Abstract
Bladder cancer is one of the common human cancers and also has a very high recurrence rate. There is a great need for agents capable of inhibiting bladder cancer development and recurrence. Here, we report that allyl isothiocyanate (AITC), an ingredient of many common cruciferous vegetables, potently inhibited the proliferation of bladder carcinoma cell lines in vitro [half maximal inhibitory concentration (IC50) of 2.7–3.3 μM], which was associated with profound G2/M arrest and apoptosis. In contrast, AITC was markedly less toxic to normal human bladder epithelial cells (IC50 of 69.4 μM). AITC was then evaluated in two rat bladder cancer models in vivo (an orthotopic model and a subcutaneous model). The orthotopic model closely mimics human bladder cancer development and recurrence. We show that a low oral dose of AITC (1 mg/kg) significantly inhibited the development and muscle invasion of the orthotopic bladder cancers but was ineffective against the subcutaneous xenografts of the same cancer cells in the same animals. This differential effect was explained by our finding that urinary levels of AITC equivalent were two to three orders of magnitude higher than that in the plasma and that its levels in the orthotopic cancer tissues were also three orders of magnitude higher than that in the subcutaneous cancer tissues. Moreover, we show that AITC is a multi-targeted agent against bladder cancer. In conclusion, AITC is selectively delivered to bladder cancer tissue through urinary excretion and potently inhibits bladder cancer development and invasion.
Introduction
Urinary bladder cancer is one of the common human cancers and originates predominately from the epithelial cells on the inner surface (1). Although the majority of bladder cancers (∼80%) are initially diagnosed without muscle invasion, known as superficial bladder cancers (2), which are typically treated with transurethral resection, most patients experience recurrence. No agent is currently available for prevention of primary bladder cancer and existing prophylactics of bladder cancer recurrence, such as attenuated Bacillus Calmette–Guerin bacteria and chemotherapeutic agents (3), have limited utility and efficacy (4). It is noteworthy, however, that these prophylactics are delivered intravesically to the bladder, taking advantage of the superficial nature of the cancer and to reduce systemic side effects of the agents, and urethral catheterization is required.
Several epidemiological studies have shown that consumption of cruciferous vegetables is significantly associated with reduced risk of bladder cancer (5–7). We recently showed that broccoli sprout extracts inhibited bladder carcinogenesis in a rat model (8). Allyl isothiocyanate (AITC, see Figure 1A for its chemical structure) occurs in many commonly consumed cruciferous vegetables and are particularly abundant in mustard, horseradish and wasabi (9,10). Although literature data are somewhat ambiguous about the chemopreventive activity of AITC (11), there is some indication that AITC may potentially prevent bladder cancer. Particularly, studies in rodents showed that >90% of orally dosed AITC was absorbed; up to 80% of the administered dose could be recovered in the urine within 24 h and tissue levels of AITC in the bladder appeared to be >10-fold higher than in other organs (12–14). Moreover, AITC is primarily metabolized through the mercapturic acid pathway to finally form the N-acetylcysteine (NAC) conjugate, which is excreted in the urine (11). We previously showed that AITC and its NAC conjugate possessed similar growth-inhibitory activity against human bladder cancer cells in culture, apparently due to the ability of the latter compound to dissociate to AITC (15,16).
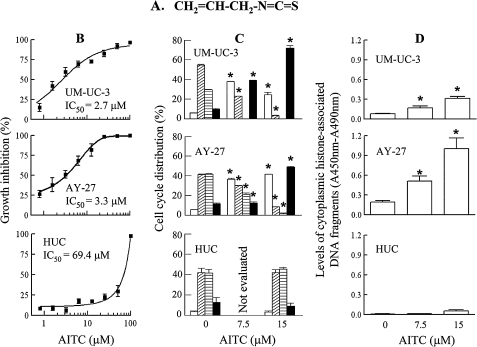
Fig. 1.
The effect of AITC on cell survival and proliferation. AITC was evaluated at the indicated concentrations in UM-UC-3, AY-27 and HUC. (A) Chemical structure of AITC. (B) Cell growth, measured by 3-(4,6-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, 72 h AITC treatment. IC50 was calculated from non-linear regression curve fit. (C) Cell cycle ( , subG1;
, subG1;  , G1;
, G1;  , S;
, S;  ; G2/M), measured by flow cytometry, 24 h AITC treatment. (D) Apoptosis, measured by an enzyme-linked immunosorbent assay, 24 h AITC treatment. Mean ± SE (n = 3–6), *P < 0.05.
; G2/M), measured by flow cytometry, 24 h AITC treatment. (D) Apoptosis, measured by an enzyme-linked immunosorbent assay, 24 h AITC treatment. Mean ± SE (n = 3–6), *P < 0.05.
