dramatically hampers the DNA repair efficiency. Observation of reduced clonogenic survival and proliferation in DNA-PK-suppressed MO59K cells after Zebularine exposure seems to illustrate the importance of DNA-PK in cellular protection against Zebularine-induced cytotoxicity. Further studies are required to determine the precise role of DNA-PK in Zebularine-induced DNA damage response pathway.
DNA-demethylating agents such as 5-azadeoxycytidine and Zebularine have been shown to induce G2/M arrest in a wide variety of cancer cells types (27,40). Earlier studies have shown that the release of cells from G2 arrest as well as the DNA repair efficiency (removal of γ-H2AX foci) correlated well with phosphorylation of ATM, p53 and Chk1 proteins (27,32,33). A prolonged G2/M arrest has been observed in cells lacking DNA-PK activity following exposure to DSB inducing agents such as bleomycin and ionizing radiation (38,39,46). In this study, enrichment of polyploid cells observed in DNA-PK-defective cell line after treatment with either Zebularine or nocodazole illustrates the lack of G2/M checkpoint in DNA-PK-deficient MO59J cell line. If DNA damage containing cells are allowed to proceed to mitosis due to G2/M checkpoint failure, mitotic catastrophe can occur in the form of aneuploidy. An elevated frequency of polyploid and apoptotic cells observed in MO59J cell line after Zebularine treatment indicates that DNA-PK is critical for an efficient G2/M checkpoint response after Zebularine exposure.
The extent of DNA demethylation observed at the gene promoter-specific level and at the genome overall level in MO59J cells after Zebularine exposure was distinctly higher than in MO59K cells. Consistent with this finding, the deoxy-Zebularine adduct level measured by HPLC-MS/MS was 3- to 6-fold higher in MO59J than MO59K cells. The reason for the enhanced Zebularine adduct level in the absence of functional DNA-PK in MO59J cells is not entirely clear. Given the fact that Zebularine is incorporated into genomic DNA as deoxy-Zebularine adduct during DNA replication, we speculate that unperturbed DNA replication due to S-phase checkpoint deficiency may lead to increased Zebularine incorporation in MO59J cells. Existence of S-phase checkpoint deficiency was recently observed by us in MO59J cells after exposure to cisplatin treatment (P.Carminati, J.A.Meador, E.T.Sakamoto-Hojo and A.S.Balajee, unpublished data). An alternate possibility is that DNA-PK has the potential to remove or resolve the Zebularine-DNA adducts and in the absence of DNA-PK, Zebularine adduct may simply persist leading to impaired DNA repair and cell cycle checkpoint activities. Further, Zebularine incorporation has been shown to be 7-fold higher for RNA as compared with DNA (29). Therefore, persistence of elevated deoxy-Zebularine adduct level in the genomic DNA and RNA is likely to affect the fidelities of DNA replication, transcription and translation processes leading to the sensitization of MO59J cells. On the basis of our findings, we propose that the mechanism for Zebularine-mediated sensitization of DNA-PK-deficient cells is likely to be independent of either the status of DNA methylation or the ability of Zebularine to increase the expression of tumor suppressor genes. Clearly, further studies are required to understand the molecular mechanisms by which DNA-PK protects the cancer cells against Zebularine. Taken together, our study suggests that the functional status of DNA-PK is the major determining factor for cellular response to Zebularine. The enhanced sensitivity of DNA-PK-defective MO59J cells to Zebularine is due to a combination of DNA repair and cell cycle checkpoint deficiencies. Therefore, development of clinically applicable DNA-PK inhibitor may be of use in the effective sensitization of gliomas to exogenous DNA damage.
Supplementary material
Supplementary data and Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
Office of Science (Biological and Environmental Research); US Department of Energy (DE-FG02-05ER64055); National Institutes of Health/National Cancer Institute (5P01CA49062-16).