In the present study, we show that AITC causes strong cell cycle arrest and apoptosis in bladder cancer cells but is markedly less toxic to normal human bladder epithelial cells. We also demonstrate that oral administration of AITC at a low dose level significantly inhibits cancer growth and muscle invasion in a rat model that mimics the development and recurrence of bladder cancer in humans. Evidence is also presented to show that AITC is a multi-targeted agent against bladder cancer. Moreover, we show that orally administered AITC is selectively delivered to bladder cancer tissue through urinary excretion. Thus, AITC is a highly promising agent for bladder cancer prevention and/or treatment, and represents a novel and attractive approach in the management of recurrence of superficial bladder cancer, since all currently available intravesicle therapies against bladder cancer recurrence mandate urethral catheterization.
Materials and methods
Chemicals and antibodies
AITC was purchased from Sigma–Aldrich (St Louis, MO). Antibodies specific for cyclin B1, caspase-3 and cleaved caspase-3 and glyceraldehyde-3-phosphate dehydrogenase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), Cell Signaling Technology (Beverly, MA) and Chemicon (Temecula, CA), respectively.
Cell culture and cell proliferation assay
Human bladder carcinoma UM-UC-3 cells were obtained from American Type Culture Collection (Manassas, VA). Rat bladder carcinoma AY-27 cells were a gift of Dr R.B.Moore of the University of Alberta in Canada. The cell line was originally established from a bladder tumor in a F344 rat (17). Normal human urothelial cells (HUCs), isolated from normal human bladder, were purchased from ScienCell Research Laboratories (Carlsbad, CA). All cells were maintained in 75 cm2 flasks at 37°C and 5% CO2 in a humidified incubator. AY-27 cells were grown in RPMI 1640 medium, containing 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1.0 mM sodium pyruvate and 10% fetal bovine serum. UM-UC-3 cells were grown in McCoy's 5α medium with 10% fetal bovine serum. HUC were grown in urothelial cell medium purchased from ScienCell (Carlsbad, CA).
For cytotoxicity assay, cells were grown in 96-well plates (5 × 103 cells per well with 0.15 ml of medium) for 24 h and then grown for 72 h in fresh medium (0.2 ml per well) containing AITC or solvent. Cell density was measured at the end of the incubation by 3-(4,6-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (18), with which the growth curve was constructed to calculate the half maximal inhibitory concentration (IC50) of AITC.
For western blot analysis and flow cytometry analysis, as described below, 1 × 106 to 1.5 × 106 cells were grown in 10 cm plate with 10 ml medium for 24 h and then treated with AITC or solvent for 24 h before analysis. The cells were trypsinized and harvested by centrifugation. For enzyme-linked immunosorbent assay-based detection of apoptosis, cells were grown in 96-well plates (1 × 104 cells per well with 0.2 ml medium) for 24 h and then grown in the same volume of fresh medium containing AITC or solvent for 24 h before analysis.
In all experiments, AITC was dissolved in acetonitrile and the final acetonitrile concentration in the medium was ≤0.025% (vol/vol).
Animal bladder cancer models
AITC was evaluated in two rat bladder cancer models: an orthotopic model and a subcutaneous model. Female Fisher 344 rats at 8–10 weeks of age were purchased from Harlan (Indianapolis, IN) and acclimatized for ∼1 week before use. AY-27 cells were simultaneously inoculated both orthotopically and subcutaneously in each rat. The rats were anesthetized with ketamine and xylazine before AY-27 cell inoculation.
Bladder instillation of AY-27 cells was based on the protocol developed by Xiao et al. (19). Briefly, each rat bladder was catheterized via the urethra with an 18 gauge plastic intravenous cannula, and 0.4 ml of 0.1 N HCl was instilled intravesically and retained for 15 s, followed immediately with 0.4 ml of 0.1 N KOH and retained for 15 s in order to neutralize the acid. The bladder was then completely drained, flushed with phosphate-buffered saline (PBS), and 1 × 106 cells in 0.5 ml serum-free medium was instilled. The cell suspension was held in the bladder for 1 h, during which the rat body was turned 90° every 15 min to facilitate exposure of the entire bladder surface to the cells. The catheter was then removed and the rat was allowed to void the suspension spontaneously. All rats were subsequently administered a subcutaneous dose of analgesic flunixin meglumine (2 mg/kg).
For subcutaneous inoculation, AY-27 cell suspension in serum-free medium was mixed 1:1 with Matrigel (BD Biosciences, San Jose, CA), and 1 × 106 cells in 0.15 ml volume were injected subcutaneously to the right flank of each rat.
One day after tumor cell inoculation, the rats were randomly assigned to receive by gavage either soy oil (3 ml/kg) or AITC (freshly dissolved in an equal volume of soy oil) once daily for 21 days. The animals were euthanized 24 h after the last dose, and both subcutaneous tumors and bladders were removed and weighed. Half of each specimen was fixed in formalin for histological analysis and the other half frozen in liquid nitrogen for western blot analysis.
All animal protocols and procedures were approved by the Roswell Park Cancer Institute Animal Care and Use Committee.
Western blot analysis
Cells after harvest were washed with ice-cold PBS, suspended in radio-immunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich) and further disrupted by sonication. Tissue specimens were thoroughly washed in PBS and homogenized in RIPA buffer supplemented with protease inhibitor cocktail, in glass homogenizers. After removal of the debris from both cell lysates and tissue homogenates by centrifugation and measurement of protein contents using the bicinchoninic acid kit from Pierce (Rockford, IL), the samples were each resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (8–12%), followed by transfer to polyvinylidene difluoride membranes. The membranes were then probed by specific antibodies and the bands of interest visualized using SuperSignal West Pico Chemiluminescence detection system from Thermo Scientific (Rockford, IL).
Enzyme-linked immunosorbent assay-based detection of apoptosis
Apoptosis was detected using the Cell Death Detection ELISAplus kit from Roche Diagnostics (Indianapolis, IN), according to manufacturer's instruction. Briefly, cells growing in 96-well plates, after removing the medium (the media were assayed by the same kit to rule out necrosis), were lysed with cell lysis buffer, and after low speed centrifugation, a portion of the supernatant was transferred to a streptavidin-coated microplate well and incubated with a mixture of anti-histone-biotin and anti-DNA-peroxidase monoclonal antibodies. The wells were then thoroughly rinsed to remove the unbound antibodies. Quantitative determination of the amount of nucleosomes retained in the immuocomplex was made by spectroscopic measurement of a peroxidase-catalyzed color reaction (measured at 405 nm, background at 490 nm).
Flow cytometry
Cell cycle was analyzed by flow cytometry, as described previously (15), using FACScan (BD Biosciences, San Jose, CA) and ModFit software (Verity Software House, Topsham, ME).
Pharmacokinetic study
Groups of four to six female F344 rats (10–12 weeks of age) were administered a single oral dose of AITC in ∼0.5 ml of soy oil per rat. The rats were immediately transferred to metabolism cages (1 rat per cage), with free access to food and water, for urine collection for 1.5, 3, 6, 12 and 24 h. One group of rats were killed for blood collection and plasma preparation at each time point. A control group was administered the same volume of soy oil and similarly kept in metabolism cages for 24 h urine collection and blood collection at the end. The plasma and urine specimens were subsequently analyzed for concentrations of AITC levels.
In a separate experiment, Groups of four F344 rats bearing both orthotopic bladder cancer and subcutaneous bladder cancer, 3 weeks after inoculation of AY-27 cells, were administered a single oral dose of AITC. The animals were sacrificed at 0, 1.5 and 6 h, and tumors from both sites were removed and thoroughly washed with ice-cold PBS for subsequent analysis of AITC levels.
AITC content in the plasma, urine and tumor tissues were determined using the high-performance liquid chromatography-based cyclocondensation assay, as described previously (8) and is expressed as AITC equivalent because the assay detects both AITC and its metabolites formed in the mercapturic acid pathway.
Histological analysis
Rat bladder specimens fixed in formalin were paraffin embedded, cut at ∼4 μm and stained with standard hematoxylin and eosin. The slides were examined for bladder and tumor histology using a Nikon 50i light microscope.
Statistical analysis
All results are expressed as mean ± SE. Unpaired two-tailed Student t-test was used for data analysis, with a P value < 0.05 being considered significant (GraphPad Version 5.00, GraphPad Software, San Diego, CA).
Results
The Effect of AITC on survival and proliferation of normal and malignant bladder cells
Treatment of human bladder carcinoma UM-UC-3 cells and rat bladder carcinoma AY-27 cells with AITC led to dose-dependent inhibition of cell proliferation with an IC50 of 2.7 and 3.3 μM, respectively (Figure 1B). It is important that AITC was similarly effective against both cell lines because only AY-27 cells were later used in the animal studies. Inhibition of cell proliferation by AITC was associated with profound cell cycle arrest and induction of apoptosis. Up to 72% of UM-UC-3 cells and 49% of AY-27 cells were in G2/M phase after treatment with AITC at 7.5–15 μM for 24 h compared with 9.9–11.5% of control cells that were in G2/M phase (Figure 1C). Both cell lines also showed strong apoptosis induction by AITC, as indicated by up to 6.5-fold (UM-UC-3) and 7.8-fold (AY-27) increases in sub-G1 population (Figure 1C) and up to 3.9 fold (UM-UC-3) and 5.2-fold (AY-27) increases in cytoplasmic levels of histone-associated DNA (Figure 1D).