Supplementary Material
Acknowledgments
We thank Benoit Duroux of Laboratory des lésions des Acides Nucléiques, Institut Nanosciences et Cryogénie CEA/Grenoble, France for the analysis of 5-methylcytosine detection using HPLC-tandem mass spectrometry. Financial support received from the Office of Science (BER), U.S. Department of Energy and NIH/NCI is gratefully acknowledged.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ATM
ataxia telangiectasia mutated
- DMSO
dimethyl sulfoxide
- DNA-PK
DNA-dependent protein kinase
- DNMT
DNA methyltransferase
- DSB
double-strand break
- HPLC
high-performance liquid chromatography
- MS/MS
tandem mass spectrometry
- PCNA
proliferating cell nuclear antigen
- RPA
replication protein A
- UCK
uridine cytidine kinase
References
- 1.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 2.Johannessen TC, et al. DNA repair and cancer stem-like cells—Potential partners in glioma drug resistance? Cancer Treat Rev. 2008;34:558–567. doi: 10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, et al. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 5.Goll MG, et al. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 6.Karagiannis TC, et al. Clinical potential of histone deacetylase inhibitors as stand alone therapeutics and in combination with other chemotherapeutics or radiotherapy for cancer. Epigenetics. 2006;1:121–126. doi: 10.4161/epi.1.3.3328. [DOI] [PubMed] [Google Scholar]
- 7.Szyf M. The role of dna hypermethylation and demethylation in cancer and cancer therapy. Curr. Oncol. 2008;15:72–75. doi: 10.3747/co.v15i2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones PA, et al. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyko F, et al. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J. Natl Cancer Inst. 2005;97:1498–1506. doi: 10.1093/jnci/dji311. [DOI] [PubMed] [Google Scholar]
- 10.Issa JP, et al. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J. Clin. Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 11.Momparler RL, et al. Comparison of the antileukemic activity of 5-AZA-2′-deoxycytidine, 1-beta-D-arabinofuranosylcytosine and 5-azacytidine against L1210 leukemia. Leuk. Res. 1984;8:1043–1049. doi: 10.1016/0145-2126(84)90059-6. [DOI] [PubMed] [Google Scholar]
- 12.Santini V, et al. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann. Intern. Med. 2001;134:573–586. doi: 10.7326/0003-4819-134-7-200104030-00011. [DOI] [PubMed] [Google Scholar]
- 13.Leone G, et al. Inhibitors of DNA methylation in the treatment of hematological malignancies and MDS. Clin. Immunol. 2003;109:89–102. doi: 10.1016/s1521-6616(03)00207-9. [DOI] [PubMed] [Google Scholar]
- 14.Cihak A. Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974;30:405–422. doi: 10.1159/000224981. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 16.Jones PA, et al. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JC, et al. Continuous zebularine treatment effectively sustains demethylation in human bladder cancer cells. Mol. Cell. Biol. 2004;24:1270–1278. doi: 10.1128/MCB.24.3.1270-1278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng JC, et al. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J. Natl Cancer Inst. 2003;95:399–409. doi: 10.1093/jnci/95.5.399. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JC, et al. Preferential response of cancer cells to zebularine. Cancer Cell. 2004;6:151–158. doi: 10.1016/j.ccr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Meador J, et al. Histone H2AX is a critical factor for cellular protection against DNA alkylating agents. Oncogene. 2008;27:5662–5671. doi: 10.1038/onc.2008.187. [DOI] [PubMed] [Google Scholar]
- 22.Frelon S, et al. High-performance liquid chromatography–tandem mass spectrometry measurement of radiation-induced base damage to isolated and cellular DNA. Chem. Res. Toxicol. 2000;13:1002–1010. doi: 10.1021/tx000085h. [DOI] [PubMed] [Google Scholar]
- 23.Ravanat JL, et al. Isotope dilution high-performance liquid chromatography-electrospray tandem mass spectrometry assay for the measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in biological samples. J. Chromatogr. B Biomed. Sci. Appl. 1998;715:349–356. doi: 10.1016/s0378-4347(98)00259-x. [DOI] [PubMed] [Google Scholar]
- 24.Ravanat JL, et al. Cellular background level of 8-oxo-7,8-dihydro-2′-deoxyguanosine: an isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis. 2002;23:1911–1918. doi: 10.1093/carcin/23.11.1911. [DOI] [PubMed] [Google Scholar]