In contrast, AITC was markedly less toxic to HUC. Under similar experimental conditions, the IC50 of AITC in HUC was 69.4 μM (Figure 1B), which was 21.0–25.7 times higher than the corresponding IC50 of AITC in their malignant counterparts. Moreover, AITC at 7.5 and/or 15 μM failed to cause cell cycle arrest and apoptosis in HUC (Figure 1C and D).
Inhibition of bladder cancer growth and muscle invasion by AITC in vivo
We next asked if AITC could inhibit bladder cancer development in vivo. AITC was evaluated in two rat models, an orthotopic bladder cancer model and a subcutaneous bladder cancer model, in order to understand if AITC is more effective against cancer growing in the bladder. AY-27 cells were used in both models, and both types of cancers developed simultaneously in the same animals. Subcutaneous cancer and orthotopic cancer formed in 96 and 90% of female F344 rats respectively, following AY-27 cell inoculation. The cancers in both sites grew extremely fast. The subcutaneous tumors weighed on average 1.02 g, 3 weeks after inoculation of 1 × 106 cells per rat. In the orthotopic model, 3 weeks after inoculation of the same amount of cells, the tumors weighed on average 0.30 g, which was >4.4 times the normal bladder weight (67.7 ± 4 mg), but was smaller than the subcutaneous tumors. The smaller size of orthotopic tumors, compared with the subcutaneous tumors, probably reflects to a large degree the difficulty for intravesically inoculated cells to attach to the bladder epithelium and grow into a tumor.
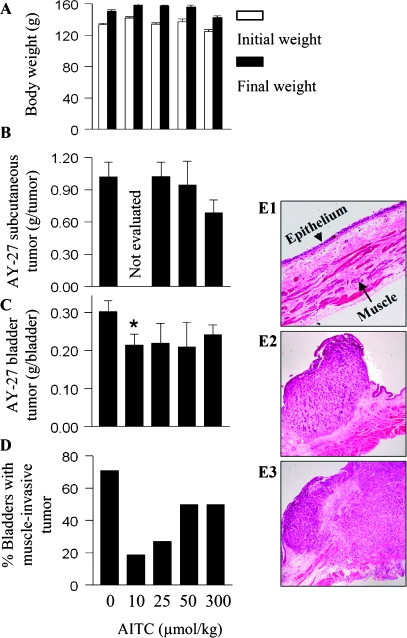
Daily oral administration of AITC was initiated 1 day after the animals were inoculated with AY-27 cells and continued for 3 weeks. Longer treatment time was not chosen in view of the heavy tumor burden in the animals at that time. AITC was evaluated at four dose levels: 10, 25, 50 and 300 μmol/kg. All animals behaved normally during AITC treatment, and none of the AITC doses caused significant body weight loss (Figure 2A). AITC at 300 μmol/kg inhibited subcutaneous cancer growth by 33%, albeit not statistically significant but became progressively ineffective as the dose was lowered (Figure 2B). The lowest dose of AITC was not evaluated in this model, in view of its lack of effect at 25 and 50 μmol/kg. In contrast, while AITC at 300 μmol/kg also inhibited the orthotopic bladder cancer growth by 20%, it became somewhat more efficacious at the lower dose levels, inhibiting tumor growth by 30%, with its effect at 10 μmol/kg showing statistical significance (P = 0.034) (Figure 2C). The three lower doses of AITC, however, seem to show similar efficacy in reducing tumor burden, suggesting that the maximal anticancer efficacy of AITC in this model might have been reached. The seemingly modest inhibitory efficacy of AITC might be related to the explosive tumor growth rate. Interestingly, histological examination of the tumors showed increasing inhibition of muscle invasion with decreasing AITC dose. There were only 18.9% of the tumor-bearing bladders showing muscle invasion in rats treated with AITC at 10 μmol/kg compared with 27% in rats treated with AITC at 25 μmol/kg, 50% in rats treated with AITC at 50 or 300 μmol/kg and 71% in the control rats (Figure 2D). Shown in Figure 2E are representative images of normal rat bladder wall (E1), a superficial AY-27 rat bladder tumor (E2) and a muscle-invasive AY-27 rat bladder tumor (E3).
Fig. 2.
Inhibition of cancer development by AITC. Female F344 rats were inoculated with AY-27 cells to initiate simultaneous development of both orthotopic bladder tumor and subcutaneous tumor in each rat. Oral adminstration of AITC at 0, 10, 25, 50 and 300 μmol/kg once daily was started 1 day after cancer cell inoculation and ended 3 weeks later. The number of rats per group varied from 6 to 33; some of the data, particularly the control group (n = 33) and the group treated with the lowest dose of AITC (n = 23), were pooled from several experiments. (A) The initial and final body weights. (B and C) The weights of subcutaneous tumors and bladder tumors. Bladder tumor weight was calculated by subtracting the average normal bladder weight from tumor-bearing bladder weight; *P < 0.05. (D) Percentage of bladders where the tumor invaded the muscle tissue. (E) Hematoxylin and eosin staining of the normal rat bladder wall (E1), a superficial rat bladder tumor (E2) and a rat bladder tumor that invaded the muscle layer (E3).
Molecular targets of AITC
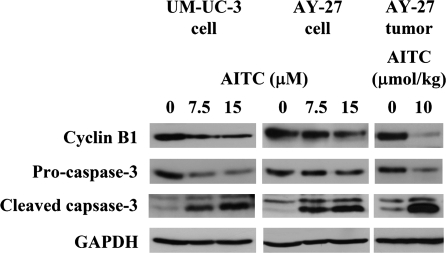
Several proteins that are known to be important for G2/M phase and apoptosis were assessed in AITC-treated bladder cells and tissues. In cultured AY-27 cells and UM-UC-3 cells, after treatment with AITC at the concentrations (7.5 and 15 μM) that caused marked and dose-dependent G2/M arrest and apoptosis (see Figure 1), there were marked down regulation of cyclin B1 (a key G2/M regulator) and activation of caspase 3 (a key executioner caspase) (Figure 3). More importantly, strong downregulation of cyclin B1 and activation of caspase 3 were also consistently detected in AY-27 cell-derived bladder tumors from rats treated with AITC at 10 μmol/kg (Figure 3). The modulation of cyclin B1 and caspase 3 by AITC in the tumor tissue in vivo suggests that AITC was able to penetrate the tumor tissue in vivo (the tumors were removed from rats after 3 weeks of AITC treatment).
Fig. 3.
Molecular targets of AITC in bladder cancer. UM-UC-3 cells and AY-27 cells in culture treated with AITC for 24 h. The results are representative of triplicate experiments. The bladder tumors were removed from rats, which were treated with AITC orally at 10 μmol/kg once daily for 3 weeks, starting 1 day after intravesicle inoculation of AY-27 cells. The results are representative of tumors from other rats. Cell lysates and tumor tissue homogenates were analyzed by western blot analysis, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control.
Two proteins, which are known to be important in cancer invasion and metastasis, matrix metalloproteinase (MMP)-2/-9 were also assessed. Despite strong inhibition by AITC of muscle invasion of bladder tumor (Figure 2D), AITC had no impact on the levels of MMP-2 and MMP-9 in either AY-27 cells and UM-UC-3 cells in vitro or AY-27 cell-derived bladder tumors in vivo (results not shown).
AITC levels in the plasma, urine and tumor tissues
Pharmacokinetic studies of AITC were subsequently undertaken, in order to better understand the exceptional anticancer potency of AITC in the bladder. Rats were administered a single oral dose of AITC at 10 or 300 μmol/kg, and urine and blood were collected at several time intervals up to 24 h. To compare AITC levels between the orthotopic bladder tumor tissue and the subcutaneous tumor tissue, rats bearing both types of tumors (3 weeks after AY-27 cell inoculation) were administered a single oral dose of AITC at 300 μmol/kg, and the tumor-bearing bladders and subcutaneous tumors were removed at different time points. All specimens were subjected to the cyclocondensation assay for measurement of AITC equivalent.
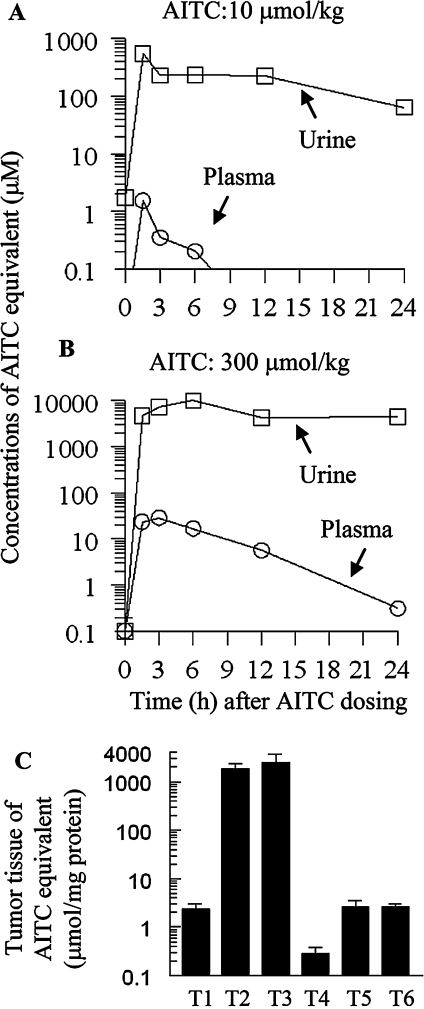
Oral administration of AITC led to dose-dependent increase in the levels of AITC equivalent in both plasma and urine (Figure 4A and B). Peak plasma concentrations of AITC equivalent of 1.5 μM and 23.4 μM were reached within 3 h of AITC dosing at 10 and 300 μmol/kg, respectively. The corresponding urinary concentrations of AITC equivalent of 0.6 and 9.9 mM were reached within 6 h of dosing. Thus, urinary peak concentrations of AITC equivalent were 400- to 423- fold higher that that in the plasma. Moreover, while the plasma concentrations of AITC equivalent declined rapidly thereafter (half-life of <3 h at the low AITC dose and ∼6 h at the higher dose), urinary concentrations of AITC equivalent declined slowly. For example, the average 24 h urinary concentrations of AITC equivalent were 63.8 μM (low AITC dose) and 4.5 mM (high AITC dose), which were 11.6–45.2% of the peak urinary concentrations, but were 4911- to 14 501-fold higher than corresponding plasma concentrations at 24 h after dosing.