- 25.Nutley BP, et al. Preclinical pharmacokinetics and metabolism of a novel prototype DNA-PK inhibitor NU7026. Br. J. Cancer. 2005;93:1011–1018. doi: 10.1038/sj.bjc.6602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortusewicz O, et al. Recruitment of DNA methyltransferase I to DNA repair sites. Proc. Natl Acad. Sci. USA. 2005;102:8905–8909. doi: 10.1073/pnas.0501034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palii SS, et al. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol. Cell. Biol. 2008;28:752–771. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Gac G, et al. DNA damage-induced down-regulation of human Cdc25C and Cdc2 is mediated by cooperation between p53 and maintenance DNA (cytosine-5) methyltransferase 1. J. Biol. Chem. 2006;281:24161–24170. doi: 10.1074/jbc.M603724200. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Kasus T, et al. Metabolic activation of zebularine, a novel DNA methylation inhibitor, in human bladder carcinoma cells. Biochem. Pharmacol. 2005;70:121–133. doi: 10.1016/j.bcp.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Stiff T, et al. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, et al. Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J. Cell. Physiol. 2005;202:492–502. doi: 10.1002/jcp.20141. [DOI] [PubMed] [Google Scholar]
- 32.Chan DW, et al. Lack of correlation between ATM protein expression and tumour cell radiosensitivity. Int. J. Radiat. Biol. 1998;74:217–224. doi: 10.1080/095530098141591. [DOI] [PubMed] [Google Scholar]
- 33.Gately DP, et al. Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol. Biol. Cell. 1998;9:2361–2374. doi: 10.1091/mbc.9.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Block WD, et al. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res. 2004;32:997–1005. doi: 10.1093/nar/gkh265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, et al. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene. 2005;24:4728–4735. doi: 10.1038/sj.onc.1208674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balajee AS, et al. Oxidative damage-induced PCNA complex formation is efficient in xeroderma pigmentosum group A but reduced in Cockayne syndrome group B cells. Nucleic Acids Res. 1999;27:4476–4482. doi: 10.1093/nar/27.22.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balajee AS, et al. Chromatin-bound PCNA complex formation triggered by DNA damage occurs independent of the ATM gene product in human cells. Nucleic Acids Res. 2001;29:1341–1351. doi: 10.1093/nar/29.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holgersson A, et al. Different G2/M accumulation in M059J and M059K cells after exposure to DNA double-strand break-inducing agents. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:915–921. doi: 10.1016/j.ijrobp.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Lee SE, et al. Evidence for DNA-PK-dependent and -independent DNA double-strand break repair pathways in mammalian cells as a function of the cell cycle. Mol. Cell. Biol. 1997;17:1425–1433. doi: 10.1128/mcb.17.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dote H, et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by the DNA methylation inhibitor zebularine. Clin. Cancer Res. 2005;11:4571–4579. doi: 10.1158/1078-0432.CCR-05-0050. [DOI] [PubMed] [Google Scholar]
- 41.Marquez VE, et al. Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy. Ann. N. Y. Acad. Sci. 2005;1058:246–254. doi: 10.1196/annals.1359.037. [DOI] [PubMed] [Google Scholar]
- 42.Klecker RW, et al. Zebularine metabolism by aldehyde oxidase in hepatic cytosol from humans, monkeys, dogs, rats, and mice: influence of sex and inhibitors. Bioorg. Med. Chem. 2006;14:62–66. doi: 10.1016/j.bmc.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 43.Dejmek J, et al. DNA-dependent protein kinase (DNA-PK)-dependent cisplatin-induced loss of nucleolar facilitator of chromatin transcription (FACT) and regulation of cisplatin sensitivity by DNA-PK and FACT. Mol. Cancer Res. 2009;7:581–591. doi: 10.1158/1541-7786.MCR-08-0049. [DOI] [PubMed] [Google Scholar]
- 44.Lee SH, et al. DNA-dependent protein kinase complex: a multifunctional protein in DNA repair and damage checkpoint. Mol. Cells. 2002;13:159–166. [PubMed] [Google Scholar]
- 45.Weterings E, et al. DNA-dependent protein kinase in nonhomologous end joining: a lock with multiple keys? J. Cell Biol. 2007;179:183–186. doi: 10.1083/jcb.200705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sturgeon CM, et al. Effect of combined DNA repair inhibition and G2 checkpoint inhibition on cell cycle progression after DNA damage. Mol. Cancer Ther. 2006;5:885–892. doi: 10.1158/1535-7163.MCT-05-0358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.