Fig. 4.
Pharmacokinetics of AITC in F344 rats. (A and B) Five groups of four to six female rats were administered a single oral dose of AITC at 10 or 300 μmol/kg and then kept in metabolism cage for urine collection (1 rat per cage) for 1.5, 3, 6, 12 and 24 h. At the end of each time period, blood was drawn from one group of rats, from which plasma was prepared. (C) Rats bearing both orthotopic (T1, T2 and T3) and subcutaneous bladder tumors (T4, T5 and T6) 3 weeks after AY27 cell inoculation, were administered a single oral dose of AITC at 300 μmol/kg. Both orthotopic and subcutaneous tumors were removed from the rats at 0 h (T1 and T4), 1.5 h (T2 and T5) and 6 h (T3 and T6) after AITC dosing. Concentrations of AITC equivalent in plasma, urine and tumor tissue homogenates were determined by the cyclocondensation assay. Each value is a mean ± SE.
Low basal levels of AITC equivalent were detected in both orthotopic and subcutaneous bladder tumor tissues, and the level in the orthotopic tumors (2.4 μM) was significantly higher than that in the subcutaneous tumors (0.3 μM) (Figure 4C). It is possible that the rat diet might contain a trace amount of AITC or related compounds, which were preferentially delivered to the bladder and detected by the cyclocondensation assay. Levels of AITC equivalent in the orthotopic bladder tumors were 1.9 and 2.5 mM at 1.5 and 6 h after the rats were given a single oral dose of AITC at 300 μmol/kg, which were 790–1071 times higher than the basal level. In contrast, the concentrations of AITC equivalent in the subcutaneous tumor tissues at both time points were 2.6 μM, which, albeit nine times higher than the basal concentration, was 720-fold to 986-fold lower than that in the orthotopic tumor tissues.
These data show clearly that orally administered AITC is selectively delivered to the cancer tissue in the bladder through urinary excretion.
Discussion
AITC potently inhibited the growth of both human bladder cancer cells and rat bladder cancer cells in the present study. Similar results were obtained in several other human bladder cancer cell lines (results not shown). Our previous study showed that AITC also potently inhibited the growth of human bladder cancer RT-4 cells (16), which were derived from a low-grade superficial bladder cancer (20). Thus, the growth-inhibitory activity of AITC against bladder cancer cells is not cell line-specific. The growth-inhibitory activity of AITC undoubtedly resulted at least in part from its ability to elicit cell cycle arrest and apoptosis, as AITC-treated bladder cancer cells were strongly arrested in G2/M phase and showed significant formation of sub-G1 population and cytoplasmic accumulation of histone-associated DNA.
However, AITC had much less effect on normal human bladder epithelial cells as follows: its IC50 value in these cells was >21 times higher than that in bladder cancer cells (Figure 1B), and it failed to elicit significant cell cycle arrest and apoptosis in the normal cells at the concentrations that were highly effective against the cancer cells (Figure 1C and D). These data suggest that AITC may selectively target malignant cells versus normal cells in the bladder in vivo. Interestingly, two previous studies have also shown that AITC is significantly more toxic to human prostate cancer cells and colon cancer cells than to their normal counterparts (21,22). Further study is warranted to understand how AITC selectively targets cancer cells. In addition to its selective toxicity toward bladder cancer cells, it also appears probable that AITC may be delivered more readily to bladder cancer tissue than to the normal bladder tissue. The normal bladder epithelium is believed to be not readily penetrated by an intervention agent due to its protective mechanisms, including tight junctions, thickened apical membrane and coverage by a mucopolysaccharide layer (23). Such barriers do not appear to exist in the bladder cancer tissue (see the supplementary Figure 1, available at Carcinogenesis Online).
In the orthotopic rat bladder cancer model, which mimics the development and recurrence of bladder cancer in humans, AITC significantly inhibited cancer growth and muscle invasion at the oral dose of 10 μmol/kg/day (1 mg/kg/day). This dose is ∼30 times lower than what was previously used to inhibit the growth of subcutaneous xenografts of human prostate cancer cells and dimethyhydrazine-induced formation of aberrant crypt foci in the colonic mucosa (24,25). Indeed, we found that AITC at 25 μmol/kg/day was totally ineffective against the growth of subcutaneous xenografts of the same bladder cancer cells. The exceptional potency of AITC against the cancer in the bladder is explained by our discovery that AITC is selectively delivered to bladder through urinary excretion.
The metabolites of isothiocyanates formed in the mercapturic acid pathway, including those of AITC, are believed to be biologically inactive themselves but serve as carriers of their parent compounds by dissociation (26,27). The half-life of dissociation of NAC conjugates of isothiocyanates, the main urinary metabolites, in an aqueous environment at pH 7.4 and 37°C is ∼1 to 3 h (27). Increased storage time of urine in the bladder probably facilitates the formation of free isothiocyanates from the conjugates and increases the exposure of cancer cells to the compounds present in the urine. In this connection, it seems reasonable to speculate that AITC may be more efficacious in humans than in rats because rats are known to urinate frequently, e.g. approximately every 15 min in female Lewis rats (28), whereas humans may hold up their urine for several hours.
Two well-known chemopreventive/chemotherapeutic targets were modulated by AITC in both cultured bladder cancer cells and bladder cancer tissues in vivo, including cyclin B1 and caspase 3. Since cyclin B1 is critical for G2/M phase progression and caspase 3 is key to apoptosis, our findings provide insight into the molecular basis of AITC-induced G2/M arrest and apoptosis. However, it remains unclear how AITC inhibited muscle invasion of bladder tumor in vivo. MMP-2 and MMP-9 are well known to promote cancer invasion and metastasis, and a previous study showed that AITC as well as its NAC conjugate inhibited MMP-2 and MMP-9 in cultured SK-Hep1 human hepatoma cells (29). But AITC had no impact on these proteins in the bladder cancer cells and tissues in our study.
Several important questions about AITC remain. In the present study, the lowest AITC dose tested turned out to be the most effective dose. The reason is not known, nor is it known if AITC dose can be lowered further without compromising or perhaps even increasing its anticancer efficacy. Moreover, AITC treatment in the present study was initiated one day after cancer cell inoculation. Although this protocol seems to resemble the current clinical approach where a transurethral intravesicle therapy is initiated soon after surgical resection of a bladder tumor, additional studies where AITC is administered to the animals either before cancer cell inoculation or after bladder cancer is fully established is probably to shed additional light on the anticancer efficacy of this compound. Also, AITC is known to exist in a variety of cruciferous vegetables. It will be important to determine if some of these vegetables or their extracts also inhibit bladder cancer development. In a previous study, in which F344 rats and B6C3F1 mice (both male and female) were administered orally with AITC at 120 and 250 μmol/kg 5 days per week for 103 weeks, urinary bladder cancer was detected in 4 and 8% of male rats treated with the low and high doses of AITC, respectively, but no bladder tumor was detected in any other groups (30). Although this result raises a concern of potential toxicity of AITC in human bladder, those doses are 12- to 25-fold higher than the anticancer dose recorded in the present study. No apparent AITC-induced toxicity in normal bladder epithelium was detected in our study.
In summary, AITC is a highly promising bladder cancer chemopreventive/therapeutic agent, with a particular clinical potential for use against disease recurrence and progression. It causes strong cell cycle arrest and apoptosis in bladder cancer cells but is markedly less toxic to normal human bladder epithelial cells. It inhibits cancer development in the bladder at a very low dose level and is selectively delivered to the bladder cancer tissue through urinary excretion. In view of these findings, clinical assessment of AITC for prevention of bladder cancer recurrence appears to be warranted. Although AITC at 1 mg/kg seems to be beyond the average dietary consumption level of AITC in humans, which is estimated at <1 mg/day (∼10 μg/kg) (31), such a dose may be readily achievable in humans, since cruciferous vegetables such as fresh wasabi may yield as much as 3.4 mg AITC per gram (10).
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (R01CA124627) to Y.Z.; R25 Postdoctoral Fellowship from the National Cancer Institute (CA114101) to L.T.
Supplementary Material
Acknowledgments
We wish to thank Dr Karoly Toth of Roswell Park Cancer Institute for valuable advice and Dr Ronald B.Moore of University of Alberta in Canada for providing AY-27 cells.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AITC
allyl isothiocyanate
- HUC
human urothelial cells
- IC50
half maximal inhibitory concentration
- MMP
matrix metalloproteinase
- NAC
N-acetylcysteine
- PBS
phosphate-buffered saline
References
- 1.Negri E, et al. Epidemiology and prevention of bladder cancer. Eur. J. Cancer Prev. 2001;10:7–14. doi: 10.1097/00008469-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lapham RL, et al. Pathology of transitional cell carcinoma of the bladder and its clinical implications. Semin. Surg. Oncol. 1997;13:307–318. doi: 10.1002/(sici)1098-2388(199709/10)13:5<307::aid-ssu4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Pow-Sang JM, et al. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7:335–339. doi: 10.1177/107327480000700402. [DOI] [PubMed] [Google Scholar]
- 4.Amling CL. Diagnosis and management of superficial bladder cancer. Curr. Probl. Cancer. 2001;25:219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 5.Michaud DS, et al. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J. Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 6.Nagano J, et al. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. Int. J. Cancer. 2000;86:132–138. doi: 10.1002/(sici)1097-0215(20000401)86:1<132::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Tang L, et al. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 2008;17:938–944. doi: 10.1158/1055-9965.EPI-07-2502. [DOI] [PubMed] [Google Scholar]
- 8.Munday R, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 9.Uematsu Y, et al. Determination of isothiocyanates and related compounds in mustard extract and horseradish extract used as natural food additives. Shokuhin Eiseigaku Zasshi. 2002;43:10–17. doi: 10.3358/shokueishi.43.10. [DOI] [PubMed] [Google Scholar]
- 10.Sultana T, et al. Effects of fertilisation on the allyl isothiocyanate profile of above-ground tissue of New Zealand-grown wasabi. J. Sci. Food Agric. 2002;82:1477–1482. [Google Scholar]
- 11.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2009 doi: 10.1002/mnfr.200900323. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannou YM, et al. Allyl isothiocyanate: comparative disposition in rats and mice. Toxicol. Appl. Pharmacol. 1984;75:173–181. doi: 10.1016/0041-008x(84)90199-6. [DOI] [PubMed] [Google Scholar]
- 13.Bollard M, et al. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem. Toxicol. 1997;35:933–943. doi: 10.1016/s0278-6915(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 14.Munday R, et al. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr. Cancer. 2006;54:223–231. doi: 10.1207/s15327914nc5402_9. [DOI] [PubMed] [Google Scholar]
- 15.Tang L, et al. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J. Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, et al. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SM, et al. Transplantation and cell culture of rat urinary bladder carcinoma. Invest. Urol. 1981;19:136–141. [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z, et al. Characterization of a novel transplantable orthotopic rat bladder transitional cell tumour model. Br. J. Cancer. 1999;81:638–646. doi: 10.1038/sj.bjc.6690741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Carbayo M, et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–6980. [PubMed] [Google Scholar]
- 21.Xiao D, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 22.Musk SR, et al. Allyl isothiocyanate is selectively toxic to transformed cells of the human colorectal tumour line HT29. Carcinogenesis. 1993;14:2079–2083. doi: 10.1093/carcin/14.10.2079. [DOI] [PubMed] [Google Scholar]
- 23.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava SK, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- 25.Smith T, et al. Allyl isothiocyanate seletively kills undifferentiated HT29 cells in vitro and suppresses aberrant crypt foci in the colonic mucosa of rats. Biochem. Soc. Trans. 1996;24:381S. doi: 10.1042/bst024381s. [DOI] [PubMed] [Google Scholar]
- 26.Bruggeman IM, et al. Glutathione- and cysteine-mediated cytotoxicity of allyl and benzyl isothiocyanate. Toxicol. Appl. Pharmacol. 1986;83:349–359. doi: 10.1016/0041-008x(86)90312-1. [DOI] [PubMed] [Google Scholar]
- 27.Conaway CC, et al. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem. Res. Toxicol. 2001;14:1170–1176. doi: 10.1021/tx010029w. [DOI] [PubMed] [Google Scholar]
- 28.Luber-Narod J, et al. Role of substance P in several models of bladder inflammation. Urol. Res. 1997;25:395–399. doi: 10.1007/BF01268854. [DOI] [PubMed] [Google Scholar]
- 29.Hwang ES, et al. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp. Biol. Med. 2006;231:421–430. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- 30.Dunnick JK, et al. Carcinogenesis bioassay of allyl isothiocyanate. Fundam. Appl. Toxicol. 1982;2:114–120. doi: 10.1016/s0272-0590(82)80091-2. [DOI] [PubMed] [Google Scholar]
- 31.NAS/NRC. Poundage Update of Food Chemicals 1982. Washington, DC: National Academy of Sciences and the National Research Council; 1982. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.